Abstract
Focusing and separation of cells by microfluidic techniques are significant steps in many applications, such as single-cell analysis and disease diagnosis. Among the microfluidic techniques, passive magnetophoresis, as a label-free manner, can manipulate samples by means of magnetic field. Nowadays, most magnetic fields are generated by permanent magnets and electromagnets with large size. However, it is difficult to assemble a magnetic array using permanent magnets or electromagnets to optimize the field distribution. To produce a flexible magnetic field, a micro-magnet made by NdFeB powder and polydimethyl siloxane is proposed in this paper, and those magnetized micro-magnets are arranged into different arrays according to the arrangements of their magnetization directions. Meanwhile, a microfluidic chip containing magnetized micro-magnet arrays is designed for focusing and separating polystyrene microbeads with different diameters. The focusing and separation behaviors of microbeads in the designed microfluidic system are numerical and experimental investigated. In addition, the effects of flow rate and the arrangement of the magnetic micro-magnet array on microbead focusing and separation are discussed. Finally, a multistage microfluidic chip is designed to successfully isolate 5 μm-diameter, 10 μm-diameter, and 15 μm-diameter microbeads from their mixture at a flow rate of 240 μL/min with high purity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Microfluidic technology has proven to precisely and tunably manipulate micro-scale fluids, microbeads, and cells (Nilsson et al. 2009; Pachmann et al. 2008; Zhou and Wang 2016a). Over the past several decades, microfluidics has attracted attention in many applications, such as disease diagnostics (Li et al. 2013; Hyun et al. 2015), single cell analysis (Yin and Marshall 2012; Merrin 2019) and environmental monitoring (Marle and Greenway 2005; Jokerst et al. 2012). Nowadays, external forces (active methods) or using intrinsic hydrodynamic forces (passive methods) are always used in microfluidic system to focus and separate microbeads or cells. Passive methods are mainly dependent on the geometrical structure of the microchannel, including inertial microfluidic (Xu et al. 2021; Zhou and Papautsky 2013), pinched flow fractionation (Lu and Xuan 2015) and deterministic lateral displacement (Tottori and Nisisako 2018). They allow to control the behavior of targets at a high flow rate. However, the low separation precision and a risk of clogging in microfluidic systems (McGrath et al. 2014; Shen et al. 2019) limit their applications.
On the other hand, active technologies, which generally introduce external driven forces, such as magnetophoretic, dielectrophoretic, optical, or acoustic force, into microfluidic system, can control the motion of microbeads precisely (Samantaa et al. 2017; Çetin and Li 2011; Augustsson et al. 2012). In particular, magnetophoresis is able to avoid generating heat and does not affect properties of the sample solution such as pH value, ion concentration, or surface charge (Suwa and Watarai 2011; Shi et al. 2020). Based on the difference in magnetization coefficient between carrier fluid and microbeads, the magnetophoresis is divided into active and passive (Alnaimat et al. 2018). The active magnetophoresis will happen when the susceptibility of microbeads is larger than that of carrier fluid. The microbeads will be drawn towards the maxima of the non-uniform magnetic field. On the other hand, when the diamagnetic microbeads are dispersed in a magnetic medium, then microbeads will be drawn towards the minima of the nonuniform magnetic field and this is specifically referred to as passive magnetophoresis. Compared with the active magnetophoresis, the passive magnetophoresis has been attracting increasing attention because most cells and viruses are diamagnetic. Furthermore, as a label-free technology, the passive magnetophoresis could effectively reduce the operation steps and avert contamination of samples.
In the passive magnetophoresis, permanent magnets and electromagnets are widely used to generate magnetic fields. Chung et al. proposed a simple, fully integrated, and two-dimensional electromagnet using MEMS, which sorts particles efficiently (Chung et al. 2016). Due to Joule heating, the microfluidic systems with the electromagnets were usually complicated and large in size. Hence, it is necessary to design a magnetic field that does not generate joule heat. Except for the above microfluidic systems with electromagnets, Zeng et al. enriched yeast cells in a straight microchannel combined with two attracting and two offset permanent magnets and separated 3 μm and 10 μm-diameter diamagnetic polystyrene particles in the same microfluidic chip using a water-based ferrofluid (EMG 408) (Zeng et al. 2013a, 2013b). Besides, Shiriny et al. proposed a simple microfluidic device to fractionate WBCs and RBCs from whole blood using passive and active magnetophoretic force applied by Halbach array of three permanent magnets when flow rate is 7 μL/h (Shiriny and Bayareh 2020). Even though some achievements have been made using permanent magnets, the accuracy of separation and the flow rate still needs to be improved due to the larger size of the permanent magnet compared with the microchannel. Therefore, the micro-magnet with mini size is widely used for driving microbeads precisely in recent years. So far, several methods for fabricating micro-magnets have been developed and applied. Huang et al. adopted thermal deposition to make cylindrical micro-magnets, which captured target cells (Huang et al. 2015). Besides, Chen et al. reported an inkjet-printed microscale magnetic structure that can be integrated on regular glass slides for the immunomagnetic screening of rare circulating tumor cells (Chen et al. 2015). These methods are complicated and need special equipment. In 2016, Zhou et al. proposed and demonstrated a simple and low-cost technique to fabricate microscale permanent magnetic microstructures and integrate them into microfluidic devices (Zhou and Wang 2016b). By injecting a mixture of iron powder and PDMS into a prefabricated channel, an iron–PDMS microstructure was fabricated next to a microfluidic channel. However, this method limits the distance between the micro-magnet and the fluid. Descamps et al. reported a method to fabricate arrays of micrometer-sized permanent magnets consisting of mixing a NdFeB magnetic powder with PDMS and designed the MagPure chip to sort cancer tumor cells (CTCs), which was performed on 7.5 mL of blood and takes between 3 and 4 h (Descamps et al. 2022). This paper will draw inspiration from the simple manufacturing method proposed by Zhou et al. to make micro-magnets and assemble them into the microchannel to drive samples closely.
In this work, a micro-magnet that was made of NdFeB powder and PDMS was proposed, and arrays of micro-magnets with different directions of magnetization were fabricated to form external magnetic field. This magnetic field can avoid the production of external heat because there is no electricity. Then, a microfluidic chip containing micro-magnet arrays was designed to focus and separate two sizes of diamagnetic microbeads. In this microfluidic chip, the focusing and separation of microbeads were investigated in numerical simulation and experiment simultaneously. Moreover, the ferrofluid flow rate and the arrangement of micro-magnet array were also discussed for enhancing the separation of two sizes of microbeads. Finally, based on our previous research (Chen et al. 2022), the multistage microfluidic chip was designed to separate three sizes of diamagnetic microbeads at a high flow rate.
2 Theoretical background
The ferrofluid is regarded as a uniform magnetic fluid for which the steady-state flow is described by the Navier–Stokes equations (Zhu and Nguyen 2012; Khashan and Furlani 2013),
where ρf is the density of the ferrofluid, u is the velocity of the ferrofluid, P is the pressure of the ferrofluid, η is the viscosity of the ferrofluid. In the Eq. (2), fm is the magnetic body force given by (Rosensweig 1987),
where µ0 is the permeability of the free space. Mf is the magnetization of ferrofluid, which is collinear with the magnetic field H and is determined using the Langevin function (Rosensweig 1987). In the Eq. (4), c is the concentration of the ferrofluid, Md is the saturation moment of magnetic nanoparticles with Md being the magnitude, d is the diameter of magnetic nanoparticles in the ferrofluid, H is the magnitude of H, kB is the Boltzmann constant, and T is the ferrofluid temperature in kelvin.
The fourth term on the right-hand side of Eq. (2), fp, is the force density caused by the counter-drag force exerted by the diamagnetic microbeads on the fluid, which is written as (Khashan and Furlani 2013)
where \(\dot{m}_{p}\) is the mass flow rate (kg/s) of each parcel of microbeads in the simulation, up is the velocity of diamagnetic microbeads, ∆t is the time that a parcel resides in one computational cell (Gomez-Pastora et al. 2018), Dp is the diameter of diamagnetic microbeads, CD is the drag coefficient, and Re is the relative Reynolds number. In the Eq. (6), the sum is taken over all microbeads that reside in the computational element. In our simulation, the fp is used to reflect the influence of the motion of microbeads on the surrounding fluid.
The diamagnetic microbeads in the ferrofluid are driven by passive magnetic force, viscous drag force, gravity and buoyant force. In this model, the Peclet number (Pe = Lup/D, where L is the characteristic length of the microchannel, up is the mean velocity of particles and D is the mass diffuse coefficient) is large enough (Pe ≫ 1) so that Brownian motion of microbeads is not taken into account (Lim et al. 2014). The trajectories of diamagnetic microbeads are governed by Eq. (8) based on the Newton’s second law (Maxey and Riley 1983; Zhu et al. 2012).
where mp is the mass of diamagnetic microbeads.
Due to smaller magnetization of microbeads than the suspending ferrofluid, the magnetic force acting on a diamagnetic microbead is given by (Friedman and Yellen 2005),
where Mp is the magnetization of microbeads, which is given by (Liang et al. 2013)
where χp is the susceptibility of microbeads. In the case that the susceptibility of diamagnetic microbeads is much less than 1, the passive magnetic force is simplified as
For spherical microbeads, the viscous drag force is written as
where fD is the hydrodynamic drag force coefficient that accounts for the wall retardation effects (Gijs et al. 2010).
The gravitational and buoyant force acting on the microbead is given by
where Vp is the volume of the microbead, g is the gravitational constant, ρp is the density of the microbead.
The numerical simulation is carried out using the discrete phase model (DPM) of the ANSYS FLUENT program, in which the diamagnetic microbeads are treated as discrete phase and the ferrofluid is treated as continuous phase. Meanwhile, the simulations are performed in 3D. The positions of particles are obtained with the variation of time by transient solver. In the model, the inlet of the microchannel is set as velocity-inlet calculated by flow rate and the cross-sectional area of the microchannel, and the outlet is at zero-gauge pressure. A no-slip boundary condition is applied to the walls of microchannel.
3 Experimental method
3.1 Design of microchannels
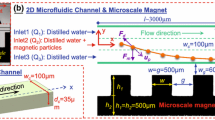
Figure 1 shows the schematic diagram of the designed single microchannel with magnetized micro-magnet arrays. The width of microchannel (wc) was 1.6 mm, the width of the main flow channel (wmc) was 0.74 mm, and the length of microchannel (lc) was 25 mm. The sides of the micro-magnet were equal, w = h = 430 μm. Near the inlet of microchannel, two micro-magnet arrays were positioned symmetrically on both sides of the channel and each array was composed of sixteen micro-magnets. The length of bilateral magnetized micro-magnet arrays (lm1) was 6.88 mm. The length of unilateral magnetized micro-magnet array (lm2) was 8.6 mm and the number of micro-magnets was twenty. The length of space between bilateral and unilateral magnetized micro-magnet arrays (lcc) was 2 mm. The flow rate of main inlet was equal to the flow rate of sheath inlet, which was Q1. The flow rate on the outlet side was Q2, which was equal to 2Q1.
Considering that the micro-magnet arrays could be assembled by micro-magnets with freedom, three types, including the same directional array (type A), the traditional alternating array (type B), and the Halbach array (type C), as shown in Fig. 2, would be studied in this paper. Among the three types of arrays, the Halbach array was actually a specific arrangement of a series of permanent magnets, which was first designed by the physicist Klaus Halbach in 1980. In the Halbach array of magnets, the magnets’ polarization angles rotated as much as 90°, and four magnets were in a cycle. The red-dash rectangles represented the observation areas where the images would be captured by the microscope.
Figure 3 shows the multistage microfluidic chip containing three parts marked by red dotted lines, and Fig. 4 shows the enlarged views of three parts separately. Firstly, the primary part was the circular split-recombination microchannel, which has been investigated in our previous paper (Chen et al. 2022). The left end was the flow inlet, which was the inlet of the multistage microfluidic chip, as shown in Fig. 4a. All of the microbeads with different diameters would be injected into microchannel form inlet. The purpose was to separate microbeads with significant size differences. Then the secondary part was a combination of straight channels with different cross-sections. The inlet of the secondary part was connected to the outlet ③. ④ ~ ⑧ all represented outlets of the secondary part. The purpose was to focus microbeads into a thin beam and reduce the flow rate for passive magnetophoretic separation in the next part.
Finally, the tertiary part was the microchannel with magnetized micro-magnet arrays as shown in Fig. 4c. The difference between the tertiary part and the single microchannel (Fig. 1) was the size of the microchannel and the micro-magnets. The width of microchannel changed from wc = 1.6 mm to Wt = 2.2 mm. Besides, in the tertiary part of the multistage microfluidic chip, the sides of the micro-magnet were W = H = 860 μm, which were different from Fig. 1. The inlet of the tertiary part was connected to the outlet ④. The rate of sheath flow nearly equaled the flow rate of the tertiary part inlet. In the tertiary part with micro-magnet arrays, the different diameters of microbeads would be focused, distributed in different locations, and separated successfully. In addition, the red-dash rectangles were outlets for three parts, where the images would be captured by the microscope. The detailed parameters of the multistage microfluidic chip were given in the Support Information.
3.2 Device fabrication and materials
In this paper, standard soft lithography was adopted to fabricate the microchannel (Chen et al. 2022). Briefly, the SU-8 photoresist (SU-8 2050, MicroChem, USA) was spun onto a 4-inch Si wafer, and the coating thickness was about 50 μm. After soft baked, the wafer was exposed through a photomask with the microchannel geometry using an exposure machine (UV KUB2, Kloe, France). The wavelength was 365 nm and the exposure does was 100.2 mJ/cm. The exposed wafer was then developed in propylene glycol methyl ether acetate (PGMEA, Xi'an Boyan Nano Information Technology Co., Ltd, China) and iso-propyl alcohol (IPA, Xi'an Boyan Nano Information Technology Co., Ltd, China), and rinsed and dried to obtain the master mold. Next, the polydimethyl siloxane (PDMS, Sylgard 184, Dow Corning, USA) was casted on the SU-8 master mold to replicate microchannel features. Finally, the PDMS layer was peeled off from the SU-8 master mold after curing and it was bonded to a glass slide by a plasma cleaner (PDC-32G-2, Harrick Plasma, USA).
And then, micro-magnets were manufactured according to six steps as shown in Fig. 5. Firstly, the positive mold was made as above and it was placed on test bed. Next, the polydimethyl siloxane (PDMS and Sylgard 184, Dow Corning, USA) was dumped on the positive mold. After casting and solidification of the mixture, a negative mold with a concave structure was obtained. Then, the NdFeB powder (MQFP, Magnequench, USA), the particle diameter of which was 5 μm and the shape of which was spherical, and PDMS were put into a beaker and mixed by a stirring rod for a while. The mixture of PDMS and NdFeB powder was fully poured into the negative mold. And then, a blade was used to scrape the redundant mixture. Finally, micro-magnets were cured, picked up by a tweezer and magnetized.
Micro-magnets were assembled together with PDMS applied to connect neighboring micro-magnets. The micro-magnet array was fabricated and carefully placed into the PDMS layer by a tweezer and an assistant model (Fig. S2). In this way, the micro-magnet array is responsible for the function of the channel walls. Besides, for perfectly bonding the PDMS layer and the glass slide, the thickness of the micro-magnet is about 50 μm, which is equal to that of the microchannel.
The fluorescent polystyrene microbeads (Huge Biotechnology, China) with diameters of 5 μm, 10 μm and 15 μm were used in the passive magnetophoretic experiment, which had similar sizes to red blood cells (3–8 μm) (Kabacaoğlu and Biros 2019), white blood cells (8–14 μm) and CTCs (15–20 μm) (Munaz et al. 2018). The colors of the microbeads are green, red and green, respectively. The density of the microbeads is 1.062 × 103 kg/m3. Microbeads were suspended in ferrofluid (EMG 707, Ferrotec Corp., USA), whose initial concentration was 2%vol. In experiments, the concentration of ferrofluid was 1%vol. by dilution with deionized (DI) water. Then 0.5 wt% of surfactant (Tween 20, Sigma Aldrich, USA) was added to the solution to avoid the aggregation of microbeads. Finally, the solution was injected into the microchannel at different flow rates by a syringe pump (Elite 11, Harvard Apparatus, USA). The image of fluorescent microbeads was captured from the bottom wall of microchannel by the inverted fluorescence microscope (AE31E, Motic, China). Adobe Photoshop (Adobe Systems Inc., USA) and ImageJ software (NIH, USA) were used to process and analyze the captured images.
3.3 Micro-magnet testing
The pulsed magnetizer (PM2520, Hangzhou Feiyu Magnetoelectric Facilities Corp., China) was used to form a strong magnetic field and magnetize micro-magnets by releasing energy, which was generated by a magnetizing coil. The micro-magnets were put into the magnetizing coil and the magnetization process could be finished by operating the panel of the pulsed magnetizer. Then, the surface magnetic distribution test system (TD 8410, Tunkia Corp., China) was used to measure the residual magnetic flux density of the magnetized micro-magnet under different magnetizing fields.
4 Results and discussion
4.1 Testing
4.1.1 Residual magnetic flux density
The residual magnetic flux density of the magnetized micro-magnet under different magnetizing fields has been plotted in Fig. 6. The sample used for testing was a cuboid magnet with a side length of 1 cm and the measurement position was the center of the vertical plane in the direction of magnetization, where the residual magnetic flux density was the largest. With the increase of the magnetizing magnetic field, the residual magnetic flux density gradually increased. When the magnetizing magnetic field was greater than 1600 kA/m, the residual magnetic flux density of the magnet basically remained unchanged.
In addition, as the mass ratio of PDMS to NdFeB was 1:2, the magnetic flux density of the magnet was about twice that of the ratio of 1:1. Based on the measurement, the residual magnetic flux density of micro-magnet with a ratio of 1:2 was about 60 mT, which was used in the numerical simulation of magnetic field.
4.1.2 Magnetic force
For different arrangements of micro-magnet array, the magnetic force Fdiay acting on 10 μm-diameter microbeads in different locations of the y axis was calculated firstly. The parameters in simulation were listed in Table 1. Figure 7a shows a conceptual diagram of 10 μm-diameter microbeads in different locations of the y axis. For example, when y = 0.1 mm, the Fdiay from x = – 3 mm to x = 3 mm was simulated. The distributions of Fdiay under the three types of micro-magnet arrays are shown in Fig. 7b–d based on the Eq. (4), (5) and (11). It was observed that the micro-magnet array with type C generated the largest Fdiay, while the Fdiay produced by the micro-magnet array with type A was similar to that of type B and was smaller. The phenomenon was caused by that the Halbach array can strengthen the magnetic field on one side of the magnet (Wu et al. 2018). Besides, type A and type B were similar, which as shown in Fig. S3 of Supporting Information.
On the one hand, the force Fdiay showed a decreasing trend when the x-axis exceeds about ± 1 mm in all results because the width of the micro-magnet array was 0.43 mm × 4 = 1.72 mm, and the left and right borders were 0.86 mm and -0.86 mm, respectively. When the microbeads exceeded the range of the magnet array on the x-axis, the force Fdiay would decrease to about zero. On the other hand, for all arrangement of micro-magnets, the magnetic force Fdiay decreased rapidly with the increasing y coordinate. When y = 0.2 mm, the magnetic force Fdiay generated by the micro-magnet array with type C was still the largest, followed by the micro-magnet array with type A, and the smallest was the micro-magnet array with type B. Based on Eq. (8), the acceleration of microbeads would be faster when the magnetic force was higher. This phenomenon showed that the weakening degree of the magnetic field generated by the micro-magnet array with type C was the slowest with the increase in the value of y, while that of the micro-magnet array with type B was the fastest. Therefore, although the variation in Fdiay of type C along the x-axis was significant when y = 0.1 mm, the driven capability of type C was the strongest. In other words, the magnetic force in the y-axis direction had a wider range, which was generated by the micro-magnet array with type C.
4.2 Microbead focusing under bilateral micro-magnet arrays
4.2.1 Effect of flow rate
Based on standard soft lithography, the manufactured microfluidic chips are shown in Fig. 8, which will be employed in subsequent experimental studies.
Firstly, the single microchannel (Fig. 8a) was adopted to carry out the experimental and numerical investigation on the separation of two sizes of microbeads. Before the separation of two sizes of microbeads, focusing of microbeads is a very important step. Thus, the behavior of 10 μm-diameter microbeads in the microchannel containing bilateral magnetized micro-magnet arrays was firstly investigated. The microbead converging process was observed in Fig. S4. Figure 9 shows the distribution of 10 μm-diameter microbeads at the ends of the bilateral micro-magnet arrays with different ferrofluid flow rates. The micro-magnet array of type A was first employed as shown in Fig. 9a, which was observed under bright field of the microscope. The solid white lines represented microchannel walls and the dotted white lines represented micro-magnet array edge.
The experimental results demonstrated that the width of the microbead stream became thinner with the decreasing of ferrofluid flow rate. Meanwhile, the variation of the microbead stream in numerical simulations exhibited similar variation tendency. In order to quantitatively analyze the focusing of microbeads, the parameter Δym, describing the width of the microbead stream, was introduced as indicated in Fig. 9b to evaluate the focusing efficiency. Correctly, Δym referred to the distance between the upper and lower edges of the microbead beam at the end of the bilateral micro-magnet arrays.
Figure 10 depicted the variation of Δym for 10 μm-diameter microbeads under the bilateral magnetized micro-magnet arrays as shown in Fig. 9a at different ferrofluid flow rates. Even though the tendency of Δym variation with the flow rate was similar, a comparison of experimental and numerical results showed that as Q1 > 7 μL/min, the numerical width of the microbead stream was larger than experiments, and when Q1 < 7 μL/min, the result was reversed. The reason of the phenomenon is that the concentration of ferrofluid becomes lower due to the accumulation of magnetic nanoparticles near micro-magnets. Based on the Eq. (4) and (11), the passive magnetic force will weaken with the decreasing concentration of ferrofluid. In the simulation of this paper, the variation in concentration of ferrofluid that happened in the experiment was not taken into consideration. Therefore, the difference in width of the microbead stream was generated, as shown in Fig. 10.
4.2.2 Effect of micro-magnet array
The focusing of microbeads was greatly related with the external magnetic field, which was determined by the arrangement of the magnetized micro-magnets. The distribution of 10 μm-diameter microbead stream width (Δym) was plotted under three types of magnetized micro-magnet arrays at different flow rates Q1, as shown in Fig. 11. The number of measurements for the data was five.
In general, the value of Δym decreased gradually with the decreasing of flow rate. Besides, under the same flow rate, the value of Δym increased successively by the arrangement of magnetized micro-magnet array with type C, A, and B, respectively. Combined with the magnetic force distribution in Fig. 7b, c, although the magnetic force generated by the Halbach micro-magnet array (C type) was fluctuant, the maximum value of the magnetic force was the largest, and the magnetic force attenuation along y-axis was the weakest. It was speculated that these two reasons together made the microbead stream width Δym smallest under the Halbach micro-magnet array.
4.3 Multi-lateral micro-magnet arrays for microbead separation
Based on the research above, the Halbach micro-magnet array generates a greater magnetic field. Therefore, a combination of bilateral and unilateral Halbach micro-magnet arrays was used to focus and separate the microbeads with diameters of 10 μm and 15 μm. It should be noted that the 10 μm-diameter microbeads were red fluorescence and the 15 μm-diameter microbeads were green fluorescence.
Firstly, Fig. 12 shows the distribution of two sizes of microbeads flowing through the bilateral Halbach micro-magnet arrays at different flow rates Q1. The experimental results showed that the two kinds of microbeads were gathered in the microchannel center, and the width of the microbead stream became thinner with the deceasing of the flow rate. Based on Eq. (11), the negative magnetic force would be larger with a greater diameter. Hence, the negative magnetic force acting on the 15 µm-diameter microbeads would be larger, which caused more microbeads to move to the center of the microchannel. However, when the flow rate was high, the microbeads with a diameter of 10 µm couldn’t arrive at the center of the microchannel due to a lack of driving time. Therefore, when the flow rate was 10 µL/min, microbeads with a diameter of 15 µm appeared to converge more than those with a diameter of 10 µm.
The distribution of 10 μm- and 15 μm-diameter microbeads once they flowing into the region with a unilateral Halbach micro-magnet array is shown in Fig. 13a–f. The experimental results indicated that microbead streams remained relatively thin. This phenomenon mainly relied on the fact that the sheath flow was equal to the main inlet and the flow field of ferrofluid wasn’t disturbed as shown in Figs. 13g and 14. The flow lines didn’t change significantly. In addition, when the flow rate of ferrofluid was greater than 8 μL/min, the two sizes of microbeads were overlapped. As the flow rate decreased, the acting time of the passive magnetic force in the vertical direction of flow increased, so that the difference in the balance positions of two sizes of microbeads was more obvious. Then two sizes of microbeads were gradually separated, and a small gap appeared between two microbead streams.
At different sheath flow rates, the separation of 10 μm- (red) and 15 μm- (green) diameter microbeads under a unilateral Halbach micro-magnet array (a bright field image; b Q1 = 10 μL/min; (c) Q1 = 8 μL/min; d Q1 = 6 μL/min; e Q1 = 4 μL/min; f Q1 = 2 μL/min); g The flow field of ferrofluid when Q1 = 10 μL/min
For quantifying and comparing the difference in microbead separation with different parameters, the fluorescence intensity distribution of microbeads at the end of the unilateral Halbach micro-magnet array was plotted. The separation distance Δys was employed as shown in Fig. 15. Meanwhile, an enlarged view of the separation part was provided, which was located in the area indicated by the red dashed line.
After drawing fluorescence intensity profiles, the separation distances at different flow rates were plotted as shown in Fig. 16. When the flow rate was 2–4 μL/min, the average value of Δys was positive. It indicated that two sizes of microbeads were completely separated and that there was a certain separation distance. Besides, the average value of Δys was negative when the flow rate increased to 6 μL/min. It indicated that the two sizes of microbeads are not completely separated. In fact, with the decreasing of flow rate, the time of magnetic force on microbeads would increase and the difference in migration of different sizes of microbeads would be more obvious. When the flow rate was 10 µL/min, the movement of the microbeads was fast, and the number of microbeads whose trajectories could be captured through the microscope was relatively small. Therefore, the width of the overlap actually decreased at 10 µL/min.
4.4 Multistage microfluidic chip for microbeads separation
Based on the research above, the arrangement of micro-magnet arrays is the type C in the tertiary part of the multistage microfluidic chip. In this section, microbeads with diameters of 5 μm, 10 μm and 15 μm were adopted, and the multistage microfluidic chip was used to carry out research. The sides of micro-magnet were 860 μm in the tertiary part. The separation of the microbeads was investigated experimentally, and the distributions of microbeads at all outlets were recorded as shown in Figs. 17, 18 and 19.
Figure 17 shows the distribution of three sizes of microbeads at the outlet of the primary part at a flow rate of 270 μL/min. To better distinguish the distribution of the three sizes of microbeads, each size of microbead was injected into the primary part separately, since the colors of the three sizes of microbeads were green, red, and green, respectively. It could be seen from the experimental results that most microbeads with a diameter of 5 μm flowed to the middle outlet, and a small part of microbeads flowed to the two side outlets. For microbeads with a diameter of 10 μm, most of them flowed to the two side outlets. Different from 5 μm- and 10 μm-diameter microbeads, 15 μm-diameter microbeads were focused into thin streams, and all of them flowed to the two side outlets. The phenomenon was caused by the balance of the inertial lift force and the Dean drag force in the split-recombination microchannel (Chen et al. 2022).
As the sample containing three sizes of microbeads flowed out of the primary part, most of 5 μm-diameter microbeads were separated from the sample, and the flow rate Qm2 was nearly equal to a third of the flow rate Qm1, which was measured in Supporting Information. Figure 18 shows the distribution of remnant 10 μm- and 15 μm-diameter microbeads at the end of the secondary part at different flow rates. Meanwhile, the normalized fluorescence intensity was curved based on the experimental results.
The flow rate Qm2 was 80 μL/min, 90 μL/min and 100 μL/min, which corresponded to the flow rate Qm1 of 240 μL/min, 270 μL/min and 300 μL/min, respectively. As could be seen from Fig. 18 (a-c), almost all microbeads gathered in the middle of the microchannel and flowed to the tertiary part through the middle outlet of the secondary part. Besides, it could be seen that the peak position of the intensity curve was about y = 0.25 mm, which also indicated that most of the microbeads gathered in the middle of the microchannel. With the increase in the flow rate, the half bandwidth (Whb) of the intensity curve gradually decreased. It indicated that the width of the microbead stream became smaller. Similarly to the primary part, the secondary part effectively reduced the flow rate of the sample. Because the action time of the passive magnetic force would be longer with a decrease in flow rate, the design of the outlets in the secondary part was more conducive to the separation operation based on the passive magnetophoresis in the tertiary part.
Figure 19 shows the distribution of microbeads at the gradually expanded outlets of the tertiary part. Based on the volume of the collected solution from each outlet of the multistage microfluidic chip in the Supporting Information, the flow rate Qm3 was 16 μL/min, 18 μL/min and 20 μL/min, which corresponded to the flow rate Qm1 of 240 μL/min, 270 μL/min and 300 μL/min, respectively. The flow rate of sheath flow was equal to Qm3. It can be seen that the 10 μm- and 15 μm-diameter microbeads were clearly divided into two microbead streams. When the flow rate was 20 μL/min, the two sizes of microbeads flow out from the same outlet. As the flow rate gradually decreased, the two fluxes of microbeads were shifted downward. When Qm3 = 16 μL/min, red and green microbeads flowed out from different outlets. The microbeads collected at outlet 4 were counted using a cell counting plate when the flow rate of the inlet was 240 μL/min. After five same experiments, the sorting efficiency and purity were 88.3% ± 5.2% and 93.4% ± 2.1%, respectively. It would be possible to collect beads with a diameter of 10 µm at outlet 3. However, the purity would be not very high due to residual 5 μm-diameter microbeads. In one word, the 15 μm-diameter microbeads can be collected with high purity at the outlet 4.
5 Conclusion
In this work, micro-magnets were proposed and fabricated using NdFeB powder and PDMS. And then, a single microfluidic chip containing magnetized micro-magnet arrays and sheath flow was designed, in which the micro-magnet array was spliced by many micro-magnets. Numerical simulation showed that the magnetic force of the Halbach micro-magnet array was the largest, and the attenuation of the magnetic force was the weakest compared with the same directional array and the traditional alternating array. Based on the numerical simulations and experiments, diamagnetic microbeads 10 μm and 15 μm in diameter were focused into thin streams and separated successfully with the help of sheath flow. In addition, the flow rate and the arrangement of micro-magnet arrays affected the separation of two-size microbeads. At a flow rate of 2 μL/min, 10 μm- and 15 μm-diameter microbeads were separated completely, and the separation distance Δys was 20 μm under the Halbach micro-magnet arrays. Furthermore, in primary part of the multistage microfluidic chip, 5 μm-diameter microbeads were separated from 10 μm-, and 15 μm-diameter microbeads. And then, in tertiary part of the multistage microfluidic chip, 10 μm-, and 15 μm-diameter microbeads were separated successfully. When the flow rate Qm1 is 240 μL/min, 15 μm-diameter microbeads were collected with high purity at a single outlet.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Alnaimat F, Dagher S, Mathew B, Hilal AH, Khashan S (2018) Microfluidics based magnetophoresis: a review. Chem Rec 18:1–18
Augustsson P, Magnusson C, Nordin M, Lilja H, Laurell T (2012) Microfluidic, label-free enrichment of prostate cancer cells in blood based on acoustophoresis. Anal Chem 84:7954–7962
Çetin B, Li D (2011) Dielectrophoresis in microfluidics technology. Electrophoresis 32:2410–2427
Chen P, Huang Y, Bhave G, Hoshino K, Zhang X (2015) Inkjet-print micromagnet array on glass slides for immunomagnetic enrichment of circulating tumor cells. Ann Biomed Eng 44(5):1710–1720
Chen S, Shi ZQ, Sun JJ, Jia SL, Zhong MJ, Ma YX (2022) High-throughput particle focusing and separation in split-recombination channel. J Micromech Microeng 32:025007
Chung YC, Wu CM, Lin SH (2016) Particles sorting in micro channel using designed micro electromagnets of magnetic field gradient. J Magn Magn Mater 407:209–217
Descamps L, Garcia J, Barthelemy D, Laurenceau E, Payen L, Roy DL, Deman AL (2022) MagPure chip: an immunomagnetic-based microfluidic device for high purification of circulating tumor cells from liquid biopsies. Lab Chip 22:4151–4166
Friedman G, Yellen B (2005) Magnetic separation, manipulation and assembly of solid phase in fluids. Curr Opin Colloid Interface Sci 10:158–166
Gijs MAM, Lacharme F, Lehmann U (2010) Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem Rev 110:1518–1563
Gomez-Pastora J, Gonzalez-Fernandez C, Real E, Iles A, Bringas S, Furlani EP, Ortiz I (2018) Computational modeling and fluorescence microscopy characterization of a two-phase magnetophoretic microsystem for continuous-flow blood detoxification. Lab Chip 18:1593–1606
Huang Y, Chen P, Wu C, Hoshino K, Sokolov K, Lane N, Liu H, Huebschman M, Frenkel E, Zhang J (2015) Screening and molecular analysis of single circulating tumor cells using micromagnet array. Sci Rep 5:16047
Hyun KA, Lee TY, Lee SH, Jung HI (2015) Two-stage microfluidic chip for selective isolation of circulating tumor cells (CTCs). Biosens Bioelectron 67:86–92
Jokerst JC, Emory JM, Henry CS (2012) Advances in microfluidics for environmental analysis. Analyst 137:24–34
Kabacaoğlu G, Biros G (2019) Sorting same-size red blood cells in deep deterministic lateral displacement devices. J Fluid Mech 859:433–475
Khashan SA, Furlani EP (2013) Coupled particle–fluid transport and magnetic separation in microfluidic systems with passive magnetic functionality. J Phys D: Appl Phys 46:125002
Li P, Stratton ZS, Dao M, Ritz J, Huang T-J (2013) Probing circulating tumor cells in microfluidics. Lab Chip 13:602–609
Liang L, Zhang C, Xuan X (2013) Enhanced separation of magnetic and diamagnetic particles in a dilute ferrofluid. Appl Phys Lett 102:234101
Lim J, Yeap SP, Low SC (2014) Challenges associated to magnetic separation of nanomaterials at low field gradient. Sep Purif Technol 123:171–174
Lu X, Xuan X (2015) Inertia-enhanced pinched flow fractionation. Anal Chem 87:4560–4565
Marle L, Greenway GM (2005) Microfluidic devices for environmental monitoring. TrAC Trends Anal Chem 24:795–802
Maxey MR, Riley JJ (1983) Equation of motion for a small rigid sphere in a nonuniform flow. Phys Fluids 26:883–889
McGrath J, Imenez M, Bridle H (2014) Deterministic lateral displacement for particle separation: a review. Lab Chip 14:4139–4158
Merrin J (2019) Frontiers in microfluidics, a teaching resource review. Bioengineering 6:109
Munaz A, Shiddiky M, Nguyen NT (2018) Magnetophoretic separation of diamagnetic particles through parallel ferrofluid streams. Sens Actuators B Chem 275:459–469
Nilsson J, Evander M, Hammarstrom B, Laurell T (2009) Review of cell and particle trapping in microfluidic systems. Anal Chim Acta 649:141–157
Pachmann K, Camara O, Kavallaris A, Krauspe S, Malarski N, Gajda M, Kroll T, Jorke C, Hammer U, Altendorf-Hofmann A, Rabenstein C, Pachmann U, Runnebaum I, Hoffken K (2008) Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J Clin Oncol 26:1208–1215
Rosensweig RE (1987) Magnetic fluids. Ann Rev Fluid Mech 19:437–463
Samantaa A, Gangulyb R, Dattab A, Modak N (2017) Separation of magnetic beads in a hybrid continuous flow microfluidic device. J Magn Magn Mater 427:300–305
Shen YG, Yalikun Y, Tanaka Y (2019) Recent advances in microfluidic cell sorting systems. Sens Actuators B Chem 282:268–281
Shi ZQ, Chen S, Sun JJ, Li MJ, Jia SL (2020) Three-dimensional numerical analysis of focusing and separation of diamagnetic particles in ferrofluid. J Phys D: Appl Phys 53:315002
Shiriny A, Bayareh M (2020) On magnetophoretic separation of blood cells using halbach array of magnets. Meccanica 55:1903–1916
Suwa M, Watarai H (2011) Magnetoanalysis of micro/nanoparticles: a review. Anal Chim Acta 690:137–147
Tottori N, Nisisako T (2018) High-throughput production of satellite-free droplets through a parallelized microfluidic deterministic lateral displacement device. Sens Actuators B 206:918–926
Wu J, Cui Y, Xuan S, Gong X (2018) 3Dprinted microfluidic manipulation device integrated with magnetic array. Microfluid Nanofluid 22:103
Xu X, Huang X, Sun J, Wang R, Yao J, Han W, Wei M, Chen J, Guo J, Sun L, Yin L (2021) Recent progress of inertial microfluidic-based cell separation. Analyst 146:7070–7086
Yin H, Marshall D (2012) Microfluidics for single cell analysis. Curr Opin Biotechnol 23:110–119
Zeng J, Chen C, Vedantam P, Tzeng TR, Xuan XC (2013a) Magnetic concentration of particles and cells in ferrofluid flow through a straight microchannel using attracting magnets. Microfluid Nanofluid 15:49–55
Zeng J, Deng YX, Vedantam P, Tzeng TR, Xuan XC (2013b) Magnetic separation of particles and cells in ferrofluid flow through a straight microchannel using two offset magnets. J Magn Magn Mater 346:118–123
Zhou J, Papautsky I (2013) Fundamentals of inertial focusing in microchannels. Lab Chip 13:1121–1132
Zhou R, Wang C (2016a) Multiphase ferrofluid flows for micro-particle focusing and separation. Biomicrofluidics 10:034101
Zhou R, Wang C (2016b) Microfluidic separation of magnetic particles with soft magnetic microstructures. Microfluid Nanofluid 20:48
Zhu G, Nguyen NT (2012) Magnetofluidic spreading in microchannels. Microfluid Nanofluid 13:655–663
Zhu J, Liang L, Xuan X (2012) On-chip manipulation of nonmagnetic particles in paramagnetic solutions using embedded permanent magnets. Microfluid Nanofluid 12:65–73
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China under project 52307015, the Fundamental Research Funds for the Central Universities under project sxzy012022014, and the Natural Science Foundation of Shaanxi Province under project 2023-JC-QN-0488.
Funding
National Natural Science Foundation of China, 52307015, Fundamental Research Funds for the Central Universities, sxzy012022014, Natural Science Foundation of Shaanxi Province, 2023-JC-QN-0488.
Author information
Authors and Affiliations
Contributions
S.C. and J.S. wrote the main manuscript text along with simulation, fabrication, and testing of the microfluidic device. Z.S., X.L. and K.W. revised and reviewed the manuscript. X.L., Y.M. and R.L. performed the experiments. S.X. and N.W. analyzed simulation data.
Corresponding authors
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Sun, J., Shi, Z. et al. Investigation on the focusing and separation of polystyrene microbeads in an integrated microfluidic system using magnetized functionalized flexible micro-magnet arrays. Microfluid Nanofluid 28, 51 (2024). https://doi.org/10.1007/s10404-024-02749-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-024-02749-5