Abstract
Sealing is one of the inevitable process in microfluidic chip fabrication to form complex networks for the biosensing applications. Currently, only a few materials can be used in microfluidic biosensor due to the lack of reliable bonding technique for most materials. To solve the problem of versatility for chip sealing, a novel adhesive bonding method as simple as “tear off–paste on” is developed. PDMS is mixed with a small amount of polyethylenimine solution to prepare a sticky thin layer, which works like a tape to paste on different materials. Various substrates including glass, plastic, metal and ceramics are used for preparation of microfluidic chips with good bonding strength. This method is appealing for its compatibility to traditional replication method using PDMS and SU8 channel mold while the small surface structures of channel walls can be retained. This method is reliable and versatile for microfluidic biosensor sealing, especially for those with biological sensitive recognition elements on the surfaces since neither aggressive chemicals, high temperature nor high-energy plasma is used. The applicability of the developed method is demonstrated to fabricate a novel long-term cell culture 3D microfluidic chip which keeps bacteria viable for more than 7 days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microfluidic chip is considered as one of the most promising platform for biosensing applications due to the advantages including low cost, little reagent consumption, fast reaction time, high sensitivity, large integration and good portability, as compared to the conventional analytical instruments and techniques (Shamsi and Chen 2017). The microfluidic-based biosensors have shown their unique advantages in point-of-care diagnostics, environmental monitoring, and food safety (Luka et al. 2015). Wang et al. developed an optical biosensor for high throughput diagnostics with the sensitivity comparable to that of the lab instrument (Wang et al. 2017). Zhang et al. reported a capillary biosensor for detection of Salmonella using Fe-nanocluster amplification and smart phone imaging (Zhang et al. 2019). Recently, Ma et al. showed microfluidic channel could lower down the detection limit of endotoxin with the confined space and enhanced Van der Waals force (Ma et al. 2020). All of them showed superior advantages of microfluidic biosensor in detections.
To fabricate the microfluidic biosensors, the sample handling units—filtration, mixing, separation, and reaction have to be included. More specifically, the biological sensitive recognition element such as antibodies, nucleic acids, enzymes, or aptamers has to be immobilized on a physicochemical transducer. However, the commonly used materials such as PDMS, glass, silicon, and plastics are all difficult to integrate different units of immobilizing biological elements or cumbersome steps have to be conducted. On the contrary, the easily coating materials like gold or paper have difficulties in fabricating microfluidic chip due to the lack of applicable bonding method to seal microchannels.
As developed for decades since the birth of microfluidics, lots of bonding techniques have been reported, which are mainly divided into four categories—plasma bonding, hot-press bonding, chemical assisted bonding, and adhesive bonding. Plasma bonding (Cira et al. 2012; Ye et al. 2020) for PDMS–PDMS, PDMS–glass, and PDMS–silicon chips are the most commonly used method for channel sealing. The bonding strength reported for PDMS–PDMS plasma bonding is around 300 kPa (Eddings et al. 2008). Hot-press bonding with well controlled temperature and time is good for plastics such as poly(methyl methacrylate) (PMMA) (Nayak et al. 2010), polystyrene (PS) (Fan et al. 2013), polycarbonate (PC) (Xu et al. 2003) and cyclic olefin copolymer (COC) (Jena et al. 2011). The bonding strength of device using hot-press bonding varies from 110 kPa to 1.6 MPa depending on the molecular weight of materials and the process temperature. The chemical assisted bonding applies isopropanol, ethylene glycol (Umbrecht et al. 2009), tetraethyl orthosilicate (TEOS) (Suzuki et al. 2010), and wax (Gong et al. 2010) etc. to seal two slabs of various materials to achieve high bonding strength (> 300 kPa). However, all of the reported methods are not applicable for biosensors due to the use of high energy (plasma bonding), high temperature (hot-press bonding), as well as chemical residues (chemical assisted bonding), which will definitely destroy the biological coating on the surfaces of transducers. Adhesive bonding with simple method and low cost material is the appealing technique to seal the channel in microfluidic biosensor. UV-curable adhesives (Carroll et al. 2008; Lu et al. 2010; Pan and Yang 2006) are most frequently applied for adhesive bonding, which bring acceptable bonding strength around 180 kPa. However, the UV light in the bonding process can probably denature the bioreagents or biomolecules in the chip. Therefore, epoxy adhesives (Dang et al. 2005; Flachsbart et al. 2006; Riegger et al. 2010) and double-sided tapes (Patko et al. 2014; Thompson and Abate 2013) with bonding strength varying from 50 to 300 kPa depending on materials used are most promising materials for bonding purposes in biosensors. However, it is too difficult to retain the channel structure from mold as PDMS does in softlithography for both using epoxy and double-sided tapes.
As mentioned above with respect to the bonding techniques used to seal the microchannel, it is highly desired for sealing microfluidic biosensors under the promise of preserving the small structures in the channel, with simple and low-cost process and without damaging the biomolecules. PDMS is the commonly used material to replicate channel structure from mold and modifying the surface by adding chemicals in it to enhance the adhesion is a promising way to develop PDMS-based adhesive bonding technique. Kersey et al. reported the addition of adhesion promoter GE SS4120 to enhance the adhesion strength of PDMS to aluminum, silicon and glass (Kersey et al. 2009). Inspired by the sticky elastomer for epidermal electronics (Jeong et al. 2016), heterogeneous crosslinking of PDMS could be applied to enhance adhesion of PDMS to various substrates. The nanostructure heterogeneity has been widely discovered in polymers and gels (Di Lorenzoab and Seiffert 2015). PDMS is composed by silicone base and crosslinker (curing agent) and the polymerization process is catalyzed by a platinum complex. When polyethylenimine, ethoxylated solution (PEIE) is added in the system, part of platinum complex will be depleted around PEIE, resulting in heterogeneous crosslinking. In this scenario, the topological defects of polymer chains are formed and incomplete curing of PDMS is achieved to produce a sticky material. Here, a universal bonding method is developed for microfluidic biosensor fabrication using PEIE to adjust the heterogeneous crosslinking of PDMS. This partially cured PDMS works as adhesive to seal microchannels on various substrates including glass, PMMA, Polyethylene terephthalate (PET), ceramics and metal. The prepared chip has good bonding strength for controlling fluid flow in a wide range. The generation of droplets with different sizes and the culture of bacteria for a week are demonstrated for the applicability of the proposed method.
2 Materials and methods
2.1 Chemicals
Polydimethylsiloxane (PDMS, Sylgard 184) was obtained from Dow Corning (Midland, USA). 1H,1H,2H,2H-Perfluorooctyltrichlorosilane, red color dye and fluorescein isothiocyanate isomer I (FITC) were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Paraffin oil was supplied by Chengdu Kelong Chemical Co. Ltd. (Sichuan, China). Polyethylenimine, 80% ethoxylated solution (PEIE) and Span 80 were obtained from Sigma-Aldrich (USA). MTT assay kit—M1020 was provided by Solarbio (Beijing, China).
2.2 Instruments
The mask aligner URE-2000/35 (Institute of Optics and Electronics, Chinese Academy of Science, China) and plasma cleaner PDC-32G (HARRICK PLASMA, USA) were used for photolithography process and PDMS-PDMS bonding respectively. Spin coater SJL-1 (Shanghai Guangze optical machinery Co., Ltd., China) was used for thin film preparation. Laser cutter was used to cut PMMA for placing microfluidic chip. FE-SEM (Tescan Mira 3, Czech Republic) was used to observe the structure and surface roughness of microfluidic chip. Syringe pump LSP02-1B (Longer Pump, China) was applied to generate constant flow rate in microfluidic chip.
2.3 Fabrication of the microfluidic chip
The main fabrication technique used for microfluidic chip preparation was the commonly used photo-lithography and soft-lithography except the plasma bonding step. Briefly, the photolithography processes including spin coating, pre-baking, exposure, post-baking, develop were conducted using SU8 2035. The SU8 mold was replicated by PDMS with desired channel structures. To prepare a sticky material for bonding, PEIE was mixed thoroughly with standard PDMS solution (base:curing agent = 10:1) under a series of ratios from 20 μl: 10 g to 50 μl: 10 g with an interval of 10 μl for finding the best combination for application. The mixture was then degassed in a vacuum chamber to get rid of bubbles generated during mixing, followed by pouring onto the SU8 mold to have a sticky layer of about 500 μm. The mixture along with the mold was then put in the oven at 95 °C for 30 min and PDMS was poured onto the half-cured mixture to obtain a thick layer (about 3 mm), followed by baking in the oven at 95 °C for another 20 min. Finally, the prepared PDMS channel was peeled off from the mold and bond to different substrates—glass, PMMA, ceramics and steel plate by contact pressing and chip sealing was achieved.
2.4 Characterization of microfluidic chip
The cross section of the microchannel was characterized by scanning electron microscope (SEM). The chip was cut along the channel width by sharp blade and mounted to the SEM stab, followed by gold thin film coating with thickness of 2 nm. Then, the cross section of the channel was imaged under high voltage of 10 kV.
The microfluidic chip used for the demonstration of the developed method was the well-known “T-junction” chip, which has a main channel with width of 315 µm, height of 140 µm and length of 3 cm and a side channel with width of 100 µm, height of 140 µm and the narrow junction width of 25 µm. The bonding strength was tested by pumping water into the main channel under different flow rates. The maximum flow rate was recorded for estimation of the bonding strength by simulation using COMSOL multiphysics. The build-in equations are uncompressible Navier–Stokes equation:
where, ρ is the density, which is 1 g/cm3 for water, u is the linear flow rate, P is the pressure, η is the dynamic viscosity at 22 °C, which is 0.001 Pa s.
Droplet generation was performed by this “T-junction” microfluidic chip. For better visualization of the generated droplets, water phase was dyed with red color and pumped into the side channel using a syringe pump with flow rate of 1 µl/min, while the paraffin oil was introduced into the main channel with flow rate of 20 µl/min, 30 µl/min, 40 µl/min and 50 µl/min, respectively. The whole chip was placed under optical microscope for monitoring the droplets generated.
2.5 The biocompatibility test of the composite material
The toxicity test of the PEIE and PDMS composite was conducted by culturing liver cancer cells MHCC97H in small reservoirs. The ratio used for the toxicity test was 40 μl PEIE in 10 g PDMS as a representative. The composite was prepared using the same procedure in microfluidic chip fabrication method and holes were punched to be a cylinder shape with diameter of 6 mm and thickness of 5 mm, followed by contact press to a flat slab made by PEIE mixed PDMS composite. As comparison, the toxicity of PDMS to cells was also conducted using the similar reservoir made by pure PDMS as the control experiment. Both the composite and pure PDMS reservoirs were UV sterilized for 30 min and placed in petri-dishes for cell culture. Cells with initial concentration of 105 cells/ml and culture medium were added into the reservoirs and put in the incubator with 5% CO2 and humid condition to avoid water evaporation and constant temperature at 37 °C for 2 days. Optical pictures of cells before and after culture were taken to compare the cell proliferation in reservoirs made by PDMS and PEIE mixed PDMS. The quantitative analysis of cell toxicity for materials made by PDMS and PEIE mixed PDMS was then performed following the MTT assay protocol. The culture medium was removed and cells were washed with PBS. Fresh medium with 0.5 mg/ml MTT was added in the reservoir and incubated at 37 °C for 2 h. The medium with MTT was then removed and Formazan solubilizing solution was added. The light absorbance at 492 nm of solution in 96-well plate was performed using microplate reader (Thermo scientific) after purple crystals were dissolved.
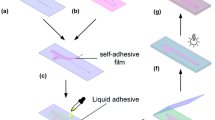
2.6 Design, fabrication, and test of a 3D microfluidic chip for cell culture
To extend the culture period of cells in microfluidic chip, a polycarbonate (PC) thin film with nanopores was integrated to separate the culture chamber and the medium channel, resulting in a 3D sandwiched chip. The fabrication of the 3D microfluidic chip was achieved by assembling three layers together (Fig. 1). For the preparation of the bottom channel layer, PEIE mixed PDMS was poured onto a PDMS mold which had five parallel channels with width of 500 μm, height of 500 μm and length of 27 mm. The thickness of the PEIE mixed PDMS composite layer was about 500 μm. Then baking was conducted for 30 min at 95 °C until the surface is semi-solidified, followed by adding PDMS on the surface to the mold to prepare the bottom substrate with thickness of 3 mm. The second baking was then conducted for 30 min at 95 °C to solidify PDMS. The outlets were punched after peeling off from the mold. The cell culture layer was prepared by spin coating twice on a clean and flat PDMS slab with perfluorooctylsiloxane on it using the method developed previously. A layer of PDMS is firstly spin-coated on the surface at a speed of 850 rpm for 20 s, and it is baked at 95 °C for 20 min. After that, a layer of PEIE mixed PDMS composite was spin-coated on the surface at a speed of 900 rpm for 20 s, and then it is baked in an oven at 92 °C for 25 min. The two layers of PDMS spin-coated on the PDMS substrate are combined into a layer of PDMS with a thickness of about 150 μm. Then, an array (9 × 5) of the culture chambers was punched on the thin layer of PDMS. The thin layer, the polycarbonate nanoporous layer, and the bottom channel layer were assembled by contact press. The inlets of the microfluidic chip were prepared by bonding a flat PDMS slab with a thickness of 1 mm on the top and holes were punched through from the top to the bottom channel layer.
The performance of the cell culture chip was tested with E. coli—BL21 with GFP strains for easy visualization. Briefly, the chip was firstly sterilized under UV for 30 min and surface of the culture chamber was treated by plasma for 1 min to render hydrophilic. The bacteria were then loaded into the chamber by pouring onto the surface and the excess solution was removed by swiping through the whole area using glass rod. After that, paraffin oil was added to cover the surface of the culture chamber to avoid evaporation. The bottom channel was filled with culture medium by syringe and continuous flow with flow rate of 10 µl/min was kept using the syringe pump.
3 Results
3.1 Optimization of the PEIE ratio
The amount of PEIE added in PDMS is critical to have an applicable sticky layer for “tear off–paste on” bonding to prepare microfluidic chip. Too much of PEIE will cause the failure of structure replication and deformation of microchannels, while too less will result in weak bonding strength and fluid leakage. Therefore, the PEIE concentration was optimized by mixing PEIE (Sigma-Aldrich) with PDMS under a series of ratios from 20 μl: 10 g to 50 μl: 10 g (PEIE: PDMS) with an interval of 10 μl for chip fabrication. The chip has three layers—the substrate layer, the PEIE mixed PDMS composite layer for adhesive bonding and the pure PDMS layer. PMMA was used as a represented substrate for chip fabrication as PDMS could not bond to it by plasma treatment. Figure 2 is the optical images of microchannel with different PEIE to PDMS ratios. It is clearly seen that the channel is well defined as good as pure PDMS chip and no deformation was observed when the ratio is no larger than 40 μl: 10 g as shown in Fig. 2a–c. However, the channel structure could not be properly replicated and lots of residues are found on the mold when 50 μl: 10 g is used (Fig. 2d). The maximum flow rates before leakage and the corresponding pressures are shown in Table 1. When the flow rate is larger than the maximum number, leakage is always happened at the inlet of the channel shown in Fig. 2a3, b3 and c3 since it is the highest pressure point. The results show that higher bonding strength can be obtained with the increase of PEIE to PDMS ratio.
The optical images of the fabricated microfluidic chip during leakage testing. a PEIE: PDMS = 20 μl: 10 g; b PEIE: PDMS = 30 μl: 10 g; c PEIE: PDMS = 40 μl: 10 g; d PEIE: PDMS = 50 μl: 10 g; a1–c1 just prepared; a2–c2 fluid flowing in channels; a3–c3 leakage happened, marked by light blue squares. The scale bars are 600 μm in the images
To show this bonding method can preserve the channel profile as pure PDMS does, the SEM images of the cross section of PDMS bonded chip and the composite chip were taken. Figure 3a shows the cross section of PDMS chip which is bonded by plasma treatment. A very clear line can be observed between two layers in this figure, illustrating the interface between PDMS slab and PDMS microchannel. Figure 3b–d show the cross section of composite chip prepared by adhesive bonding using a thin layer of PDMS mixed with PEIE with the ratios of 20 µl: 10 g, 30 µl: 10 g and 40 µl: 10 g, respectively. The substrates, the bottom layers are all flat slab made by pure PDMS in Fig. 3. It is clearly seen that the channel walls almost have the same dimension as the conventional bonded PDMS chip (Fig. 3a), suggesting that the channel profile is preserved (no deformation) by mixing PEIE with PDMS under the ratio smaller than 40 µl: 10 g. It is also noted that with the increase of PEIE to PDMS ratio, the sharp corner becomes smoother as seen from Fig. 3b–d, which is not surprising since it becomes softer with increase of PEIE content when bonding to the substrate. Additional experiments were performed to obtain the minimum channel size using the developed adhesive bonding method. Figure S1 in the supplementary shows the optical pictures of channels with sizes of 10 µm, 20 µm, 30 µm, and 40 µm. It is clearly seen that there is a little distortion at the wall for 10 µm channel and others have straight lines. Therefore, the PEIE mixed PDMS sticky material can preserve the channel profile if the channel size is larger than 10 µm. To check the hydrophobicity change after addition of PEIE into PDMS, the contact angle is also measured and the results are shown in supplementary Fig. S2. It is seen that the composite material has nearly the same wettability as PDMS, due to the addition of very small amount of PEIE in PDMS networks. The hydrophobicity of microfluidic chip can also be changed by oxidation using UV–ozone (Ma et al. 2011) or oxygen plasma (Li et al. 2016) or coating with hydrophilic materials (Trantidou et al. 2017).
SEM images of the cross sections of prepared microfluidic chips. a Pure PDMS bonded by plasma treatment; b the composite chips made by adhesive bonding with PEIE to PDMS ratio of 20 μl: 10 g; c the composite chips made by adhesive bonding with PEIE to PDMS ratio of 30 μl: 10 g; d the composite chips made by adhesive bonding with PEIE to PDMS ratio of 40 μl: 10 g; the substrates are all pure PDMS slabs. The scale bars are 200 μm in the images
Droplet generation experiment was then conducted to verify the applicability of the bonded chip prepared by the presented method when PMMA substrate and PEIE-to-PDMS ratio with 40 µl: 10 g was used. The microfluidic chip that generates droplets has high requirements for the tightness of the chip structure, the ability to withstand the fluid pressure, and the flatness of the channel, since any leakage or even a little shape change under pressure would result in perturbation for generating non-uniform droplets. Therefore, it is necessary to conduct a droplet experiment by fabricating a microfluidic chip with “T-junction” structure using the developed method. Paraffin oil with 1 wt% Span 80 surfactant is pumped into the main channel and aqueous solution with red dye for easy visualization is pumped into the side channel for generating water droplets. The flow rate of the aqueous solution in the side channel remains constant at 1 μl/min, and the flow rate of the liquid paraffin oil in the main channel is varied between 20, 30, 40, and 50 μl/min. Figure 4 shows the snap-shots of the moving droplets under different flow rates and it confirms the prepared microfluidic chip with developed bonding method can be successfully used in generating droplets with different sizes when PMMA is selected as the substrate.
The optical images of droplets generated by a “T-junction” microfluidic chip prepared by adhesive bonding using PEIE mixed PDMS composite material under flow rates of 20 μl/min (a), 30 μl/min (b), 40 μl/min (c) and 50 μl/min (d) in the main channel. Paraffin oil is used to generate water droplets dyed with red color. The flow rate of water in the side channel is kept at 1 μl/min. The scale bars are 200 μm in the images
3.2 Universal bonding of the developed method
To verify the versatility of this bonding method with different substrate materials, glass, PET, steel, and ceramics are selected to prepare chips. The microfluidic chip used for demonstration is the same as that for PEIE ratio optimization. The real pictures of microfluidic chips are shown in supplementary Fig. S3. The bonding strength is also calculated by measuring the maximum flow rate under which each chip can withstand. Due to the opacity of metals and ceramics, fluorescent aqueous solution with 1 mg/ml of FITC is used as the injection sample solution, and the pumping and leakage process is observed under a fluorescent microscope and shown in Fig. 5. It is clearly seen that all the channels have well-defined channel walls and the maximum flow rates are 6000 μl/min, 6000 μl/min, 6000 μl/min and 4000 μl/min for glass, PET, steel plate, and ceramics respectively.
The images of microchannels made by 10 g PDMS mixed with 40 μl PEIE based on different materials: a glass substrate; b PET substrate; c steel substrate; d ceramic substrate. a1–d1 The channels are filled with aqueous solutions; a2–d2 the fluid flow in channels under different flow rates; a3–d3 the images of channels when flow rates exceed the maximum. The scale bars are 600 μm in the figures
Following the same method employed to assess the bonding strength of prepared microfluidic chip with PMMA substrate, the maximum pumping pressure were also calculated using COMSOL multiphysics. The results are listed in Table 2. The chips with glass slide, steel plate, and PET film as substrate have the same bonding strength as high as 184 kPa. The ceramic plate has the lowest bonding strength. It can be seen from the above results that the bonding strength of this developed method meets the application requirements with different substrate materials.
3.3 Biocompatibility test of the composite material
Biocompatibility is a critical character for biosensor. PDMS is the well-known material with good biocompatibility (Peterson et al. 2005), while PEIE is reported as a toxic substance for cells (Hunter 2006). Thus, the biocompatibility of the composite material of PEIE and PDMS mixture was tested using the liver cancer cell line MHCC97H. Two reservoirs with diameters of 2 mm and height of 1 mm made by pure PDMS and composite material, respectively, were prepared and cancer cells were cultured in these reservoirs. Optical pictures of cells before and after culture were taken and the results were shown in Fig. 6. Low densities of cells suspended in culture media are observed before culture and large numbers of cells are found grown on the surfaces after 2 days’ culture for both PDMS reservoir and the composite reservoir made by PEIE mixed PDMS. The quantitative viability assay is performed using the MTT assay which is broadly used to measure the in vitro cytotoxic effects of drugs on cell lines. This colorimetric assay uses reduction of a yellow tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, or MTT) to measure cellular metabolic activity as a proxy for cell viability. Viable cells contain NAD(P)H-dependent oxidoreductase enzymes will reduce the MTT reagent to formazan, which is an insoluble crystalline product with a deep purple color. Formazan crystals are then dissolved using a solubilizing solution and absorbance is measured at 492 nm using a plate-reader. The darker the solution, the greater the number of viable cells. Each MTT assay are performed three times and the average absorbance are 0.71 and 0.63 for PDMS reservoir and PEIE mixed PDMS reservoir. It confirms the biocompatibility does not change too much after addition of PEIE. It is speculated that the toxic PEIE is trapped in PDMS networks and cannot leak into the culture solution. Therefore, the proposed PEIE mixed PDMS composite has good biocompatibility for biosensor applications.
3.4 Preparation and test of 3D microfluidic chip for long-term cell culture
To demonstrate the applicability of the developed bonding method for microfluidic biosensor, a more complex chip—the 3D long-term cell culture chip—is designed, prepared, and tested. For most of the microfluidic chip with microchambers or microdroplets, the cells cannot live for too long due to the depletion of nutrition and unable to replace the waste (Kimura et al. 2008). Here, a 3D microfluidic chip including the culture chamber layer, the nano-membrane layer and the underneath channel layer is designed and shown in Fig. 7a. The whole chip is placed in a well made by PMMA and filled with paraffin oil to prevent the evaporation of water in the culture chamber (Fig. 7b). The inlets are punched on the top of the chip and the outlets are arranged on the bottom layer. A top PDMS layer is added for the connection of tubing and a bottom PDMS layer is added for ease of pumping out culture medium and waste from the channels. There are five channels parallel to each other and each of them has nine culture chambers with diameter of 600 μm and height of 150 μm. The key of this 3D microfluidic chip is the polycarbonate nano-membrane layer show in Fig. 7c, which has lots of pores with fixed diameter of 800 nm, permitting the transport of nutrition and waste but isolating the bacteria or cells on the upper side. The intermediate polycarbonate layer cannot be bonded to PDMS using plasma treatment and the UV glue bonding has too much residuals which may go to the culture chamber.
The fabricated microfluidic device is then used to culture E. coli BL-21 which has GFP plasmid for easy visualization. To demonstrate the advantages in cell culture, a traditional droplet trapping microfluidic chip (Wang et al. 2009) was prepared and used to compare their differences. The chip structure is shown in the supplementary Fig. S4. The diameter of the circular trapping chamber is 600 μm, and the height is about 50 μm. E. coli with density of 3720 CFU/ml is loaded into the prepared 3D chip and the droplet trapping chip, respectively. Fluorescent images are taken continuously and typical ones are shown in Fig. 8. It is clearly seen that the bacteria grow to the stationary phase in 1 day and they will go to death phase in the following 2 days in traditional droplet trapping chip as shown in Fig. 8a. It is because the nutrition will be depleted during growth and reproduce. However, the bacteria can grow faster and are still viable as long as 7 days, suggesting the maintaining of stationary phase in the prepared 3D chip with a nano-membrane for providing nutrition continuously. Therefore, the proposed bonding method can be used to prepare the 3D microfluidic chip which shows superior in long-term cell culture. It is also noted that the bonding between PDMS and substrate material is reversible, making it useful to recover cells from the culture chamber for further analysis.
4 Discussions
For the fabrication of microfluidic biosensors, hard materials such as glass, silicon and gold metal are commonly used for their good mechanical properties and easy of surface modification to immobilize affinity tags for binding of target molecules on surfaces (Pijanowska et al. 2003). Self-assembly is usually applied for surface modification and lots of biomolecules such as enzyme, antibody, nucleotide, aptamer etc. have been successfully demonstrated (Deng et al. 2008; Krenkova and Foret 2004). However, these materials are not good for constructing microstructures in microfluidic chip due to the high cost, cumbersome process, and use of large equipment. On the contrary, polymers such as PDMS, PMMA, PS, PET, and COC are attractive materials for fluidic channels and reactors. PDMS is the most commonly used material for microchannels for its easy replication from SU8 mold. Therefore, the bonding method for sealing the substrate and channel is an inevitably step. To protect biomolecules on the surface of chip substrate, adhesive bonding is believed to be a good choice. However, most of these reported adhesive bonding methods are not compatible with softlithography replication process, leading to lots of difficulties in preparation of microfluidic biosensor with well-defined microstructures. The proposed method here has the same preparation process as PDMS chip. By comparing the SEM images of the pure PDMS plasma bonded chip and that prepared by PDMS mixed with PEIE (Fig. 3), this thin adhesive layer has a very good bonding effect. The structure of the chip prepared by adding 20 μl and 30 μl PEIE per 10 g PDMS is basically the same as that of pure PDMS, and there is no deformation. The chip prepared by adding 40 μl PEIE per 10 g PDMS undergoes slight deformation, but the structure of the channel is unchanged, which does not have much impact on sample injection and fluid flow. The results showing in Figs. 2, 4 and 4, also demonstrate the chip has a good sealing performance for most applications.
The materials used in biosensor are extremely diverse (Dou et al. 2019; Sanjay et al. 2020), thus a universal method that can be used to bond to most existing substrates is extreme helpful in preparation of microfluidic biosensors. The representative substrate materials including glass, metal, plastics, and ceramics all have been demonstrated to have good performance using the proposed “tear off–paste on” method. Especially, a 3D cell culture chip with polycarbonate nonporous membrane as the intermediate layer for the transport of culture solution and waste has been designed and prepared, which is unachievable with conventional bonding method. The cell culture experiment shows the bacteria could stay viable as long as weeks, while they can only growth for 1 day in the enclosed chamber without fresh nutrition provided. The non-toxic property to cells is also required for biosensors. Indeed, PEIE itself is a toxic substance to cells. However, the biocompatibility test confirmed the PDMS mixed PEIE composite material has no harm to cells (Fig. 6). It may be because small amount of PEIE molecules are embedded in the PDMS networks and not much of them could leak into the solution.
5 Conclusions
A universal bonding method by simply mixing PDMS with a small portion of PEIE to form sticky thin layer is presented for microfluidic biosensors fabrication. This method has advantages including low cost, simple operation, large equipment-free, which is expected to apply in preparation of microfluidic biosensors in non-specialized laboratories. The mixing ratio of PEIE to PDMS ratio and the incubation time and temperatures have been optimized. This method is appealing for its compatibility to traditional replication method using PDMS and the surface structures can be retained. It can be used for different materials and typical ones such as glass, PMMA, plastics, ceramics, and metal have been demonstrated and the bonding force is applicable for most usage as droplet generation with different sizes shows. The bonding effect is expected to help the preparation of a large number of different materials of microfluidic chips. The biocompatibility of prepared microfluidic chip by composite material has almost the same as that using pure PDMS. Based on this method, a novel three-dimensional microfluidic chip with a nanopore membrane for providing fresh culture medium was designed and fabricated, which can be used for long-term culture of bacteria. Compared to the isolated microwells which can only be used for bacteria culture for 1 day, the designed three-dimensional microfluidic chip can make sure the bacteria are still viable for as long as 7 days. It is envisioned the developed universal bonding method has wide applications in biosensors for cell culture, environmental monitoring, clinical diagnostics etc., which permits the use of different materials with biomolecules as substrates to prepare microfluidic chips.
References
Carroll S, Crain MM, Naber JF, Keynton RS, Walsh KM, Baldwin RP (2008) Room temperature UV adhesive bonding of CE devices. Lab Chip 8:1564–1569
Cira NJ, Ho JY, Dueck ME, Weibel DB (2012) A self-loading microfluidic device for determining the minimum inhibitory concentration of antibiotics. Lab Chip 12:1052–1059
Dang F, Shinohara S, Tabata O, Yamaoka Y, Kurokawa M, Shinohara Y, Ishikawa M, Baba Y (2005) Replica multichannel polymer chips with a network of sacrificial channels sealed by adhesive printing method. Lab Chip 5:472–478
Deng L, Wang YZ, Shang L, Wen D, Wang F, Dong SJ (2008) A sensitive NADH and glucose biosensor tuned by visible light based on thionine bridged carbon nanotubes and gold nanoparticles multilayer. Biosens Bioelectron 24:951–957
Di Lorenzoab F, Seiffert S (2015) Nanostructural heterogeneity in polymer networks and gels. Polym Chem 6:5515–5528
Dou M, Macias N, Shen F, Bard JD, Dominguez DC, Li X (2019) Rapid and accurate diagnosis of the respiratory disease pertussis on a point-of-care biochip. EClinMed 8:72–77
Eddings MA, Johnson MA, Gale BK (2008) Determining the optimal PDMS-PDMS bonding technique for microfluidic devices. J Micromech Microeng 18:1171–1185
Fan Y, Li H, Yi Y, Foulds IG (2013) PMMA to polystyrene bonding for polymer based microfluidic systems. Microsyst Technol 20:59–64
Flachsbart BR, Wong K, Iannacone JM, Abante EN, Vlach RL, Rauchfuss PA, Bohn PW, Sweedler JV, Shannon MA (2006) Design and fabrication of a multilayered polymer microfluidic chip with nanofluidic interconnects via adhesive contact printing. Lab Chip 6:667–674
Gong X, Yi X, Xiao K, Li S, Kodzius R, Qin J, Wen W (2010) Wax-bonding 3D microfluidic chips. Lab Chip 10:2622–2627
Hunter AC (2006) Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv Drug Deliv Rev 58:1523–1531
Jena RK, Yue CY, Lam YC (2011) Micro fabrication of cyclic olefin copolymer (COC) based microfluidic devices. Microsyst Technol 18:159–166
Jeong SH, Zhang S, Hjort K, Hilborn JN, Wu ZG (2016) Stretchable electronic devices: PDMS-based elastomer tuned soft, stretchable, and sticky for epidermal electronics. Adv Mater 28:5765–5765
Kersey L, Ebacher V, Bazargan V, Wang R, Stoeber B (2009) The effect of adhesion promoter on the adhesion of PDMS to different substrate materials. Lab Chip 9:1002–1004
Kimura H, Yamamoto T, Sakai H, Sakai Y, Fujii T (2008) An integrated microfluidic system for long-term perfusion culture and on-line monitoring of intestinal tissue models. Lab Chip 8:741–746
Krenkova J, Foret F (2004) Immobilized microfluidic enzymatic reactors. Electrophoresis 25:3550–3563
Li SB, Gong XQ, Mc Nally CS, Zeng ML, Gaule T (2016) Rapid preparation of highly reliable PDMS double emulsion microfluidic devices. RSC Adv 6:25927–25933
Lu CM, Lee LJ, Juang YJ (2010) Packaging of microfluidic chips via interstitial bonding technique. Electrophoresis 29:1407–1414
Luka G, Ahmadi A, Najjaran H, Alocilja E, DeRosa M, Wolthers K, Malki A, Aziz H, Althani A, Hoorfar M (2015) Microfluidics integrated biosensors: a leading technology towards lab-on-a-chip and sensing applications. Sensors 15:30011–30031
Ma K, Rivera J, Hirasaki GJ, Biswal SL (2011) Wettability control and patterning of PDMS using UV-ozone and water immersion. J Colloid Interface Sci 363:371–378
Ma WR, Liu LL, Xu Y, Wang L, Li SB (2020) A highly efficient preconcentration route for rapid and sensitive detection of endotoxin based on an electrochemical biosensor. Analyst 145:4204–4211
Nayak NC, Yue CY, Lam YC, Tan YL (2010) Thermal bonding of PMMA: effect of polymer molecular weight. Microsyst Technol 16:487–491
Pan YJ, Yang RJ (2006) A glass microfluidic chip adhesive bonding method at room temperature. J Micromech Microeng 16:2666–2666
Patko D, Martonfalvi Z, Kovacs B, Vonderviszt F, Kellermayer M, Horvath R (2014) Microfluidic channels laser-cut in thin double-sided tapes: Cost-effective biocompatible fluidics in minutes from design to final integration with optical biochips. Sens Actuators B 196:352–356
Peterson SL, Mcdonald A, Gourley PL, Sasaki DY (2005) Poly(dimethylsiloxane) thin films as biocompatible coatings for microfluidic devices: cell culture and flow studies with glial cells. J Biomed Mater Res Part A 72a:10–18
Pijanowska DG, Remiszewska E, Lysko JM, Jazwinski J, Torbicz W (2003) Immobilisation of bioreceptors for microreactors. Sens Actuators B 91:152–157
Riegger L, Strohmeier O, Faltin B, Zengerle R, Koltay P (2010) Adhesive bonding of microfluidic chips: influence of process parameters. J Micromech Microeng 20:87003–87007
Sanjay ST, Li MH, Zhou W, Li XC, Li XJ (2020) A reusable PMMA/paper hybrid plug-and-play microfluidic device for an ultrasensitive immunoassay with a wide dynamic range. Microsyst Nanoeng 6:1044–1053
Shamsi MH, Chen S (2017) Biosensors-on-chip: a topical review. J Micromech Microeng 27:083001–083017
Suzuki Y, Yamada M, Seki M (2010) Sol–gel based fabrication of hybrid microfluidic devices composed of PDMS and thermoplastic substrates. Sens Actuators B 148:323–329
Thompson CS, Abate AR (2013) Adhesive-based bonding technique for PDMS microfluidic devices. Lab Chip 13:632–635
Trantidou T, Elani Y, Parsons E, Ces O (2017) Hydrophilic surface modification of PDMS for droplet microfluidics using a simple, quick, and robust method via PVA deposition. Microsyst Nanoeng 3:16091–16100
Umbrecht F, Müller D, Gattiker F, Boutry CM, Neuenschwander J, Sennhauser U, Hierold C (2009) Solvent assisted bonding of polymethylmethacrylate: characterization using the response surface methodology. Sens Actuators A 156:121–128
Wang W, Yang C, Li CM (2009) On-demand microfluidic droplet trapping and fusion for on-chip static droplet assays. Lab Chip 9:1504–1506
Wang LJ, Chang YC, Sun RR, Li L (2017) A multichannel smartphone optical biosensor for high-throughput point-of-care diagnostics. Biosens Bioelectron 87:686–692
Xu YC, Vaidya B, Patel AB, Ford SM, Mccarley RL, Soper SA (2003) Solid-phase reversible immobilization in microfluidic chips for the purification of dye-labeled DNA sequencing fragments. Anal Chem 75:2975–2984
Ye Z, Wang K, Lou M, Jia X, Ye G (2020) Consecutive synthesis of gold nanobipyramids with controllable morphologies using a microfluidic platform. Microfluid Nanofluid 24:38–46
Zhang H, Xue L, Huang FC, Wang SY, Wang L, Liu N, Lin JH (2019) A capillary biosensor for rapid detection of Salmonella using Fe-nanocluster amplification and smart phone imaging. Biosens Bioelectron 127:142–149
Acknowledgements
This research was funded by the Fundamental Research Funds for the Central Universities, grant number 2020CDJYGGD004, 2020CDJGFGDZD016, 2020CDJXZ001, the National Natural Science Foundation of China, grant number 61904021, 61971074, Key project of science and technology research program of Chongqing Education Commission of China, grant number KJZD-K201900103, the Pioneer Natural Science Foundation of Chongqing, grant number cstc2019jcyj-xfkxX0003 and National Key Research and Development Program of China, grant number 2019YFC0214400. We thank Mr. Hong He from College of Physics, Chongqing University for the help in calculation of bonding strength using COMSOL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, P., Lv, Z., Sun, B. et al. A universal bonding method for preparation of microfluidic biosensor. Microfluid Nanofluid 25, 43 (2021). https://doi.org/10.1007/s10404-021-02445-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-021-02445-8