Abstract
This paper presents a non-equilibrium multiscale molecular dynamics simulation method to investigate the effects of periodic wall surface roughness on the structure and mass transfer of methane fluid through the silicon nano-channels. In order to accurately capture the trajectories and microstructure of methane nano-fluidics, the present modification of OPLS fully atomic model is employed. Meanwhile, we introduce the corresponding coarse-grained model to solve the problem of wall–fluid interaction for methane Poiseuille flow within silicon atomic walls using the classical Lorentz–Berthelot mixing rules. The geometries of the upper wall roughness are modeled by rectangular waves with different amplitudes and wavelengths. The three-dimensional number densities of C (H) atom and kinetic energy distribution plots give a clear observation of the impacts of surface roughness on the localization micro-information of methane fluid. Moreover, the slip length of fluid over rough surface decreases with the increase in amplitude. The diffusion coefficients appear anisotropic, and the radial distribution functions decrease with the increase in the amplitude. These properties should be taken into account in the design of energy-saving emission reduction nano-fluidic devices. All numerical results also indicate that the presented method not only can well solve the issue of wall–fluid interactions, but also could accurately predict the micro-information and dynamic properties of methane Poiseuille flow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent decades, considerable attention has been focused on the rough nano-channel walls in fluid mechanics and contact mechanics. In order to understand the atomic processes of fluids occurring at the rough solid interface of two materials, the scientific community attempts to bridge models at different scales through restudying the classical theory (Bhushan et al. 1995; DelRio et al. 2005; Giannakopoulos et al. 2014; Granick 1991; Kasiteropoulou et al. 2012; Sparreboom et al. 2010). Moreover, the theoretical and experimental studies have been carried out in innovative technological applications and various engineering devices, such as friction and lubrication, drug delivery, water desalination and purification (Asproulis et al. 2012; Bernardo et al. 2009; Bhushan 2000; Choi et al. 2008; Corry 2008; Gargiuli et al. 2006; Kumar et al. 2008; Liakopoulos et al. 2016; Mantzalis et al. 2011; Menezes et al. 2013; Wang et al. 2007). However, experimental researches at the atomic scale (few atomic diameters) are difficult to accomplish and, thus, atomistic molecular dynamics (MD) simulation techniques play a very important role in these fields that cannot be accessed by experiment. Although many studies of micro- and nano-flows were reported using the MD simulations in the literature (Allen and Tildesley 1989; Cao et al. 2009; Delhommelle and Evans 2001a, b; Hartkamp et al. 2012; Jiang et al. 2016a; Karniadakis et al. 2006; Kim and Strachan 2015; Ranjith et al. 2013; Svoboda et al. 2015), there are still numerous problems to be resolved. First, at the atomic scale, because any solid surface is intrinsically rough on the micro- and nanoscale, how to describe the roughness of nano-channel walls is the urgent need to be addressed (Cao et al. 2009; Menezes et al. 2013). Second, the classical no-slip condition breaks down when downsizing to the nanoscale (Asproulis and Drikakis 2011; Karniadakis et al. 2006). Furthermore, the classical Lorentz–Berthelot (L–B) mixing rules fail for the complex atomistic fluids flow through the roughness nano-channel walls (Kong 1973), due to the fact that the potential function of complex atomistic models is inconsistent with the wall, such as water, methane and methyl alcohol.
The effect of roughness of wall is of particular importance in nano-flows, since the various rough wall structures induce complex atom force at the near wall, which will affect the flow and mass transfer. Several classifications of rough wall structures have been available in the literature (Cao et al. 2006b; Galea and Attard 2004; Jabbarzadeh et al. 2000; Kim and Darve 2006; Kim and Strachan 2015; Kumar et al. 2011; Malijevský 2014; Mo and Rosenberger 1990; Noorian et al. 2014; Priezjev 2007; Sbragaglia et al. 2006; Sofos et al. 2010; Svoboda et al. 2015; Zhang 2016a; Ziarani and Mohamad 2006). The impacts of sinusoidally and randomly roughened wall on momentum transfer of gas argon were studied using MD simulations by Mo and Rosenberger (1990). The research of Jabbarzadeh et al. (2000) demonstrated that the slip length of flow of liquid hexadecane increases at near sinusoidal-wall channel with the increase in the roughness period. Later, Priezjev (2007) defined randomly and periodically roughened wall that depended on the kind of forces of the wall atoms, and discussed the relation between the variation of slip length and the roughness amplitude of the wall surface. Noorian et al. (2014) reported the effect of wall roughness on the Poiseuille flow behavior of liquid argon through the randomly and periodically roughened wall using the non-equilibrium MD simulations. The effects of surface roughness on squeeze-film damping for three different random and periodical surfaces were discussed by Kim and Strachan (2015). But these rough walls were implemented rather complex in numerical simulation. In particular, the randomly rough wall is not a quantitative analysis of the wall roughness.
The periodical and rectangular waves are usually considered as a key pattern in depicting the roughness of nano-channel walls and are further used to investigate the various properties (such as transport properties, potential distribution, velocity fluctuations and wetting properties) of fluids in the nano-channels (Cao et al. 2006a; Kim and Darve 2006; Kumar et al. 2011; Sofos et al. 2009a,b, 2010, 2016; Zhang 2016a). Kim and Darve (2006) indicated that the maximum magnitude of the polarization density inside the groove is higher than for the smooth wall, and the flow rate decreases with an increasing amplitude and a decreasing period of surface roughness. Cao et al. (2006a, b) reported that the velocity of gaseous argon decreases near the submicron platinum wall as the roughness amplitude increases. Sofos et al. (2009a, b, c, 2010, 2012 and 2016) demonstrated the effects of periodic wall roughness on the flow of liquid argon through krypton nano-channels and discussed the transport properties and slip length near the rough wall. Kumar et al. (2008, 2011) discussed the wetting properties of LJ atomistic fluid systems by Monte Carlo simulation at geometrically rough interfaces. Zhang (2016a, b) studied the effects of wall surface roughness on the fluid mass flow rate through a nano-slit pore in the Poiseuille flow using the flow factor approach model.
It should be noted that all the researches mentioned above are mainly confined to rough wall for simple Lennard-Jones (LJ) fluid using traditional numerical methods. Some microstructural information (distribution of atom or charge) of complex fluid (water or methane) will be lost. The interaction of wall–fluid was supposed to be LJ potential, where the parameters were determined by the fluid (generally independent of the wall material except the research of Sofos et al. (2016) and Cao et al. (2006a)). This leads to the strength of wall–fluid interaction independent on the material of wall. However, the properties of wall material should not be ignored, since it determines the wall–fluid interaction, which characterizes a wetting (weak interaction of wall–fluid) or a non-wetting (strong interaction of wall–fluid) surface (Cao et al. 2009). The research of Priezjev et al. (2005) indicated that the larger peak maxima and more oscillations of the density profile are generated near wall, while the wall–fluid interaction increases. Asproulis and Drikakis (2011) reported the effects of the surface stiffness and wall particles’ mass on the slip length. To the best of our knowledge, studies of the effect of rough wall on the methane nano-fluidics are scarce because the interaction of wall–fluid depends on the properties of wall material. Though the viscosity of different resolution-level methane models has been studied by Jiang et al. (2016a) using Poiseuille flow in the silicon nano-channels, it is very important to study the impacts of wall roughness on the flow behavior and microstructural properties in the nano-channels, which will be helpful for the design of energy-saving emission reduction nano-fluidic devices, since methane is considered as a very key fuel with environmental advantages and industrial importance (Schiermeier 2006).
In order to explore the impacts of silicon atomic wall roughness on microstructural and dynamical properties of complex methane fluid in the rough nano-channels, we present a non-equilibrium multiscale molecular dynamics (MSMD) method in this work. As a promising multiscale method, it plays a key role in investigating the complex molecular fluid systems (Jiang et al. 2016a; Mashayak and Aluru 2012a, b; Noid 2013) The advantage of this method is that the potential function of the coarse-grained (CG) fluids and wall atoms is always consistent, which ensures the validity of the classical L–B mixing rules for solving the problem of wall–fluid interaction. Moreover, the potential of mean force of CG model is in good agreement with the corresponding fully atomistic model (Noid 2013; Shell 2008), which ensures the strength of wall–CG interaction close to realistic interaction of wall–fluid. It is useful for observing the slip length of fluid through the rough nano-channel walls. Furthermore, the non-equilibrium MSMD method can be used not only to tackle the physical boundary, but also to capture the micro-information of methane nano-fluidics, such as localization atomic density distributions (C and H atom), localization velocity distribution and radial distribution functions (RDFs) \(g_{\text{CC}} (r)\), \(g_{\text{CH}} (r)\) and \(g_{\text{HH}} (r)\). For these reasons, the non-equilibrium MSMD method is very suitable for investigating the effect of wall roughness on the microstructural and dynamical properties of complex methane fluid in nano-channels.
The primary goal of this work is to investigate the influence of the wall roughness on microstructural information and dynamical properties of methane fluid through the nano-channels in the Poiseuille flow using non-equilibrium MSMD simulations. In order to exhibit the advantage of MSMD in studying the effect of rough wall on the fluid atom behavior of the rough nano-channel walls, detailed number density distribution of C (H) atom and microstructural properties are discussed. To demonstrate the methane fluid molecules trapped into cavities of rough walls, we trace the trajectories of the trapped methane fluid molecule in the cavities using snapshots for different roughness of walls. The kinetic energy distributions are calculated over a three-dimensional computational grid inside the nano-channel in order to reveal the localization fluid velocity close to and inside the rectangular grooves. Furthermore, the effects of nano-channel walls roughness on the detailed temperature profile, velocity profile, slip length and diffusion coefficients of methane Poiseuille flow are also discussed by altering the amplitude or wavelength.
The rest of this paper is organized as follows. In Sect. 2, our models and simulation method are given. In particular, the implementation about how to describe the nano-channel walls roughness and solve the problem of wall–fluid interaction is introduced in detail. Next, simulation results are presented and discussed in Sect. 3. Finally, we draw conclusions from the present work and show some directions for future work in Sect. 4.
2 Models and simulation details
A non-equilibrium MSMD simulation is conducted to study the detailed atomic behavior of methane Poiseuille flow within silicon atomic nano-channel. The methane molecules are placed by a face-centered cubic lattice between the two silicon atomic walls at a certain distance from each other in the z-direction.
2.1 Physically rough wall models
Each wall is comprised of four layers of silicon atoms (each layer consisting of \(240\) silicon atoms). The wall atoms are placed and fixed in an ABAB stacking, since the ABAB stacking is the lowest energy configuration (Kamal et al. 2013). The lower wall of the nano-channels is smooth. For simplicity, the rough upper wall is constructed by “anchoring” extra wall atoms on the smooth wall to form periodically rectangular wave with different amplitudes and wavelengths. For a physically rough wall, we intuitively expect that the height of the surface should vary with lateral position. Therefore, the “anchoring” extra wall atoms are placed by \(\Delta z(x)\) according to the following formula for the nano-channel upper wall surfaces:
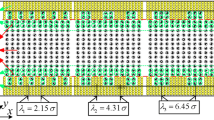
where \(A_{i}\)(\(i = 1,2,3\)) and \(\lambda_{j}\)(\(j = 1,2,3,4,5\)) are characteristics of the roughness, respectively, representing the amplitude and wavelength of the periodically rectangular wave nano-channel walls. In this work, we consider three amplitudes \(\left( {A_{i} = \{ 0.54\;\sigma ,\;1.07\;\sigma ,\;1.62\;\sigma \} } \right)\) and five wavelengths \(\left( {\lambda_{j} = \{ 1.07\;\sigma ,\;2.15\;\sigma ,\;4.31\;\sigma ,\;6.45\;\sigma ,\;12.84\;\sigma \} } \right)\) for the periodic roughness of upper walls (e.g., \(\lambda_{2} = 2.15\;\sigma\) and \(A_{2} { = }1.07\;\sigma\) are given in Fig. 1). The periodic boundary conditions are applied in the x- and y-directions only. To keep consistent with the periodicity, the length of the simulation box in the x-direction should be chosen in such a way that it accommodates an integer number of full rectangular wave depending on the wavelength \(\lambda_{j}\).
Schematic of the interactions of fluid–fluid and wall–fluid using non-equilibrium MSMD simulation. Also shown nano-channel models for periodic roughness (\(\lambda_{2} = 2.15\;\sigma ,\;A_{2} { = }1.07\;\sigma\)) of the upper walls. The coarse-graining process and the high of nano-channels are given. Channel dimensions are \(L_{x} \times L_{y} \times L_{z} = 12.4\;\sigma \times 10.3\;\sigma \times 12.4\;\sigma\)
2.2 Methane fluid models
The optimized potential for liquid simulation (OPLS) model has been reported by Jorgensen and Tirado-Rives since 1988 (Jorgensen and Tirado-Rives 1988). And the modification of OPLS (MOPLS) model was given by Jiang et al. for studying the structural and dynamical properties of dense methane fluid at the low-temperature domains (Jiang et al. 2016b). The potential function of fully atomistic methane model can be given as
where \(\varepsilon_{0}\), \(\varepsilon\), \(\sigma\), \(q\) and \(r_{ij}\) represent the permittivity of vacuum, the depth of the interaction energy well, molecular diameter, point charge and the distance between atoms \(i\) and \(j\), respectively. \(a\) and \(b\) denote different types of atoms (C and H). The subscripts \(\alpha\) and \(\beta\) are used to distinguish the interactions between different molecules. The bond length of C–H is \(l_{\text{CH}} = 1.087\;{\AA}\) in MOPLS model. The charges appearing in the potential function are \({\kern 1pt} q_{\text{C}} = - 4q_{\text{H}} = - 0.572\;e\). All other details of MOPLS are given in Table 1.
To solve the issue of wall–fluid interaction, the classical L–B mixing rule is employed in this work. The MOPLS is inconsistent with the silicon atoms wall [LJ (12-6)] (Hu et al. 2015), which leads to the failure of classical L–B mixing rule in tackling solid–fluid interactions. Therefore, we present the CG methane model to solve the problem (the formula of CG potential function depends on the atoms wall). Meantime, the LJ (12-6) interatomic pair potential is commonly used in MD simulations of solid–liquid boundaries (Karniadakis et al. 2006; Sadus 2002)
The CG methane model maps one methane molecule onto a single particle with its center of mass as the interaction point. The interactions of CG methane particles are implicitly incorporated into the effective pair interaction potential, given as
The parameters of CG potential are determined by the relative entropy minimization method (Shell 2008) in the bulk methane fluid under NVT ensemble. The obtained parameters of the CG potential are \(\sigma_{\text{CG}} = 3.645\;{\AA}\), \(\varepsilon_{\text{CG}} = 1.329\;{{\text{kJ}} \mathord{\left/ {\vphantom {{\text{kJ}} {\text{mol}}}} \right. \kern-0pt} {\text{mol}}}\) at \(\rho = 377.15\;{\text{kg/m}}^{3}\) and \(T = 140\;{\text{K}}\). The potential of mean force (\(W\left( r \right) = - k_{\text{B}} T\ln g\left( r \right)\)) and RDF of CG model are given in Fig. 2. It can be seen that the results of CGMOPLS are in better agreement with the corresponding fully atomistic model than unit atom LJ model (Poling et al. 2001). It indicates that we can obtain the ideal interactions of wall–fluid using the classical L–B mixing rule coupling the corresponding CG model (Kong 1973).
Finally, we apply the classical L–B mixing rule to determine the parameters of wall–fluid \(\sigma_{\text{MS}}\) and \(\varepsilon_{\text{MS}}\) for methane–silicon interactions
2.3 Simulation details
In this work, we use non-equilibrium MSMD to study the effects of wall roughness on methane fluid in nano-channels. Our system consists of \(1440\;(\lambda_{1} )\) \((1500\;(\lambda_{i} ,A_{1} ),\;1560\;(\lambda_{i} ,A_{2} ),\;1620\;(\lambda_{i} ,A_{3} ),\;i = 2,\; \ldots ,\;5)\) fluid methane molecules with different rough walls and is bounded by \(2160\;(A_{1} )\;(2400\;(A_{2} ),\;2640\;(A_{3} ))\) silicon atom walls which are subjected to an additional harmonic potential with respect to an ABAB stacking (Kamal et al. 2013) (shown as example (\(A_{2} ,\;\lambda_{2}\)) in Fig. 1) site \({\mathbf{r}}_{\text{eq}}\),
where \({\mathbf{r}}\left( t \right)\) is the position of a boundary particle at time \(t\). The rigidity of the wall is determined by the force constant \(k_{w}\) \(\left(= 72\varepsilon_{\text{S}} /\left( {2^{{{1 \mathord{\left/ {\vphantom {1 3}} \right. \kern-0pt} 3}}} \sigma_{\text{S}}^{2} } \right)\right)\), which is calculated by the second derivative of the interaction LJ (12-6) potential of silicon atoms at \(r = 2^{{{1 \mathord{\left/ {\vphantom {1 6}} \right. \kern-0pt} 6}}} \sigma_{\text{S}}\) (Liem et al. 1992), where the parameters are \(\sigma_{\text{S}} = 3.826\;{\AA}\), \(\varepsilon_{\text{S}} = 1.6885\;{\text{kJ/mol}}\), and the density of silicon is \(2329\;{{\text{kg}} \mathord{\left/ {\vphantom {{\text{kg}} {{\text{m}}^{ 3} }}} \right. \kern-0pt} {{\text{m}}^{ 3} }}\) (Hu et al. 2015). The system is periodic along the x- and y-directions in all cases, while in the z-direction, the distance, \(L_{\text{z}}\) (\(= 12.4\;\sigma\)), between the two silicon atom walls, is large enough for methane molecules to penetrate beyond the wall surface layers.
In the present study, our method is essentially different from that employed by Markesteijn et al. (2012) to study a complex water molecule fluid using the Poiseuille flow in solving the problem of interaction for wall–fluid. We apply the CG methane model to solve the problem of interactions of wall–fluid rather than simple ignoring the charge force and H atom interactions adjacent to wall (less than \(r_{\text{cut}}\)). And more specifically, the formula of CG potential function of complex fluid relies on solid wall. For example, the LJ (12-6) potential is employed as the CG methane potential in this work, because the potential function of solid silicon atom is LJ (12-6) potential (Hu et al. 2015).
In order to avoid compressibility effects, the external driving force \(f_{\text{ex}} = 0.1\;\left( {{\varepsilon \mathord{\left/ {\vphantom {\varepsilon \sigma }} \right. \kern-0pt} \sigma }} \right)\) is employed along the x-direction only to drive the flow in different roughness of nano-channel walls (Jiang et al. 2016a). All intermolecular interactions are subject to a continuous force (or continuous energy) cutoff radius of \(r_{\text{cut}} = 2.5\;\sigma\). The orientational coordinates of the methane tetrahedral are expressed in terms of quaternion parameters, and the center of mass motion of methane molecules is integrated via using the fourth Predictor–Corrector method (Allen and Tildesley 1989; Frenkel and Smit 2001; Hansen and McDonald 1990; Rapaport 2004). To control the temperature of system, the NVT ensemble with Nosé-Hoover thermostat is employed. Whether the thermostat follows the equipartition theorem is checked, by comparing the thermal kinetic energy per methane molecule in y- and z-directions
where \(m\), \(k_{\text{B}}\), \(T\), \(v_{\text{y}}\) and \(v_{\text{z}}\) denote the mass of molecule, Boltzmann constant, temperature, velocity of y- and z-directions, respectively. This can ensure that the thermostat effect is observed in each direction due to the intermolecular interactions, although x-direction is not explicitly considered (Bhadauria and Aluru 2013). The scaling parameters \(\sigma = 4.01{\kern 1pt} \;{\AA}\) and \({\varepsilon \mathord{\left/ {\vphantom {\varepsilon {k_{B} }}} \right. \kern-0pt} {k_{B} }} = 142.87\;\text{K}\) are used. The time step is \(\Delta t = 0.001\) (\(1.3 \times 10^{ - 15} \;{\text{s}}\)), the first \(2.0 \times 10^{5}\) MD time steps are excluded from the statistical analysis, and the relevant information of methane fluid was collected by further \(7.0 \times 10^{5}\) time steps. The streaming velocity profiles, equilibrium atom (C and H) distribution profiles and temperature profiles are calculated by dividing different channel width values (\(L_{z} + 2A_{i} \;(i = 1,\;2,\;3)\)) in 400 bins. The temperature profiles are calculated in each bin across the nano-channel using the equation (Delhommelle and Evans 2001a, b; Kasiteropoulou et al. 2012)
where \(N\) is the number of methane molecule; \({\mathbf{v}}_{i}\) and \({\bar{\mathbf{v}}}\) are velocity of molecule i and the corresponding stream velocity (macroscopic flow velocity), respectively. In order to observe the effect of different rough nano-channel walls on the localization properties of methane fluid, the three-dimensional C and H atoms number density profiles and kinetic energy distribution maps are evaluated as local values at various xoz planes of the nano-channels. To obtain these results, the domain is divided into \(n_{x} \times n_{y} \times n_{z} = (24 \times 10 \times 50)\) bins in the whole channel (include the wall) with each volume \(V_{\text{bin}} = \left( {{{L_{x} } \mathord{\left/ {\vphantom {{L_{x} } {n_{x} }}} \right. \kern-0pt} {n_{x} }}} \right) \times \left( {{{L_{y} } \mathord{\left/ {\vphantom {{L_{y} } {n_{y} }}} \right. \kern-0pt} {n_{y} }}} \right) \times \left( {{H \mathord{\left/ {\vphantom {H {n_{z} }}} \right. \kern-0pt} {n_{z} }}} \right)\). The localization kinetic energy, \(W_{\text{bin}}\), is computed by the following expression:
where \(m_{\text{bin}} = mn_{\text{bin}}\) and \(\left\langle {{\mathbf{v}}_{\text{bin}} } \right\rangle\) are mass and average velocity in the bin, respectively. \(n_{\text{bin}}\) is the number of molecule in the bin. All the simulation program codes are developed using C++ on Windows operating systems (Frenkel and Smit 2001; Rapaport 2004).
3 Results and discussion
3.1 Fluid localization properties
In order to investigate the effects of wall roughness on the methane fluid in different nano-channels, the three-dimensional number density contours (\(n_{y} = 6\)) of C and H atoms are shown in Figs. 3 and 4. Contour plots of the number density distribution of C and H atoms are obtained as values averaged in time inside the nano-channels. It is noted that the number density distribution of methane molecule is equal to that of C atom. Figures 3 and 4 illustrate that the number densities of C and H atoms present strong oscillations in density near the walls and consist of a series of number density islets. The results are similar to the research of Sofos et al. for liquid argon through krypton nano-channels (Sofos et al. 2009c). For \(\lambda = 1.07\;\sigma\), Figs. 3a(1), b(1), c(1) and 4a(1), b(1), c(1) show a series of localization number density island close to wall. The reason is that the cavities (\(\lambda = 1.07\;\sigma\)) are too small to contain a methane molecule, and therefore, each methane molecule can straightly across these small cavities without anchoring. At the protrusion domain, however, the methane molecules suffer larger repulsive force from the wall atom than the small cavities. Moreover, the interaction force exhibits periodic variation adjacent to the rough nano-channel walls, which results in the local number densities of atoms displaying cyclic variation. For \(\lambda = 2.15\;\sigma\), Figs. 3a(2), b(2) and c(2) and 4a(2), b(2), c(2) exhibit a series of regular local number density island at the protrusion domain of rough nano-channel walls. One possible reason is that the strength of wall–fluid interaction increases when the molecules come across the protrusion. Moreover, we find increasing localization densities inside the cavities as the wall amplitude (groove height) increases. It means that the trapped methane molecules become difficult to escape from the confined cavities. For \(\lambda \ge 4.31\;\sigma\), the local number densities of C and H atoms decrease with the increase in wavelength \(\lambda\) for same value of amplitude from Figs. 3a(3)–a(5), b(3)–b(5), c(3)-c(5) and 4a(3)–a(5), b(3)–b(5), c(3)–c(5) inside the cavities of rough wall. It indicates that the fluid atom localization inside the cavities increases, while the protrusion length (\(\lambda \ge 4.31\;\sigma\)) decreases, i.e., the roughness of nano-channel wall has a major influence on the fluid atom localization distribution.
The effect of wall roughness on the C and H atoms number density profiles as a total average value for all nano-channels cases is presented in Fig. 5. In general, at lower smooth wall, all nano-channels present the same C atom localization with a peak in the profile occurring at distance of about \(1.4\;\sigma\) from the lower wall limit. However, the H atom number density shows diversity in the profiles near wall except the smallest wavelength (\(\lambda = 1.07\;\sigma\)). Moreover, the localization number density peak value of smooth wall is larger than that of rough nano-channel walls, which indicates that the strength of mean wall–fluid interactions of the rough nano-channel wall is smaller than the smooth wall. For \(\lambda_{1} = 1.07\;\sigma\), C atom number density profiles are practically identical with different amplitudes in Fig. 5. However, H atom number density profiles increase as the amplitude value (groove height) increases, which shows the H atom number density distribution is very sensitive to strength of mean wall–fluid interactions. The similar conclusions of fluid density distribution are reported by Giannakopoulos (2014) for studying the density variations by non-equilibrium MD simulations in nano-channel flows. Furthermore, the C (H) atom localization number densities have significantly diversity near rough nano-channel walls with same wavelength (\(\lambda_{i} ,\;i = 2,\;3,\;4,\;5\)) and different amplitudes (\(A_{1} ,\;A_{2} ,\;A_{3}\)). And we observe that the positions of second or third peak values of H atom localization number density profile are significantly different adjacent to rough upper wall. These numerical results show that the C (H) atom distribution of methane molecule depends on the roughness (amplitudes) of nano-channel walls. Besides, we find increasing anisotropy of methane molecule as the values of rough amplitude increases at the same wavelength (or wavelength decreases at the same amplitude).
Total average C and H atoms number density profiles with different amplitudes (\(A_{1} ,\;A_{2} ,\;A_{3}\)) and wavelengths (\(\lambda_{1} ,\;\lambda_{2} ,\;\lambda_{3} ,\;\lambda_{4} ,\;\lambda_{5}\)). Note that the conclusions obtained from the molecule inside the nano-channels include the trapped molecules into the cavities
In order to exhibit the methane fluid molecules trapped into cavities of rough nano-channel walls, characteristic trajectories of the trapped methane molecules in the xoz plane for \(\lambda_{2} = 2.15\;\sigma\) are shown in Fig. 6. It can be seen that the average residence time of methane molecules inside the cavities increases with the increase in the amplitude (protrusion height). Characteristic trajectories for \(A_{2} = 1.07\;\sigma\) are also shown in Fig. 7. We observe that the methane molecules inside the cavities cost a long time to escape from the confined by the short wavelength (groove width) at same amplitude (protrusion height), which is in good agreement with the published results for fluid argon (Sofos et al. 2009c). These results demonstrate that the non-equilibrium MSMD is a credible option for studying the complex methane fluid atom distribution in the rough nano-channels. Furthermore, the numerical results indicate that the trajectories of the trapped methane molecules depend on the roughness of nano-channel walls (wavelength and amplitude).
3.2 Kinetic energy distribution and temperature profiles
To study the impact of wall roughness on the localization velocity distribution of methane fluid in nano-channels in detail, the contour plots of kinetic energy obtained from the average values in time inside the nano-channels are given in Fig. 8 for different roughness of the upper walls. The kinetic energy distributions are suitable for showing the localization distribution of velocity of methane molecule inside nano-channels from Eq. (10). In all the numerical results, adjacent to the smooth wall, the kinetic energy distributions do not obviously change. Moreover, we observe a series of kinetic energy small islands near the rough upper walls. These results show different kinetic energy distributions inside every cavity of rough upper walls, which is attributable to the mobility of molecule randomness. However, the kinetic energy distributions present the symmetry in the interior of nano-channels. It indicates that the localization velocity distribution approaches the parabolic velocity profile, which is similar to the macroscopic fluid for Poiseuille flow.
When \(\lambda_{1} = 1.07\;\sigma\), the localization kinetic energy distributions are exhibited in Fig. 8a(1), b(1), c(1). We observe that the regularity of kinetic energy small islands gradually increases with the increase in amplitude, and low kinetic energy (the lighter regions) domain increases as the amplitude increases. It indicates that the force of methane molecules obtained from wall atoms decreases, while the value of amplitude increases.
It can be seen from Fig. 8a(2), b(2), c(2) that the values of localization kinetic energy of cavities decrease as the amplitude increases (\(\lambda_{2} = 2.15\;\sigma\)). It denotes that the velocity of trapped methane molecule decreases, while it was trapped in the depth of cavities. And the trapped molecules always try escaping from the cavities, which will consume some kinetic energy.
Figure 8a(3), b(3), 8-c(3) shows the localization kinetic energy for \(\lambda_{3} = 4.31\;\sigma\). We observe that there exhibits at least one region of high kinetic energy (the darker regions) inside every cavity, and the number of regions of high kinetic energy increases with the increase in amplitude. It means that the angular velocity of methane molecule becomes larger in the cavities, and at least one attempts to accumulate much more kinetic energy (from other molecules or wall atoms) for escaping from the confined depth cavity (large amplitude).
For \(\lambda_{4} = 6.45\;\sigma\), we find that there are many regions of high kinetic energy (the darker regions) inside every cavity, and these regions of high kinetic energy show some regularity as the amplitude increases. The results are given in Figs. 8a(4), b(4) and c(4). At the same time, the regions of low kinetic energy (the lighter regions) exhibit the cavity adjacent to interior of nano-channels. It indicates that the methane molecule would be easy to escape the cavity. And their kinetic energy reaches local minimum when they escape from the cavity.
Figure 8a(5), b(5) and c(5) demonstrates that the regions of high kinetic energy are observed adjacent to the wall inside cavity for \(\lambda_{5} = 12.84\;\sigma\). And the regions of low kinetic energy (the lighter regions) increase with the increase in amplitude inside the cavity near interior of nano-channels, due to the fact that the wavelength is too long to well confine methane molecules to stay inside the cavities.
To observe the effect of the nano-channel walls roughness on the temperature of methane fluid, the temperature profiles are calculated using Eq. (9) and are illustrated in Fig. 9. It can be found that the temperature adjacent to smooth walls is higher than the system temperature. And the temperature presents complex change near the rough upper nano-channel walls. The results are similar to the simulation results of Kasiteropoulou et al. (2012) using the dissipative particle dynamics method. We attribute it to the complex wall–fluid interactions which significantly influence on the atom number density distributions (see Figs. 3, 4, 5) and kinetic density distributions (see Fig. 8). Moreover, we also observe that the values of temperature in the central part of the nano-channel are slightly less than 140 K. The numerical results are in good agreement with the reported results of Delhommelle and Evans (2001a, b) via the non-equilibrium MD simulations. The reason may be attributed to the statistical errors and the constant-temperature method which controls the temperature by comparing the thermal kinetic energy per methane molecule in y- and z-directions. All the results of temperature profiles also indicate that the non-equilibrium MSMD is reasonable and credible to study the methane nano-fluidic systems within the rough silicon atom walls.
Total average temperature profiles with different amplitudes (\(A_{1} ,\;A_{2} ,\;A_{3}\)) and wavelengths (\(\lambda_{1} ,\;\lambda_{2} ,\;\lambda_{3} ,\;\lambda_{4} ,\;\lambda_{5}\)). Note that the conclusions obtained from the molecule inside the nano-channels include the trapped molecules inside the cavities
3.3 Velocity profile and slip length
The velocity profiles of \(v_{\text{x}}\) averaged in time and space are given in Fig. 10 for different rough nano-channel walls. For \(\lambda_{1} = 1.07\;\sigma\), the values of maximum velocity are not significantly affected by the magnitude of the amplitude. And the velocity profiles do not obviously change near the rough wall, since the cavity is too small to contain one methane molecule, where the repulsive force decreases when the methane molecules are adjacent to the upper nano-channel walls. The results are similar to the liquid argon through krypton nano-channels (Sofos et al. 2009c). For the other values of wavelength, the values of maximum velocity slightly change as the amplitude increases (or decreases). However, the values of maximum velocity are obviously affected by the wavelength at the same amplitude, i.e., the values of maximum velocity increase with the increase in wavelength (\(\lambda \ne \lambda_{2}\)). When \(\lambda_{2} = 2.15\;\sigma\), the value of maximum velocity is obviously larger than other values (\(\lambda \ne \lambda_{2}\)), which indicates that the effect of wall roughness (\(\lambda = \lambda_{2}\)) on the mobility of molecule is significant. Moreover, for \(\lambda \ne \lambda_{1}\), the values of velocity near rough walls present an irregular fluctuation, which reveal that the trapped fluid molecules suffer from complex wall–fluid interaction in the cavities of upper wall. Furthermore, the velocity profiles obtained from the non-equilibrium MSMD simulations are similar to the Poiseuille flow velocity profile, with slight differences adjacent to the walls. It indicates that the mainstream regime of the methane nano-flows through the rough nano-channels obeys the continuum mechanics characterized by the Navier–Stokes equation. The velocity values fluctuate adjacent to the rough nano-channel walls. It means that the methane fluid molecules suffer from the resistance force of protrusion while they are confined to the cavities.
Total average streaming velocity profiles plotted over the whole channel region (\(L_{z} + 2A_{i} \;(i = 1,\;2,\;3)\)). Solid lines obtained by fitting \(280\) simple points in the middle of nano-channels. For the sake of brevity and readability, the velocity profile was given by shift \(0.5\;{\text{nm}}\) in z-direction
Furthermore, as shown in Fig. 11 we can obtain the slip length \(l_{\text{s}}\) [smooth (S), rough (R)] by extrapolating the velocity profile from the position in the fluid [with one atomic distance (D) from the smooth and rough solid surfaces] to where the velocity would lose the continuum mechanics characterized. It is noted that the slip length \(l_{\text{s}}\) is given as follows (Cao et al. 2006b):
where \(V_{\text{s}}\) is slip velocity, and \(\dot{\gamma } = {{dV_{\text{s}} } \mathord{\left/ {\vphantom {{dV_{\text{s}} } {dz}}} \right. \kern-0pt} {dz}}|_{{z = {\text{D}}}}\) is the shear rate.
The slip lengths of smooth and rough solid surfaces are given in Fig. 12 using Eq. (11). The comparison of the slip length of smooth wall is clearly larger than those of the rough wall at same wavelength and amplitude. And the slip length difference of between smooth wall and rough wall decreases with the increase in wavelength. It should be noted that \(V_{\text{s}}\) and \(\dot{\gamma }\) are calculated by average the velocity and shear rate adjacent to the smooth (rough) wall. These numerical predictions are in quantitative agreement with the results of the argon fluid through the rough micro- and nano-channels (Cao et al. 2006a, b; Sofos et al. 2009c). One possible reason is that the complex interaction of wall–fluid makes some trajectory of methane molecules more convoluted when the fluid molecule escapes from the cavities of rough wall. Moreover, the slip length \(l_{\text{s}}\)(R) decreases with the increase in amplitude, which indicates the velocity values of fluid decrease near rough wall, while the amplitude increases. This reason should be attributed to the complex interaction of wall–fluid, which leads to the increased friction factor with the increase in the protrusions of height (amplitude) at same wavelength. The similar results can be found in the literature (Liakopoulos et al. 2016). Though the slip length \(l_{s}\)(S) slightly increases with increased amplitude, it approaches a constant within calculation error at given value of amplitude. It demonstrates that the velocity values of methane fluid molecules are like near a smooth wall, since the interaction of wall–fluid does not significantly change. We attribute the slight difference to the fundamental statistical noise.
In a word, the effect of wall roughness is important in predicting the localization properties of the methane fluid in nano-channels. Based on the detailed investigation of three-dimensional number density (C and H atoms) distribution presented in Figs. 3 and 4, in connection with the characteristic trajectories of the trapped methane molecules (Figs. 6, 7), three-dimensional kinetic energy distributions (Fig. 8), temperature profiles (Fig. 9) and total average streaming velocity profiles (Fig. 10) inside nano-channels, there is conclusive evidence that methane fluid molecules are trapped inside wall cavities and this effect becomes more pronounced as the groove characteristic wavelength decreases or amplitude increases. The slip length \(l_{\text{s}}\)(R) is less than \(l_{\text{s}}\)(S). And \(l_{\text{s}}\)(R) decreases as amplitude increases. These results also further indicate that the non-equilibrium MSMD is an effective methodology to investigate the impact of the wall roughness on the fluid atom localization distribution, kinetic energy distribution, temperature profile, velocity profile and slip length of complex methane flow in rough nano-channels. To further explore the effect of the nano-channels wall roughness on the Poiseuille flow behavior of methane fluid, we investigate the variation of diffusion coefficient and RDFs in nano-fluidics.
3.4 Diffusion coefficient
Because of the strong layering of fluid atoms near the nano-channel walls, methane fluids are inhomogeneous. Classical fluid transport theories do not account for the inhomogeneity of the molecule fluid, and the diffusion coefficients in rough nano-channels can reflect the diffusion capacity and mass transfer for methane fluids. The channel diffusion coefficient is obtained from the Einstein expression (Diestler et al. 1991; Rapaport 2004)
or the Green–Kubo relation
where \(N\), \(t\), \(d\), \({\mathbf{r}}\), \({\mathbf{v}}\) are the numbers of molecule, calculation time, dimensionality of the system, position and velocity of molecule, respectively.
In order to use the Einstein expression to study the impact of the rough nano-channel walls on diffusion coefficient of methane fluid, we exclude the part of displacement that is due to the macroscopic flow velocity (\({\bar{\mathbf{v}}}\)) in this work (Bitsanis et al. 1987). Moreover, we also consider the diffusion coefficient as mainly flow direction \(D_{\text{x}}\) (x-direction of the velocity vector v), thermal motion direction \(D_{\text{y}}\) (y- direction of the velocity vector v) and transverse \(D_{\text{z}}\) (z-direction of the velocity vector v) to the flow as (Karniadakis et al. 2006)
The diffusion coefficients (\(D_{\text{x}}\), \(D_{\text{y}}\), \(D_{\text{z}}\) and \(D\)) of methane fluid through different nano-channel wall roughness at \(\rho = 377.15\;{\text{kg/m}}^{3}\), \(T = 140\;{\text{K}}\) are given in Fig. 13. It can be seen that the variation tendency of diffusion coefficients (\(D_{\text{x}}\), \(D_{\text{y}}\), \(D_{\text{z}}\) and \(D\)) for methane fluid are consistent with different rough nano-channel walls. The diffusion coefficient \(D_{\text{z}}\) is smallest (\(D_{\text{z}} < D_{\text{x}} < D_{\text{y}}\)), since the velocity value \(v_{\text{z}}\) is confined by the wall. The values of diffusion coefficients \(D_{\text{x}}\) are very close to \(D_{\text{y}}\) (\(D_{\text{x}} \approx 0.95D_{\text{y}}\)), since they are parallel the channel wall. We attribute this behavior to limited mobility of methane molecules trapping inside the grooves. Meanwhile, the values of diffusion coefficient \(D_{\text{y}}\) are adjacent to the bulk methane fluid, e.g., the smallest value \(D_{\text{y}} = 85.92 \times 10^{ - 10} \;{\text{m}}^{2} {\text{s}}^{ - 1}\) (\(A_{2} ,\;\lambda_{2}\)) is about \(81\%\) of the bulk value, and the biggest value \(D_{\text{y}} = 96.6 \times 10^{ - 10} \;{\text{m}}^{2} {\text{s}}^{ - 1}\)(\(A_{1} ,\;\lambda_{1}\)) is about \(91.2\%\) of the bulk value (obtained from the MOPLS model using MD simulation is about \(106.1\; \times 10^{ - 10} \;{\text{m}}^{2} {\text{s}}^{ - 1}\) (Jiang et al. 2016b)). The diffusion coefficient \(D\) is bigger than \(80\%\) of the bulk value as \(\lambda_{i} \ne \lambda_{2}\). These results are similar to those of liquid argon through the nano-channels (Bitsanis et al. 1987; Karniadakis et al. 2006; Sofos et al. 2009b). It indicates that the mass transfer behavior of methane fluid through the nano-channel is significantly affected by the wall surface roughness. For the same value of amplitude \(A_{i}\)(\(i = 1,\;2,\;3\)), all diffusion coefficients arrive at the smallest value at \(\lambda_{2} = 2.15\;\sigma\). And the variation tendency of all diffusion coefficients (\(D_{\text{x}}\), \(D_{\text{y}}\), \(D_{\text{z}}\) and \(D\)) become steady with the change of wavelength from \(\lambda_{2} = 2.15\;\sigma\) to \(\lambda_{5} = 12.84\;\sigma\) for different amplitudes. It is noted that the increasing trend of \(D_{\text{z}}\) is obviously different with the increase in amplitude. It means that the trajectories of molecules suffer from the effects of nano-channel wall roughness, due to the fact that the complex interactions of wall–fluid influence the movement of molecule in the rough nano-channels. The anisotropy of the diffusion coefficients inside rough nano-channel wall is similar to the results of liquid argon in krypton nano-channels (Sofos et al. 2009a). We attribute the difference to the effect of wall roughness on the trajectories of molecule inside rough cavity. For \(\lambda_{1} = 1.07\;\sigma\), the variation trend of diffusion coefficients does not significantly change with the amplitude. Since the cavity is too small to contain one methane molecule, the repulsive force slightly decreases when the methane molecule is adjacent to the upper nano-channel walls. Thus, the impact of rough wall on methane fluid is not significant, while it goes through the rough upper wall, i.e., the diffusion coefficients do not obviously change. For \(\lambda_{2} = 2.15\;\sigma\), we observe that the diffusion coefficients \(D_{\text{x}}\), \(D_{\text{y}}\) and \(D\) are not significantly different for amplitudes \(A_{2} = 1.07\;\sigma\) and \(A_{3} = 1.62\;\sigma\). Since every cavity only contains one methane molecule, the inner molecules are confined into cavities, and it has only minor effect on the other fluid molecules. Moreover, these results also demonstrate that the diffusion coefficients \(D\) increase, while wavelength increases (except for \(\lambda_{1}\)) at given amplitude. One possible reason is that the mobility of molecule is reduced inside the rough cavities, which leads to the decrease in diffusion coefficient in these regions. And the simulation results further show the non-equilibrium MSMD could be used to study the effects of rough walls on the dynamical diffusion properties of methane fluid through the nano-channels.
The intermolecular interactions assume significance in the regions of rough nano-channel walls where the discrepancy appears, and the diffusion coefficients no longer suffice to account for the effect of fluid–fluid intermolecular collisions in these cavities due to the differences in wavelength and amplitude. Next, we will show the variation of RDFs in nano-fluidics.
3.5 Radial distribution function
The structural properties are very important in most nano-fluidic systems. The RDF is a basic measure of the structure of a liquid. It measures the probability density of finding a particle at a distance \(r\) from a given particle position. The RDF is defined by
where \(N\) and \(\rho = {N \mathord{\left/ {\vphantom {N V}} \right. \kern-0pt} V}\) denote the total number of particles and the number density, respectively. The angular brackets represent a time average. The value of the \(\delta\) symbol is equal to one, while \({\mathbf{r}} = {\mathbf{r}}_{ij}\), otherwise, is zero.
Figure 14 shows quantitative comparisons of RDFs (\(g{}_{\text{CC}}\left( r \right)\), \(g{}_{\text{CH}}\left( r \right)\) and \(g{}_{\text{HH}}\left( r \right)\)) between the bulk systems and rough nano-channels. It can be seen that the values of RDFs (\(g{}_{\text{CC}}\left( r \right)\), \(g{}_{\text{CH}}\left( r \right)\) and \(g{}_{\text{HH}}\left( r \right)\)) obtained from the rough nano-channel walls are smaller than those of bulk system, since the methane molecules are confined inside the nano-channels in z-direction with the repulsive force of wall atoms. The results of \(g{}_{\text{CC}}\left( r \right)\) are similar to the equilibrium MD simulation in the pore fluids using LJ potential (Bhatia and Nicholson 2011). For \(\lambda_{1} = 1.07\;\sigma\), the RDFs (\(g{}_{\text{CC}}\left( r \right)\), \(g{}_{\text{CH}}\left( r \right)\) and \(g{}_{\text{HH}}\left( r \right)\)) of methane fluid do not significantly change with amplitude. For \(\lambda_{i}\) (\(i = 2,\;3,\;4,\;5\)), we observe that the values of RDFs (\(g{}_{\text{CC}}\left( r \right)\), \(g{}_{\text{CH}}\left( r \right)\) and \(g{}_{\text{HH}}\left( r \right)\)) of methane fluid decrease with the increase in amplitude. And the variation trend decreases with the increase in wavelength, as the averaged constraint of molecules confined inside the cavity decreases as the wavelength increases. For the same amplitude \(A_{2}\), the values RDFs \(g{}_{\text{CC}}\left( r \right)\), \(g{}_{\text{CH}}\left( r \right)\) and \(g{}_{\text{HH}}\left( r \right)\) of methane fluid decrease as the value of wavelength increases. However, the reducing trend is not obvious for large value of wavelength. One possible reason is that the molecules confined inside the cavity are easy to escape the constraint of cavity for large wavelength. In a word, the numerical results indicate that the microstructure of methane fluid is significantly affected by the roughness of walls. The values of RDFs of methane fluid decrease, while the amplitude increases. The effects of wavelength on the RDFs are not obvious when the wavelength is larger than \(2.15\;\sigma\). Furthermore, the numerical results further demonstrate that the non-equilibrium MSMD method can be indeed used to capture the changing microstructural properties of methane fluid flow in the rough nano-channels with different wavelengths and amplitudes.
Comparison of the radial distribution functions (CC, CH and HH) of methane fluid in the roughness nano-channels with the result as obtained from different amplitudes (\(A_{1} ,\;A_{2} ,\;A_{3}\)) and different wave lengths (\(\lambda_{1} ,\;\lambda_{2} ,\;\lambda_{3} ,\;\lambda_{4} ,\;\lambda_{5}\))
4 Conclusions
We have presented non-equilibrium MSMD simulations of methane Poiseuille flow within rough silicon nano-channel walls. And systematic research results show that the presented method not only can well solve the complex problem of wall–fluid, but also could accurately predict the micro-information and dynamic properties of Poiseuille flow for complex fluid. The main conclusions and important findings are:
-
1.
The non-equilibrium MSMD is an effective method to study the atomic behavior of complex methane fluid in the rough nano-channels. The roughness of silicon wall has an obvious impact on the atom distribution of methane molecule and temperature profiles. The anisotropy of methane molecule increases with the values of amplitudes near nano-channel walls.
-
2.
Three-dimensional number density of C (H) and kinetic energy distribution plots reveals inhomogeneity near the solid boundary, especially near the upper rough nano-channels. The number density of C (H) profiles and the snapshots of the trapped fluid atom trajectory denote that the fluid molecules tend to be localized inside rough wall cavities. This localization increases, when the cavities become narrower (the width is not less than diameter of fluid molecule) and deeper.
-
3.
The numerical results show that the effect of wall roughness on the localization properties of methane fluid is significant. The cavitation of the same dimensions induces different local density, velocity and kinetic energy patterns, while the overall average might be the same. Moreover, the slip length \(l_{\text{s}}\)(R) is less than \(l_{\text{s}}\)(S), and \(l_{\text{s}}\)(R) decreases as amplitude increases.
-
4.
Diffusivity has an anisotropic behavior along the three different coordinates, due to the effect of rough nano-channel walls on the mobility of molecule at different directions. And the diffusion coefficients \(D\) increase, while wavelength increases (except for \(\lambda_{1}\)) at given amplitude.
-
5.
The values of RDFs of methane fluid decrease as the amplitude increases for the same wavelength. The simulation results indicate that the MSMD is a powerful and effective tool to investigate the effects of wall roughness on the microstructural properties of methane fluid flow in the nano-channels.
-
6.
All study results recommend that the surface roughness is important and should be considered in investigating the nanostructures and profiles of complex methane fluid flow in the rough nano-channels. Therefore, it should be taken into consideration in the design of energy-saving emission reduction nano-fluidic devices, since it affects both flow and mass transfer.
In the future, we will use the non-equilibrium MSMD method to study the effects of rough nano-channels wall with realistic material on local transport properties of complex atomic fluids (such as water, methane, polymer, binary mixture system).
References
Allen MP, Tildesley DJ (1989) Computer simulation of liquids. Oxford University Press, Oxford
Asproulis N, Drikakis D (2011) Wall-mass effects on hydrodynamic boundary slip. Phys Rev E 84:031504
Asproulis N, Kalweit M, Drikakis D (2012) A hybrid molecular continuum method using point wise coupling. Adv Eng Softw 46:85–92
Bernardo P, Drioli E, Golemme G (2009) Membrane gas separation: a review/state of the art. Ind Eng Chem Res 48:4638–4663
Bhadauria R, Aluru N (2013) A quasi-continuum hydrodynamic model for slit shaped nanochannel flow. J Chem Phys 139:074109
Bhatia SK, Nicholson D (2011) Modeling self-diffusion of simple fluids in nanopores. J Phys Chem B 115:11700–11711
Bhushan B (2000) Mechanics and reliability of flexible magnetic media. Springer, Berlin
Bhushan B, Israelachvili JN, Landman U (1995) Nanotribology: friction, wear and lubrication at the atomic scale. Nature 374:607–616
Bitsanis I, Magda J, Tirrell M, Davis H (1987) Molecular dynamics of flow in micropores. J Chem Phys 87:1733–1750
Cao B-Y, Chen M, Guo Z-Y (2006a) Effect of surface roughness on gas flow in microchannels by molecular dynamics simulation. Int J Eng Sci 44:927–937
Cao B-Y, Chen M, Guo Z-Y (2006b) Liquid flow in surface-nanostructured channels studied by molecular dynamics simulation. Phys Rev E 74:066311
Cao B-Y, Sun J, Chen M, Guo Z-Y (2009) Molecular momentum transport at fluid-solid interfaces in MEMS/NEMS: a review. Int J Mol Sci 10:4638–4706
Choi WY, Osabe T, Liu T-JK (2008) Nano-electro-mechanical nonvolatile memory (NEMory) cell design and scaling. IEEE Trans Electron Dev 55:3482–3488
Corry B (2008) Designing carbon nanotube membranes for efficient water desalination. J Phys Chem B 112:1427–1434
Delhommelle J, Evans DJ (2001a) Configurational temperature profile in confined fluids. I. Atomic fluid. J Chem Phys 114:6229–6235
Delhommelle J, Evans DJ (2001b) Configurational temperature profile in confined fluids. II. Molecular fluids. J Chem Phys 114:6236–6241
DelRio FW, de Boer MP, Knapp JA, Reedy ED, Clews PJ, Dunn ML (2005) The role of van der Waals forces in adhesion of micromachined surfaces. Nat Mater 4:629–634
Diestler D, Schoen M, Hertzner AW, Cushman JH (1991) Fluids in micropores. III. Self-diffusion in a slit-pore with rough hard walls. J. Chem. Phys. 95:5432–5436
Frenkel D, Smit B (2001) Understanding molecular simulation: from algorithms to applications. Academic press, Cambridge
Galea T-M, Attard P (2004) Molecular dynamics study of the effect of atomic roughness on the slip length at the fluid-solid boundary during shear flow. Langmuir 20:3477–3482
Gargiuli J, Shapiro E, Gulhane H, Nair G, Drikakis D, Vadgama P (2006) Microfluidic systems for in situ formation of nylon 6, 6 membranes. J Membr Sci 282:257–265
Giannakopoulos AE, Sofos F, Karakasidis TE, Liakopoulos A (2014) A quasi-continuum multi-scale theory for self-diffusion and fluid ordering in nanochannel flows. Microfluid Nanofluid 17:1011–1023
Granick S (1991) Motions and relaxations of confined liquids. Science 253:1374–1379
Hansen J-P, McDonald IR (1990) Theory of simple liquids. Elsevier, New York
Hartkamp R, Ghosh A, Weinhart T, Luding S (2012) A study of the anisotropy of stress in a fluid confined in a nanochannel. J Chem Phys 137:044711
Hu C, Bai M, Lv J, Kou Z, Li X (2015) Molecular dynamics simulation on the tribology properties of two hard nanoparticles (diamond and silicon dioxide) confined by two iron blocks. Tribol Int 90:297–305
Jabbarzadeh A, Atkinson J, Tanner R (2000) Effect of the wall roughness on slip and rheological properties of hexadecane in molecular dynamics simulation of Couette shear flow between two sinusoidal walls. Phys Rev E 61:690
Jiang C, Ouyang J, Liu Q, Li W, Zhuang X (2016a) Studying the viscosity of methane fluid for different resolution levels models using Poiseuille flow in a nano-channel. Microfluid Nanofluid 20:157
Jiang C, Ouyang J, Zhuang X, Wang L, Li W (2016b) An efficient fully atomistic potential model for dense fluid methane. J Mol Struct 1117:192–200
Jorgensen WL, Tirado-Rives J (1988) The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J Am Chem Soc 110:1657–1666
Kamal C, Chakrabarti A, Banerjee A, Deb S (2013) Silicene beyond mono-layers—different stacking configurations and their properties. J Phys Condens Mat 25:085508
Karniadakis GE, Beskok A, Aluru N (2006) Microflows and nanoflows: fundamentals and simulation. Springer, Berlin
Kasiteropoulou D, Karakasidis T, Liakopoulos A (2012) A dissipative particle dynamics study of flow in periodically grooved nanochannels. Int J Numer Meth Fluids 68:1156–1172
Kim D, Darve E (2006) Molecular dynamics simulation of electro-osmotic flows in rough wall nanochannels. Phys Rev E 73:051203
Kim H, Strachan A (2015) Effect of surface roughness and size of beam on squeeze-film damping-Molecular dynamics simulation study. J Appl Phys 118:204304
Kong CL (1973) Combining rules for intermolecular potential parameters. II. Rules for the Lennard-Jones (12-6) potential and the Morse potential. J Chem Phys 59:2464–2467
Kumar G, Smith S, Jaiswal R, Beaudoin S (2008) Scaling of van der Waals and electrostatic adhesion interactions from the micro-to the nano-scale. J Adhes Sci Technol 22:407–428
Kumar V, Sridhar S, Errington JR (2011) Monte Carlo simulation strategies for computing the wetting properties of fluids at geometrically rough surfaces. J Chem Phys 135:184702
Liakopoulos A, Sofos F, Karakasidis TE (2016) Friction factor in nanochannel flows. Microfluid Nanofluid 20:24
Liem SY, Brown D, Clarke JH (1992) Investigation of the homogeneous-shear nonequilibrium-molecular-dynamics method. Phys Rev A 45:3706
Malijevský A (2014) Does surface roughness amplify wetting? J Chem Phys 141:184703
Mantzalis D, Asproulis N, Drikakis D (2011) Filtering carbon dioxide through carbon nanotubes. Chem Phys Lett 506:81–85
Markesteijn A, Hartkamp R, Luding S, Westerweel J (2012) A comparison of the value of viscosity for several water models using Poiseuille flow in a nano-channel. J Chem Phys 136:134104
Mashayak S, Aluru N (2012a) Coarse-grained potential model for structural prediction of confined water. J Chem Theory Comput 8:1828–1840
Mashayak S, Aluru N (2012b) Thermodynamic state-dependent structure-based coarse-graining of confined water. J Chem Phys 137:214707
Menezes PL, Ingole SP, Nosonovsky M, Kailas SV, Lovell MR (2013) Tribology for scientists and engineers. Springer, Berlin
Mo G, Rosenberger F (1990) Molecular-dynamics simulation of flow in a two-dimensional channel with atomically rough walls. Phys Rev A 42:4688
Noid W (2013) Perspective: coarse-grained models for biomolecular systems. J Chem Phys 139:090901
Noorian H, Toghraie D, Azimian A (2014) The effects of surface roughness geometry of flow undergoing Poiseuille flow by molecular dynamics simulation. Heat Mass Transfer 50:95–104
Poling BE, Prausnitz JM, John Paul OC, Reid RC (2001) The properties of gases and liquids. McGraw-Hill, New York
Priezjev NV (2007) Effect of surface roughness on rate-dependent slip in simple fluids. J Chem Phys 127:144708
Priezjev NV, Darhuber AA, Troian SM (2005) Slip behavior in liquid films on surfaces of patterned wettability: comparison between continuum and molecular dynamics simulations. Phys Rev E 71:041608
Ranjith SK, Patnaik B, Vedantam S (2013) No-slip boundary condition in finite-size dissipative particle dynamics. J Comput Phys 232:174–188
Rapaport DC (2004) The art of molecular dynamics simulation. Cambridge University Press, Cambridge
Sadus RJ (2002) Molecular simulation of fluids. Elsevier, Netherlands
Sbragaglia M, Benzi R, Biferale L, Succi S, Toschi F (2006) Surface roughness-hydrophobicity coupling in microchannel and nanochannel flows. Phys Rev Lett 97:204503
Schiermeier Q (2006) Methane finding baffles scientists. Nature 439:128
Shell MS (2008) The relative entropy is fundamental to multiscale and inverse thermodynamic problems. J Chem Phys 129:108
Sofos F, Karakasidis T, Liakopoulos A (2009a) Transport properties of liquid argon in krypton nanochannels: anisotropy and non-homogeneity introduced by the solid walls. Int J Heat Mass Transf 52:735–743
Sofos F, Karakasidis T, Liakopoulos A (2009b) Variation of transport properties along nanochannels: a study by non-equilibrium molecular dynamics. In: IUTAM symposium on advances in micro-and nanofluidics. Springer, pp 67–78
Sofos FD, Karakasidis TE, Liakopoulos A (2009c) Effects of wall roughness on flow in nanochannels. Phys Rev E 79:026305
Sofos F, Karakasidis TE, Liakopoulos A (2010) Effect of wall roughness on shear viscosity and diffusion in nanochannels. Int J Heat Mass Transf 53:3839–3846
Sofos F, Karakasidis TE, Liakopoulos A (2012) Surface wettability effects on flow in rough wall nanochannels. Microfluid Nanofluid 12:25–31
Sofos F, Karakasidis TE, Giannakopoulos AE, Liakopoulos A (2016) Molecular dynamics simulation on flows in nano-ribbed and nano-grooved channels. Heat Mass Transf 52:153–162
Sparreboom W, Van Den Berg A, Eijkel J (2010) Transport in nanofluidic systems: a review of theory and applications. New J Phys 12:015004
Svoboda M, Malijevský A, Lísal M (2015) Wetting properties of molecularly rough surfaces. J Chem Phys 143:104701
Wang J, Chen D, Pui D (2007) Modeling of filtration efficiency of nanoparticles in standard filter media. J Nanopart Res 9:109–115
Zhang Y (2016a) Effect of wall surface modification in the combined Couette and Poiseuille flows in a nano channel. Int J Heat Mass Transf 100:672–679
Zhang Y (2016b) Effect of wall surface roughness on mass transfer in a nano channel. Int J Heat Mass Transf 100:295–302
Ziarani A, Mohamad A (2006) A molecular dynamics study of perturbed Poiseuille flow in a nanochannel. Microfluid Nanofluid 2:12–20
Acknowledgements
We are very grateful to the anonymous referees who have provided us with valuable comments and suggestions for improving our study. This work is financially supported by the National Basic Research Program of China (973 Program) (Grant No. 2012CB025903), the Major Research Plan of the National Natural Science Foundation of China (Grant No. 91434201) and the National Natural Science Foundation of China (Grant Nos. 11402210, 11671321).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, C., Ouyang, J., Li, W. et al. The effects of wall roughness on the methane flow in nano-channels using non-equilibrium multiscale molecular dynamics simulation. Microfluid Nanofluid 21, 92 (2017). https://doi.org/10.1007/s10404-017-1927-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-017-1927-2