Abstract
Purpose
The usefulness of endoscopic ultrasound (EUS) in pediatric populations has been recently appreciated; however, published studies on mini-probe EUS in the diagnosis of congenital esophageal stenosis (CES) or congenital duodenal stenosis (CDS) in pre-school patients remain scarce. This study aimed to report the utility of mini-probe EUS for the diagnosis of CES or CDS in pre-school patients based on the etiology.
Methods
We retrospectively reviewed the medical records of pediatric patients with CES or CDS who underwent mini-probe EUS through the stenotic segments at our hospital between December 2006 and December 2021.
Results
Five patients with CES and one with CDS were enrolled. The median age and body weight when EUS was performed were 12.5 months and 8.5 kg, respectively. Hypoechoic lesions were observed on EUS in three patients, which were assessed as cartilage; one patient had no hypoechoic lesion but had a focal thickness of the muscular layer. They were diagnosed with tracheobronchial remnants based on EUS. The full circumferential wall thickness of the esophagus was visualized in one patient with fibromuscular hypertrophy. The histopathological findings confirmed the diagnoses. In the patient with CDS, EUS findings revealed pancreatic parenchyma encircling the stenotic part of the duodenum. The preoperative diagnosis was annular pancreas. The patient underwent duodenoduodenostomy, and intraoperative findings confirmed the diagnosis.
Conclusion
Mini-probe EUS can be recommended as a feasible and safe technique for infants and toddlers. It can effectively diagnose CES or CDS based on etiology and can inform treatment strategies for pre-school patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the introduction of endoscopic ultrasound (EUS) by DiMagno et al. in 1980 [1], it has been widely used in the diagnosis and evaluation of gastrointestinal diseases in the adult population. Attila et al. performed a review of 40 EUS studies conducted on 38 children with a mean age of 13.5 years and concluded that EUS was feasible and safe with a significant impact on the management of pediatric gastrointestinal and pancreaticobiliary diseases [2]. However, this modality is rarely used in the pediatric population, especially in infants and toddlers, owing to limitations on the use of an adult EUS probe in pre-school pediatric populations. Sharma et al. reported that this limitation could result from multiple factors, including the difficulty in passing an adult EUS probe across the oropharynx and maneuvering it around the duodenal sweep [3]. The advent of high-frequency mini-probes has made it possible to visualize individual layers of the gastrointestinal wall, and it is feasible to apply these probes in the pre-school pediatric population as the probe can be passed through a 2.8-mm channel of a standard endoscope [4].
Congenital esophageal stenosis (CES) is a rare malformation that usually presents in infancy or early childhood upon the introduction of semisolid foods. The causes of CES are divided into three entities: tracheobronchial remnants (TBR), fibromuscular hypertrophy (FMH), and membranous diaphragm (MD) [5]. The treatment for CES is determined based on the etiology, where resection of the stenotic portion of the esophagus is generally considered inevitable in TBR, and endoscopic treatment, such as dilatation, is effective in some cases of FMH or MD [6]. Analogously, congenital duodenal stenosis (CDS) can be diagnosed partly during early childhood, and it has been known to be caused by membranous stenosis or annular pancreas [7]. Furthermore, the treatment for CDS is determined based on the etiology, in which endoscopic treatment is an option for membranous stenosis [8], whereas surgical procedures, such as duodenoduodenostomy, are performed for annular pancreas [9].
The usefulness of EUS in pediatric populations has been recently appreciated; however, published studies on mini-probe EUS in the diagnosis of CES or CDS in pre-school patients remain scarce. We reported the utility of mini-probe EUS for the diagnosis of CES or CDS, based on the etiology, in infants and toddlers.
Materials and methods
Study setting and population
For this study, we retrospectively reviewed the medical records of pediatric patients with CES or CDS who had undergone EUS at our hospital between December 2006 and December 2021. Patients aged > 18 years, pediatric patients with CES or CDS who had not undergone EUS, and those with missing data were excluded. This study was approved by the Ethics Committee of the Tokyo Women’s Medical University Yachiyo Medical Center (approval number, 2021-0138) and was conducted in accordance with the Declaration of Helsinki of 1964 (as revised in 2013). The requirement for obtaining written informed consent was waived because of the retrospective nature of the study.
Equipment and procedure
All EUS procedures were performed by a single pediatric surgeon. An Olympus GIF-XQ260 endoscope (Olympus Medical Systems, Tokyo, Japan) was used for esophagogastroduodenoscopy (EGD), with an insertion diameter of 9.0 mm and an accessory channel of 2.8 mm. EUS was performed using a 20-MHz, 2.5-mm-diameter catheter US probe (UM-3R, Olympus) through the working channel of the endoscope. All procedures were performed under general anesthesia in the operating room. The patients were intubated with heads in a tilted position to reduce the risk of aspiration. All patients initially underwent EGD, and the stenotic portion of the esophagus or duodenum was identified. The EUS technique was performed as follows. After washing the stenotic lesion, the operator filled the lesion with deaerated water through an endoscope channel. Adequate deaerated water was instilled to improve the visualization of the lesion. After the lesion was submerged, the mini-probe was inserted through the working channel and negotiated across the stenotic portion under endoscopic visualization. The EUS examination was performed by gradually withdrawing the probe and passing through the stenotic portion. Initially, the layers of the normal gastrointestinal wall were identified, and the stratification, thickness (focal or extensive), as well as the hypoechoic lesions, supposedly cartilage, of the structure, were noted in terms of layer involvement. Furthermore, the identification of the normal layers facilitated the recognition of the adjacent structures, such as lymph nodes, blood vessels, and adjacent organs.
EUS findings
In children, the normal esophageal wall is observed as a seven-layered structure on EUS [10]. The first hyperechoic, second hypoechoic, and third hyperechoic layers correspond to the superficial mucosa, muscularis mucosa, and submucosa, respectively. The muscularis propria consists of three layers (fourth to sixth layers), in which the fourth (hypoechoic), fifth (hyperechoic), and sixth (hypoechoic) layers correspond to the circular smooth muscle, intermuscular connective tissue, and longitudinal smooth muscle, respectively. The seventh hyperechoic layer corresponds to the adventitia. The cartilage visualized as a hypoechoic lesion in the esophageal wall and/or the focal thickness of the muscularis propria is seen in patients with TBR [10, 11]. In contrast, the circumferential thickness of the entire muscular layer of the esophageal wall, without cartilage, is observed in FMH [12]. The normal duodenal wall is seen as a five-layered structure with a gastric wall [13], in which the first and second layers correspond to the superficial mucosa and muscularis mucosa, respectively, and the third layer corresponds to the submucosa. The fourth layer corresponds to the muscularis propria, and the fifth layer corresponds to the adventitia. The pancreatic parenchyma in annular pancreas with CDS is observed as “salt and pepper” parenchyma encircling the duodenal wall on EUS [14].
Treatment
To avoid post-dilation complications, such as perforation and bleeding, surgical resection is considered the first-line therapy for the treatment of CES in patients with TBR at our institution. Conversely, endoscopic dilation is chosen as the initial therapy for patients with FMH or MD, considering that persistent symptoms after two series of dilation for these etiologies could be an indication for surgery. In cases of CDS, diamond-shaped duodenoduodenostomy is chosen as the initial therapy in patients with annular pancreas, whereas endoscopic treatment is considered an option for membranous stenosis.
Data collection
Information regarding the patients’ sex, indication for EUS, age and body weight when the procedure was performed, associated anomalies, stenosis location, esophagogram findings, EUS findings, EUS diagnoses, complications associated with EUS, type of treatment applied, histopathological findings (including the size and type of cartilage in the esophageal wall), and final etiologic diagnoses were documented.
Results
Six patients (three boys and three girls) were included in this study. The baseline characteristics of the patients are presented in Table 1. The indications for EUS were CES in five patients and CDS in one patient. The median age and body weight when EUS was performed were 12.5 (range 2–45) months and 8.5 (range 5.4–11) kg, respectively. Three patients with CES had associated anomalies, including esophageal atresia in two patients and trisomy 21 in one patient. The location of the stenotic segment was at the lower esophagus in all five patients with CES and at the second portion of the duodenum in the patient with CDS. Esophagograms in CES patients revealed tapered narrowing in three patients and abrupt narrowing in two patients. Figure 1 shows esophagograms revealing abrupt narrowing in Case 2 and tapered narrowing in Case 5 at the site of stenosis.
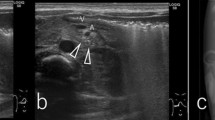
Figure 2 shows the normal part of the esophageal wall observed as a seven-layered structure on EUS in Case 4. The EUS findings of CES are also presented in Table 1. Three patients (Cases 1–3) had a hypoechoic lesion, which was assessed to be cartilage in the focal thickness of the muscularis propria (Fig. 3). One patient had no hypoechoic area but a focal thickness of the muscular layer (Case 4), and based on the EUS findings, the patient was diagnosed with TBR. Segmental resection of the stenotic region was performed, and histopathological findings showed hyaline cartilage and tracheal glands in all patients, confirming the diagnosis of TBR (Fig. 4). In Case 4, the cartilage was not observed on EUS but by microscopic examination; it was measured to be 1.4 mm, which was the smallest among the four cases (1.5 mm in Case 1, 4.9 mm in Case 2, and 1.9 mm in Case 3). In Case 5, EUS showed no hypoechoic lesions and a full circumferential thickness of the muscular layer, leading to a diagnosis of FMH (Fig. 5). The patient underwent endoscopic dilatation initially, but ended up undergoing resection because of ineffective endoscopic treatment. Histopathological findings confirmed the diagnosis of FMH (Fig. 6). Thus, the EUS-based diagnoses of CES based on the etiology were consistent with the pathological diagnoses in all cases.
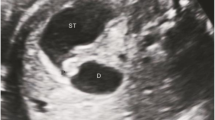
EUS findings with CDS are presented in Fig. 7a. The pancreatic parenchyma, visible as “salt and pepper” parenchyma, completely encircled the stenotic part of the duodenum, which could not be detected via transabdominal ultrasonography. The preoperative diagnosis was annular pancreas, and the patient underwent diamond-shaped duodenoduodenostomy. Intraoperative findings confirmed the annular pancreas between the proximal and distal segments of the duodenum (Fig. 7b). Additionally, the EUS diagnosis was consistent with the intraoperative diagnosis in the patient with CDS.
a EUS showed the “salt and pepper” appearance of the pancreatic parenchyma (arrow), completely encircling the stenotic portion of the duodenal wall (arrowhead) in Case 6. b Intraoperative findings in Case 6. A band of the pancreatic tissue completely encircled the stenotic part between the proximal and distal segments of the duodenum, confirming the diagnosis of annular pancreas. EUS endoscopic ultrasound
Discussion
The present study showed the use of mini-probe EUS in diagnosing CES or CDS based on the etiology in pre-school patients. Specifically, the EUS findings showed that three patients had hypoechoic lesions, which were assessed as cartilage. One patient had no hypoechoic lesions but a focal thickness of the muscular layer, leading to a preoperative diagnosis of TBR; in addition, EUS findings showed no hypoechoic lesion and an entire circumferential thickness of the muscular layer, leading to a diagnosis of FMH. In the patient with CDS, EUS revealed pancreatic parenchyma encircling the stenotic part of the duodenum, leading to a diagnosis of annular pancreas. To the best of our knowledge, this is the first pediatric case of annular pancreas diagnosed using EUS. This study highlights the use of mini-probe EUS in diagnosing CES or CDS based on etiology and reveals the treatment strategies for infants and toddlers.
The usefulness of EUS in pediatric populations has recently been appreciated; however, published studies on EUS in infants and toddlers remain limited. Sharma et al. reported that the lack of a dedicated pediatric EUS scope forced endoscopists to use adult EUS scopes in the pediatric population. However, the adult EUS scope is useful only in a subgroup of the pediatric population, and its application is limited owing to its larger diameter [3]. Moreover, Tagawa et al. reported that owing to the large diameter (outer diameter, 11–14 mm), the EUS probe can only be safely inserted in patients weighing > 15 kg [15]. Under the constraints on the applicability of endoscopes, EUS experiences in pediatric patients, especially in infants and young children aged < 4 years, are rarely reported. The present study demonstrates the feasibility and safety of using mini-probe EUS in pre-school patients. The mini-probe EUS used in this study had a 2.5-mm-diameter catheter probe with a 2.8-mm accessory channel of a standard endoscope (outer diameter, 9 mm). The American Society for Gastrointestinal Endoscopy recommends that EGD endoscopes for pediatric cases should be chosen based on the age and weight of the patient, as well as indications for the procedure [16]. In most patients aged > 12 months or weighing > 10–15 kg, standard endoscopes with an outer diameter of ≥ 8 mm may be used. In children weighing < 10–15 kg, small-caliber endoscopes are preferred, particularly in those weighing < 5 kg. For endoscopic intervention cases, a larger scope may be required [16]. In the present study, the median age and body weight of the patients where EUS was performed were 12.5 months and 8.5 kg, respectively, and the minimum age and body weight were 2 months and 5.4 kg, respectively. Both the standard endoscope and the mini-probe were used, with no complications being observed among the patients in this study.
CES is a rare malformation, with the reported incidence rate estimated to be one per 25,000–50,000 births [17]. It is categorized into three types, i.e., TBR, FMH, and MD, with reported frequencies of 30%, 54%, and 16%, respectively [17]. Therapeutic options include endoscopic dilation and surgery, based on the etiology. In cases of FMH and MD, the efficacy of endoscopic treatment has been reported, whereas TBR cases should be managed by surgery and not with dilation because of a high rate of perforation [10, 11]. To develop a therapeutic strategy, a correct diagnosis of the etiology is desirable before treatment. Contrast radiography had often been used to distinguish TBR from other etiologies. On esophagograms, TBR has been characterized by an abrupt narrowing, while FMH has shown tapered narrowing. Although these typical findings are noted, fluoroscopy does not always present typical findings [11]. In this study, two patients with TBR actually showed tapered narrowing on their esophagograms.
Meanwhile, EUS has been reported to be useful for classifying these etiologies, especially in distinguishing TBR from FMH [5, 10,11,12]. The cartilage in the esophageal wall is believed to be of the TBR type and can be visualized as a hypoechoic or hyperechoic area [10,11,12, 18]. In this study, the cartilage in the TBR was observed as a hypoechoic area on EUS. The trachea and bronchi are generally composed of hyaline cartilage, which is typically visualized as a hypoechoic area on ultrasound [19]. In the present study, the histopathological findings revealed hyaline cartilage in patients with TBR; hence, the cartilage was reasonably detected as a hypoechoic focus on EUS. In one patient with TBR (Case 4), no hypoechoic area but a focal thickness of the muscularis propria was observed on EUS. In this patient, histopathology revealed cartilage measuring 1.4 mm, which was believed to be much too small to be detected by EUS. Unlike the focal thickness of the muscular layer in TBR, the thickness of the entire circumference of the muscular layer in the esophageal wall, without cartilage, was seen in the patient with FMH (Case 5). By focusing on these findings, it is possible to distinguish the TBR type from FMH by EUS, even if no cartilage is found on EUS. Thus, EUS is useful for determining the appropriate treatment strategy.
Here, the EUS-based diagnoses of CES based on the etiology were consistent with the pathological diagnoses in all cases. However, Mochizuki et al. reported that because of its limited diagnostic reliability concerning the conflicting presentations of the cartilage visualized as a hypoechoic or hyperechoic area, EUS had not routinely been performed at their hospitals [5]. In cases where it is difficult to diagnose the histologic type of CES via esophagogram and/or EUS, they recommend endoscopic dilation as the initial therapy because low-profile, low-compliance expanding balloon dilators have become available and make the dilation procedure safer and more efficient. Persistent swallowing difficulties after dilation may be an indication for surgery [5].
Similar to CES, CDS is a rare malformation in comparison with duodenal atresia. The development of prenatal ultrasound has enabled the diagnosis of CDS during the neonatal period, although patients with CDS are only sometimes diagnosed during infancy, because incomplete duodenal obstructions have a more varied and often delayed presentation, making the diagnosis more challenging [20]. The causes of stenosis are annular pancreas and membranous stenosis [7]. According to several reports, endoscopic treatments, such as dilation or membrane incision, are options for the treatment of membranous stenosis [8, 21], although duodenal stenosis caused by annular pancreas is typically treated through surgical procedures, such as duodenoduodenostomy [22]. Gress et al. were the first to report cases of annular pancreas diagnosed using EUS in adults in 1996 [23], and we reported the first pediatric case in 2021 [14]. Papachristou et al. reported features of annular pancreas on EUS in five patients [24]. In that report, EUS detected a band of the pancreatic tissue that encircled the duodenum by 360° in three patients, 270° in one patient, and 180° in one patient. Within the tissue band, the pancreatic duct was identified in four of the five patients. In our study, EUS detected the pancreatic tissue visible as “salt and pepper” parenchyma encircling the stenotic part of the duodenum by 360°; however, the pancreatic duct was not detected within the tissue on EUS, possibly because the duct was too narrow to be detected. Features of membranous duodenal stenosis on EUS have not been reported in pediatric patients; however, preoperative diagnosis of annular pancreas based on EUS findings can help guide decisions in the surgical management of pediatric duodenal stenosis.
In this study, all procedures were performed under general anesthesia in the operating room. Although gastrointestinal endoscopy is generally considered safe, the procedure for pediatric patients during intravenous sedation and general anesthesia has a potential for adverse events, including inadequate sedation, agitation, low oxygen saturation, airway obstruction, and apnea. Thakkar et al. reported that the complication rate associated with endoscopic procedures was 1.2% under general anesthesia as compared to 3.7% under intravenous sedation, and they concluded that intravenous sedation was a risk factor for developing complications during the procedural period [25]. At our institution, well-trained anesthesiologists with a good understanding of the characteristics of pediatric physiology were in charge of providing general anesthesia for pediatric patients, especially pre-school patients. Therefore, there were no severe complications related to anesthesia and endoscopic procedures.
The current study had some limitations, which should be considered. First, this was a retrospective, single-center study consisting of case studies of six patients. Therefore, the possibility of an unintentional bias in patient selection cannot be fully excluded. Second, other etiologies, such as MD in CES or membranous stenosis in CDS, could not be evaluated using EUS because there were no cases with these etiologies. Therefore, future studies that include a prospective design from multiple medical centers in Japan, using a larger sample size and various etiologies, could aid in the validation of our findings.
Conclusion
Mini-probe EUS can be recommended as a feasible and safe technique for infants and toddlers. It can provide effective information to make the diagnosis of CES or CDS based on etiology and aid in decision-making regarding treatment in pre-school children. It is a promising technology that requires larger studies to further evaluate and strengthen our findings in future.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to contact with Tokyo Women’s Medical University but are available from the corresponding author on reasonable request.
References
DiMagno EP, Buxton JL, Regan PT, et al. Ultrasonic endoscope. Lancet. 1980;1:629–31.
Attila T, Adler DG, Hilden K, et al. EUS in pediatric patients. Gastrointest Endosc. 2009;70:892–8.
Sharma M, Wani ZA, Bansal R, et al. Utility of narrow caliber echo-bronchoscope in pre-school pediatric population: a case series (with video). Endosc Ultrasound. 2013;2:96–101.
Lakhole A, Liu QY. Role of endoscopic ultrasound in pediatric disease. Gastrointest Endosc Clin N Am. 2016;26:137–53.
Mochizuki K, Yokoi A, Urushihara N, et al. Characteristics and treatment of congenital esophageal stenosis: a retrospective collaborative study from three Japanese children’s hospitals. J Pediatr Surg. 2021;56:1771–5.
Ramesh JC, Ramanujam TM, Jayaram G. Congenital esophageal stenosis: report of three cases, literature review, and a proposed classification. Pediatr Surg Int. 2001;17:188–92.
Win MKK, Mensah C, Kaushik K, et al. Duodenal stenosis: a diagnostic challenge in a neonate with poor weight gain. Cureus. 2020;12: e8559.
Huang MH, Bian HQ, Liang C, et al. Gastroscopic treatment of membranous duodenal stenosis in infants and children: report of 6 cases. J Pediatr Surg. 2015;50:413–6.
McCollum MO, Jamieson DH, Webber EM. Annular pancreas and duodenal stenosis. J Pediatr Surg. 2002;37:1776–7.
Kouchi K, Yoshida H, Matsunaga T, et al. Endosonographic evaluation in two children with esophageal stenosis. J Pediatr Surg. 2002;37:934–6.
Takamizawa S, Tsugawa C, Mouri N, et al. Congenital esophageal stenosis: therapeutic strategy based on etiology. J Pediatr Surg. 2002;37:197–201.
Usui N, Kamata S, Kawahara H, et al. Usefulness of endoscopic ultrasonography in the diagnosis of congenital esophageal stenosis. J Pediatr Surg. 2002;37:1744–6.
Okanobu H, Hata J, Haruma K, et al. A classification system of echogenicity for gastrointestinal neoplasms. Digestion. 2005;72:8–12.
Yabe K, Kouchi K, Takenouchi A, et al. Diagnosis of annular pancreas with congenital duodenal stenosis by endoscopic ultrasound in a paediatric patient. J Pediatr Gastroenterol Nutr. 2021;73: e79.
Tagawa M, Morita A, Imagawa K, et al. Endoscopic retrograde cholangiopancreatography and endoscopic ultrasound in children. Dig Endosc. 2021;33:1045–58.
Barth BA, Banerjee S, ASGE Technology Committee, et al. Equipment for pediatric endoscopy. Gastrointest Endosc. 2012;76:8–17.
Terui K, Saito T, Mitsunaga T, et al. Endoscopic management for congenital esophageal stenosis: a systematic review. World J Gastrointest Endosc. 2015;7:183–91.
Bocus P, Realdon S, Eloubeidi MA, et al. High-frequency miniprobes and 3-dimensional EUS for preoperative evaluation of the etiology of congenital esophageal stenosis in children (with video). Gastrointest Endosc. 2011;74:204–7.
Singh M, Chin KJ, Chan VW, et al. Use of sonography for airway assessment: an observational study. J Ultrasound Med. 2010;29:79–85.
Kshirsagar AY, Sulhyan SR, Vasisth G, et al. Duodenal stenosis in a child. Afr J Paediatr Surg. 2011;8:92–4.
Goring J, Isoldi S, Sharma S, et al. Natural orifice endoluminal technique (NOEL) for the management of congenital duodenal membranes. J Pediatr Surg. 2020;55:282–5.
Wang D, Kang Q, Shi S, et al. Annular pancreas in China: 9 years’ experience from a single center. Pediatr Surg Int. 2018;34:823–7.
Gress F, Yiengpruksawan A, Sherman S, et al. Diagnosis of annular pancreas by endoscopic ultrasound. Gastrointest Endosc. 1996;44:485–9.
Papachristou GI, Topazian MD, Gleeson FC, et al. EUS features of annular pancreas (with video). Gastrointest Endosc. 2007;65:340–4.
Thakkar K, El-Serag HB, Mattek N, et al. Complications of pediatric EGD: a 4-year experience in PEDS-CORI. Gastrointest Endosc. 2007;65:213–21.
Acknowledgements
We would like to thank Editage (http://www.editage.jp) for English language editing.
Funding
No funds, grants, or other means of support were received in relation to the conduct of this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Material preparation, data collection, and analysis were performed by KY, AM, and CN. Histopathological assessment was performed by AH and TN. The manuscript was written by KY. Supervision was performed by AH and KK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Kiyoaki Yabe, Aki Matsuoka, Chikako Nakata, Atsuko Hasegawa, Tadao Nakazawa, Akira Horiuchi, and Katsunori Kouchi declare no conflicts of interest.
Ethical approval
All procedures in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki of 1964 (revised in 2013). This retrospective study was approved by the Ethics Committee of Tokyo Women’s Medical University. The requirement for obtaining written informed consent was waived because of the retrospective nature of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yabe, K., Matsuoka, A., Nakata, C. et al. Mini-probe endoscopic ultrasound for the diagnosis of congenital esophageal or duodenal stenosis. J Med Ultrasonics 50, 177–185 (2023). https://doi.org/10.1007/s10396-023-01281-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-023-01281-3