Abstract
Background
This study aimed to establish a new scoring system that combined several risk factors, including virtual touch quantification (VTQ) values and fasting plasma glucose (FPG) levels, for predicting the development of hepatocellular carcinoma (HCC) in patients with chronic liver disease.
Methods
A total of 1808 chronic liver disease patients who underwent VTQ measurement were analyzed. Risk factors for developing HCC were selected by multivariate Cox proportional hazards models.
Results
VTQ (>1.33 m/s), FPG (≥110 mg/dl), sex (male), age (≥55 years), and α-fetoprotein (AFP) level (≥5 ng/ml) were independently selected as risk factors for HCC development by multivariate analysis. Using these parameters, we established a new scoring system (0 to 5 points), based on VTQ, FPG, sex, age, and AFP level, named VFMAP. As compared with the low VFMAP score group (0 or 1 point), the hazard ratio for the incidence of HCC was 17.37 [95 % confidence interval (CI), 2.35–128.40] in the intermediate-score group (2 or 3 points) and 66.82 (95 % CI, 9.01–495.80) in the high-score group (4 or 5 points). The area under the receiver operating characteristic curve of the VFMAP score for predicting HCC development within 5 years was 0.82 (95 % CI, 0.76–0.87), indicating a moderate diagnostic value. A VFMAP cutoff value of 3 excluded HCC within 5 years with a high negative predictive value (98.2 %).
Conclusion
The VFMAP score accurately predicted HCC in patients with chronic liver disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common neoplasms in Japan, and its incidence has been increasing in Europe and the USA [1]. Chronic liver disease, including that due to persistent infection with hepatitis B (HBV) and hepatitis C (HCV), is a risk factor for the development of HCC [2]. In addition, several factors such as high age, male sex, high α-fetoprotein (AFP) levels, and heavy drinking have been reported to increase the risk of developing HCC in patients with chronic liver disease [3–5].
Advanced liver fibrosis is also a risk factor for the development of HCC [6]. Liver biopsy is still considered the gold standard for evaluating liver fibrosis even though it is painful, costly, and associated with limitations in diagnostic utility and accuracy. Moreover, since the invasiveness of liver biopsy precludes repeated examinations [7], longitudinal evaluation of liver fibrosis is difficult.
Various noninvasive imaging methods for evaluating liver fibrosis have recently emerged as alternatives to liver biopsy. Acoustic radiation force impulse (ARFI) [8–10] is a new ultrasound (US)-based technique for noninvasively evaluating liver stiffness. This method can easily and accurately assess the degree of liver fibrosis in clinical practice [11, 12]. ARFI elastography has two modes: the tissue displacement at longitudinal direction provides a qualitative response for virtual tissue imaging, which measures qualitatively by the area ratio, and a quantitative response for virtual touch quantification (VTQ), which measures transverse shear wave velocity values in meters/second (m/s).
Chronic liver disease, especially HCV infection, may be associated with the presence of diabetes. Almost 400 million people throughout the world have diabetes, 85–95 % of whom have type 2 disease [13]. Multiple studies and meta-analyses have claimed that diabetes is associated with an increased risk of cancer at several sites, including the stomach, liver, pancreas, endometrium, colorectum, breast, and bladder [14]. The observed associations between diabetes and cancer could be either causal (for example, resulting from hyperglycemia or hyperinsulinemia) or due to confounding from common risk factors such as adiposity. Type 2 diabetes was also reported to be associated with various liver-related complications such as ascites, encephalopathy, and bacterial infections in patients with chronic HCV infection [15].
In the present study, we clarified the risk factors, including VTQ value and fasting plasma glucose (FPG) level, for the development of HCC in a large number of patients with chronic liver disease. In addition, based on risk factors selected by statistical analysis, we established a new scoring system for predicting the development of HCC in this patient population.
Materials and methods
Patients
Between October 2008 and December 2013, liver stiffness was evaluated at our institution using VTQ in 3602 consecutive patients with chronic liver diseases. Of these 3602 patients, 2893 met the following eligibility criteria: (1) follow-up duration more than 1 year; (2) no previous history of HCC; (3) HCC surveillance performed during the follow-up period; (4) VTQ examination was performed more than once; (5) diagnosis of HCC more than 1 year after the start of the follow-up period. We then excluded 1085 patients because of either missing data or only one VTQ examination. Consequently, 1808 patients were analyzed in this study (Fig. 1).
Hepatitis B was diagnosed by positivity for hepatitis B surface antigen, and hepatitis C was diagnosed by positivity for HCV antibody. Non-B, non-C liver disease was diagnosed by laboratory data, US findings, or liver biopsy-based on disease-specific criteria and guidelines. Diagnosis of type 2 diabetes was made based on the American Diabetes Association criteria of an FPG level ≥126 mg/dl (≥7.0 mmol/l) or hemoglobin A1c (HbA1c) level ≥6.5 % [16], or a history of diabetes with diet therapy or pharmacotherapy.
Patient age, sex, height, and weight were recorded on the same day as VTQ examinations, and serum samples were collected in the fasting state.
The date of the first evaluation of liver stiffness using the VTQ was defined as the start of follow-up. The end of follow-up was defined as the date of the final visit in patients without HCC and as the date of HCC detection in patients in whom HCC developed during follow-up. In the present study, the clinical data for analyses were collected at the time of follow-up start.
The study protocol was in compliance with the Helsinki Declaration and was approved by our institutional review board. Prior to the start of the study, written informed consent was obtained from all patients for use of their laboratory data.
VTQ
VTQ measurement by ARFI was performed with a Siemens ACUSON S2000 (Mochida Siemens Medical Systems, Tokyo, Japan). In this procedure, patients lie in the supine position with one right upper extremity lifted. The area of the liver to be examined for elastic properties is targeted with a region-of-interest (ROI) cursor while B-mode imaging is performed. Tissue at the ROI is mechanically excited using acoustic push pulses to generate localized tissue displacements that cause propagation of shear waves away from the region of excitation. These shear waves are tracked using ultrasonic correlation-based methods. The maximal displacement is estimated for many US tracking beams laterally adjacent to the single push-beam. By measuring the time to peak displacement at each lateral location, the shear wave propagation velocity can be reconstructed. In this study the examination was performed on the right lobe of the liver with a measurement depth of 2–3 cm below the liver capsule. Six successful acquisitions at different locations were performed on each patient; the median value was calculated, and the results are expressed in m/s. The shear wave propagation velocity is considered to be proportional to the square root of tissue elasticity.
One sonologist from our institution (Y.M., 20 years of experience in sonography with 8 years of VTQ experience) performed the VTQ examinations. He was blinded to the patients’ clinical data.
HCC surveillance and diagnosis
In accordance with the Clinical Practice Guidelines for Hepatocellular Carcinoma in Japan [17], cirrhotic patients under surveillance underwent US (including VTQ examination) and monitoring of tumor markers every 3–4 months and dynamic computed tomography (CT) or magnetic resonance imaging (MRI) every 12 months. For patients with chronic hepatitis, we performed US (including VTQ examination) and monitoring of tumor markers every 6 months. The diagnosis of cirrhosis was made based on histological examination or typical US findings, e.g., superficial nodularity, a coarse parenchymal echo pattern, and signs of portal hypertension (splenomegaly >120 mm, dilated portal vein diameter >12 mm, patent collateral veins, or ascites) [18–20]. Patients who did not satisfy these criteria were classified as having chronic hepatitis. As recommended by the diagnostic algorithm of the Japan Society of Hepatology, HCC was diagnosed principally based on the results from US and dynamic CT (hyperattenuation during the arterial phase in all or part of the tumor and hypoattenuation in the portal venous phase) and/or MRI [17].
In the present study, there were no sufficient liver biopsy data for the analysis of liver fibrosis. Therefore, we used the aspartate aminotransferase (AST)/platelet ratio index (APRI) as an index for the evaluation of liver fibrosis. This index was calculated as (AST [IU/l]/upper limit of normal AST [IU/l]) × 100/platelet count [109/l], and its clinical utility in diagnosing liver fibrosis has been previously reported [21].
Statistical analysis
Continuous variables are expressed as medians (interquartile range). The Mann-Whitney U test was used for continuous variables. Curves for HCC development were constructed using the Kaplan-Meier method, and differences were tested using the log-rank test with Bonferroni correction. Multivariate Cox proportional hazards models with backward elimination were used for selecting the factors related to developing HCC.
In the multivariate Cox proportional hazards models, we used the covariates as follows, based on previous reports for factors associated with developing HCC or liver fibrosis: age, sex, etiology, albumin, AFP, FPG, APRI, and VTQ [2–6, 8–10, 14, 15, 21]. We defined the cutoff values of continuous variables based on the receiver-operating characteristic (ROC) curve analysis using maximum [sensitivity + specificity − 1] (Youden Index) [22, 23] and previously published reports.
Based on the results of the multivariate Cox proportional hazards models, we established a new scoring system for predicting the development of HCC within 5 years. Univariate Cox proportional hazards models were used to identify the hazard ratio of this system. The predictive performance of the scoring system was assessed using ROC curves and area under the ROC curve (AUC) analysis. The predictive value was classified as low (AUC = 0.50–0.70), moderate (AUC = 0.70–0.90), or high (AUC = 0.90–1.0) [24]. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated using the Youden Index as the cutoff level [22, 23] in the ROC curve analysis. In addition, we compared the discriminative ability of VTQ alone and the new scoring system using Akaike’s information criterion (AIC) [25]. The AIC is a log likelihood of model fitness by which the optimal model is associated with a lower AIC value.
Statistical significance was defined as p < 0.05. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [26]. More precisely, it is a modified version of the R commander designed to add statistical functions frequently used in biostatistics.
Results
Patient characteristics
Table 1 shows the characteristics of the patients in this study. They comprised 967 females and 841 males with a median (interquartile range) age of 59.0 (46.0–68.0) years. There were 264 patients positive for hepatitis B surface antigen (14.6 %), 635 positive for HCV antibody (35.1 %), 11 positive for both (0.6 %), and 898 negative for both (non-B, non-C) (49.7 %). In those with non-B, non-C liver disease, there were 96 patients with nonalcoholic fatty liver disease (5.3 %), 41 with autoimmune hepatitis (2.3 %), 22 with alcoholic hepatic disease (intake of ethanol ≥60 g/day for ≥5 years continuously) (1.2 %), and 15 with primary biliary cirrhosis (0.8 %). The remaining 724 patients (40.0 %) had other chronic liver diseases, including those classified as cryptogenic. There were 421 patients (23.3 %) with type 2 diabetes. The median FPGs were 95 (89–103) mg/dl in patients without diabetes and 133 (111–150) mg/dl in patients with diabetes, respectively (p < 0.001). In addition, the median HbA1cs were 5.3 (5.0–5.6) % in patients without diabetes and 6.4 (5.6–9.3) % in patients with diabetes, respectively (p < 0.001). The median follow-up period was 51.6 (38.0–66.0) months.
Development of HCC
HCC developed in 49 of 1808 patients (2.7 %) during the follow-up period. The overall 3- and 5-year rates of HCC development were 2.1 and 2.8 %, respectively. Supplementary Fig. 1 shows the cumulative incidence curve of HCC.
Decision for cutoff values
Based on the ROC curve analysis using the Youden Index for the predictive value of developing HCC within 5 years, the cutoff values of age, albumin, AFP, FPG, APRI, and VTQ were 55 years, 4.2 g/dl, 4.2 ng/ml, 107 mg/dl, 0.57, and 1.35 m/s, respectively. Supplementary Fig. 2a–f shows the respective ROC curves.
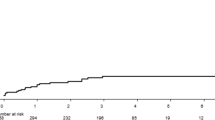
Cumulative incidence curves for HCC development by VFMAP score groups. Study patients were grouped based on their VFMAP scores as follows: 0 or 1 point, low-score group; 2 or 3 points, intermediate-score group; 4 or 5 points, high-score group. The respective 3- and 5-year cumulative incidence rates of HCC were 0.0 and 0.3 % in the low-score group, 3.0 and 3.5 % in the intermediate-score group, and 11.7 and 14.8 % in the high-score group
We defined the cutoff values of continuous variables for analysis as follows, based on the ROC curve analysis, previously published reports, or both: (including approximate values): high age was age ≥55 years; low albumin level was an albumin level <4.2 g/dl; high AFP level was defined as an AFP level ≥5 ng/ml; fasting hyperglycemia was defined as an FPG level ≥110 mg/dl; high APRI level was defined as an APRI level ≥0.5; high VTQ value was defined as a VTQ value >1.33 m/s, respectively [4, 21, 27, 28].
Factors associated with developing HCC
Multivariate analysis with Cox proportional hazards models using the covariates of age (years: <55, n = 726 vs. ≥55, n = 1082); sex (female, n = 967 vs. male, n = 841); etiology (HBV, HCV, or both, n = 910 vs. non-B, non-C, n = 898); albumin level (g/dl; <4.2, n = 186 vs. ≥4.2, n = 1535); AFP level (ng/ml; <5, n = 873 vs. ≥5, n = 440); FPG level (mg/dl; <110, n = 1302 vs. ≥110, n = 477); APRI level (<0.5, n = 780 vs. ≥0.5, n = 1003); and VTQ value (m/s; ≤1.33, n = 1181 vs. >1.33, n = 627) showed that age, sex, AFP, FPG, and VTQ were independently associated with the development of HCC (Table 2). Conversely, APRI was not significantly associated with the development of HCC.
Comparison of noninvasive liver fibrosis markers
We then compared APRI and VTQ in the study patients using multivariate analysis. Multivariate Cox proportional hazards models using the covariates of APRI level (<0.5, n = 780 vs. ≥0.5, n = 1003) and VTQ value (m/s; ≤1.33, n = 1181 vs. >1.33, n = 627) showed that VTQ was independently associated with the development of HCC (hazard ratio, 9.87; 95 % CI, 4.63–21.06, p < 0.001). APRI was also not significantly associated with the development of HCC.
Calculation of the score combining VTQ, FPG, sex, age, and AFP
Based on the results of multivariate analysis, we established a new scoring system that combined the VTQ value, FPG, sex, age, and AFP level. VTQ values of ≤1.33 and >1.33 m/s were scored as 0 and 1, respectively. FPG levels of <110 and ≥110 mg/dl were scored as 0 and 1, respectively. Female and male sex were scored as 0 and 1, respectively. Age of <55 and ≥55 years were scored as 0 and 1, respectively. AFP levels of <5 and ≥ 5 ng/ml were scored as 0 and 1, respectively. The total score was defined as the sum of the VTQ, FPG, sex, age, and AFP scores (Table 3). This new scoring system, based on VTQ, FPG, sex (male), age, and AFP, was named VFMAP.
We then compared the discriminative ability of VTQ alone and the new scoring system using AIC. The AICs of VTQ alone and the VFMAP score were 661.25 and 585.44, respectively.
Development of HCC stratified by VFMAP scores
Of the study patients with VFMAP scores, 138, 340, 373, 300, 141, and 31 had scores of 0,1, 2, 3, 4, and 5, respectively. We then grouped the study patients based on their VFMAP scores as follows: 0 or 1 point, low-score group (n = 478); 2 or 3 points, intermediate-score group (n = 673); 4 or 5 points, high-score group (n = 172). Figure 2 shows the cumulative incidence curves of HCC development for each VFMAP score group. The respective 3- and 5-year cumulative incidence rates of HCC were 0.0 and 0.3 % in the low-score group, 3.0 and 3.5 % in the intermediate-score group, and 11.7 and 14.8 % in the high-score group. After Bonferroni correction, the incidence of HCC differed significantly between the low- and intermediate-score groups (p < 0.001), the low- and high-score groups (p < 0.001), and the intermediate- and high-score groups (p < 0.001).
In univariate Cox proportional hazards models, the hazard ratio for the incidence of HCC as compared with the low-score group was 17.37 [95 % confidence interval (CI), 2.35–128.40, p = 0.005] in the intermediate-score group and 66.82 (95 % CI, 9.01–495.80, p < 0.001) in the high-score group (Table 4).
ROC curve analysis for the predictive value of developing HCC within 5 years
The ROC curve of VFMAP scores for the prediction of developing HCC within 5 years is shown in Fig. 3. The AUC value of VFMAP scores was 0.82 (95 % CI, 0.76–0.87), corresponding to moderate diagnostic value. The sensitivity, specificity, PPV, and NPV using the cutoff level (VFMAP score 3) according to the Youden Index [23] were 88.4, 63.0, 18.9, and 98.2 %, respectively.
Discussion
This study proposes a new scoring system, named VFMAP, for predicting the development of HCC in patients with chronic liver disease. In ROC analysis for the predictive ability of this system for the development of HCC within 5 years was over 0.8, which is considered to indicate moderate diagnostic value. Additionally, in an analysis of patient groups stratified by VFMAP scores, the high-score group (score 4 or 5) showed a high hazard ratio (66.82) compared with the low-score group (score 0 or 1). These results suggest that the VFMAP score accurately predicts the development of HCC in patients with chronic liver disease.
Friedrich-Rust et al. [11] performed a meta-analysis that included nine studies with a combined total of 518 patients with chronic liver disease and evaluated the diagnostic performance of ARFI imaging for the staging of liver fibrosis. The diagnostic accuracy of ARFI quantified by the AUC was 87 % for predicting significant fibrosis (F ≥ 2), 91 % for the diagnosis of severe fibrosis (F ≥ 3), and 93 % for the diagnosis of liver cirrhosis. The meta-analysis revealed good diagnostic accuracy for ARFI in the diagnosis of significant liver fibrosis and excellent diagnostic accuracy for the diagnosis of liver cirrhosis.
Advanced liver fibrosis is one of the risk factors for the development of HCC [6]. Several recent reports showed that liver stiffness measurements using US elastography were useful for predicting HCC [29–33]. However, these reports evaluated US elastography as a single predictor of HCC rather than in combination with one or more other risk factors. In patients with significant fibrosis (F ≥ 2), previously published VTQ cutoff values have ranged from 1.21–1.34 m/s (AUCs 0.85–0.89) in patients [8, 34, 35] without cirrhosis and 1.55–2 m/s (AUCs 0.89–0.93) in those with cirrhosis, [8, 12, 28, 34]. In the present study, we used a VTQ cutoff value of 1.33 m/s, which would be expected in significant liver fibrosis [28]. This cutoff value of VTQ was defined based on not only previously published reports [28], but the ROC analysis for the prediction of developing HCC within 5 years as well. Additionally, in the comparison of noninvasive liver fibrosis markers, VTQ was selected as an independent factor associated with developing HCC in the present study patients, but APRI was not statistically selected. Therefore, it was considered that VTQ is a superior noninvasive liver fibrosis marker to APRI as one of the indices of liver fibrosis.
Diabetes is associated with numerous disease types, including infectious, vascular, renal, neurological, skin, mental, and malignant [27, 36, 37]. Koh et al. [38] analyzed a large number of patients with long-term follow-up and found that a history of diabetes was associated with an approximately two-fold risk of developing HCC (hazard ratio, 2.64). In the present study, we defined fasting hyperglycemia as an FPG level ≥110 mg/dl in order to maximize the number of enrolled patients with mild hyperglycemia. In addition, this FPG cutoff value was defined based on the ROC analysis for the prediction of developing HCC within 5 years. Although 23.3 % of patients in the present study were afflicted with diabetes and there were not sufficient data regarding their treatment, the impaired glucose tolerance including diabetes was considered as a risk factor for HCC development. The level of HbA1c was not used because the lifespan of erythrocytes is shortened because of hypersplenism in patients with chronic hepatitis or cirrhosis, leading to lower HbA1c levels relative to the plasma glucose level [39].
In the present study, both high VTQ values and hyperglycemia were statistically selected as risk factors for development of HCC, together with high age, male sex, and high AFP levels. A number of studies [2–6] have already reported the association between HCC development and age, sex, and AFP level. Although each parameter might be a predictive marker of HCC alone, the new scoring system (VFMAP) established in the present study includes all of these parameters and may therefore more accurately predict the risk of developing HCC. In particular, when a cutoff value of 3 was applied, this score had a high NPV (98.2 %) for excluding HCC developing within 5 years in patients with chronic liver disease. There were extremely deviated proportions of HCC-developed and HCC-non-developed cases; therefore, it was considered that this score had a low PPV for predicting HCC developing within 5 years. VTQ measurements can evaluate liver stiffness noninvasively and repeatedly, unlike liver biopsy. In addition, the discriminative ability of the VFMAP score was superior to the VTQ alone based on the AIC analysis. Therefore, we consider that the VFMAP score, which incorporates a measure of liver fibrosis (i.e., VTQ), is a useful scoring system for predicting the development of HCC in patients with chronic liver disease.
The main limitation of this retrospective study is that the statistically analyzed parameters were limited to only age, sex, etiology, albumin, AFP, FPG, APRI, and VTQ. Because 49 patients in the present study developed HCC during the follow-up period, we used only these eight covariates in multivariate Cox proportional hazards models. Further prospective studies with a larger number of patients that analyze other potential risk factors are warranted. Another limitation of our study was that it did not consider anti-viral therapy such as nucleos(t)ide analog therapy or interferon-based therapy in patients with chronic HBV or HCV infection and cutoff vales of VTQ for each etiology. Many reports have found that anti-viral therapy suppresses hepatocarcinogenesis in these patient populations [40–42]. Further studies that account for anti-viral therapy in HBV or HCV patients and cutoff vales of VTQ for each etiology are warranted. In addition, approximately 50 % of patients in the present study had non-B, non-C (including cryptogenic) chronic liver disease. The etiology of chronic liver disease is mainly composed of HBV or HCV in Japan. Therefore, it was possible that this difference might have influenced the results of our study. Finally, in our new scoring system, aged men without liver dysfunction and impaired glucose tolerance (score 2) were classified in the intermediate-score group. Therefore, it was possible that these patients were estimated to have a higher risk of HCC development.
In conclusion, high VTQ values, fasting hyperglycemia, male sex, high age, and high AFP levels were risk factors of developing HCC in patients with chronic liver disease. In addition, a new scoring system based on these parameters, named VFMAP, had high predictive accuracy for the development of HCC. Further studies are warranted to confirm these findings in other populations.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- HBV:
-
Hepatitis B
- HCV:
-
Hepatitis C
- AFP:
-
α-Fetoprotein
- ARFI:
-
Acoustic radiation force impulse
- US:
-
Ultrasound
- VTQ:
-
Virtual Touch Quantification
- m/s:
-
Meters/second
- FPG:
-
Fasting plasma glucose
- HbA1c:
-
Hemoglobin A1c
- ROI:
-
Region of interest
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- AST:
-
Aspartate aminotransferase
- APRI:
-
Aspartate aminotransferase/platelet ratio index
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the receiver-operating characteristic curve
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- AIC:
-
Akaike’s information criterion
- CI:
-
Confidence interval
- ALT:
-
Alanine aminotransferase
- GGT:
-
γ-glutamyltransferase
References
El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37:S88–94.
Tsukuma H, Hiyama T, Tanaka S, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–801.
Chang KC, Wu YY, Hung CH, et al. Clinical-guide risk prediction of hepatocellular carcinoma development in chronic hepatitis C patients after interferon-based therapy. Br J Cancer. 2013;109:2481–8.
Oze T, Hiramatsu N, Yakushijin T, et al. Osaka Liver Forum. Post-treatment levels of α-fetoprotein predict incidence of hepatocellular carcinoma after interferon therapy. Clin Gastroenterol Hepatol. 2014;12:1186–95.
Yamada R, Hiramatsu N, Oze T, et al. Osaka Liver Forum. Impact of alpha-fetoprotein on hepatocellular carcinoma development during entecavir treatment of chronic hepatitis B virus infection. J Gastroenterol. 2015;50:785–94.
Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–75.
Castéra L, Nègre I, Samii K, et al. Pain experienced during percutaneous liver biopsy. Hepatology. 1999;30:1529–30.
Friedrich-Rust M, Wunder K, Kriener S, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595–604.
Lupsor M, Badea R, Stefanescu H, et al. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303–10.
Takahashi H, Ono N, Eguchi Y, et al. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30:538–45.
Friedrich-Rust M, Nierhoff J, Lupsor M, et al. Performance of acoustic radiation force impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212–9.
Kircheis G, Sagir A, Vogt C, et al. Evaluation of acoustic radiation force impulse imaging for determination of liver stiffness using transient elastography as a reference. World J Gastroenterol. 2012;18:1077–84.
International Diabetes Federation. Diabetes atlas. International Diabetes Federation, 2013.
Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–85.
Elkrief L, Chouinard P, Bendersky N, et al. Diabetes mellitus is an independent prognostic factor for major liver-related outcomes in patients with cirrhosis and chronic hepatitis C. Hepatology. 2014;60:823–31.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9.
The Japan Society of Hepatology. Surveillance algorithm and diagnostic algorithm for hepatocellular carcinoma: Clinical Practice Guidelines for Hepatocellular Carcinoma. Hepatology Res 2010; 40 Supplement s1: 6–7.
Shen L, Li JQ, Zeng MD, et al. Correlation between ultrasonographic and pathologic diagnosis of liver fibrosis due to chronic virus hepatitis. World J Gastroenterol. 2006;12:1292–5.
Iacobellis A, Fusilli S, Mangia A, et al. Ultrasonographic and biochemical parameters in the non-invasive evaluation of liver fibrosis in hepatitis C virus chronic hepatitis. Aliment Pharmacol Ther. 2005;22:769–74.
Caturelli E, Castellano L, Fusilli S, et al. Coarse nodular US pattern in hepatic cirrhosis: risk for hepatocellular carcinoma. Radiology. 2003;226:691–7.
Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26.
Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007;96:644–7.
Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5.
Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–93.
Yamaoka K, Nakagawa T, Uno T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–75.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(Suppl 1):S11–63.
Sporea I, Bota S, Peck-Radosavljevic M, et al. Acoustic radiation force impulse elastography for fibrosis evaluation in patients with chronic hepatitis C: an international multicenter study. Eur J Radiol. 2012;81:4112–8.
Akima T, Tamano M, Hiraishi H. Liver stiffness measured by transient elastography is a predictor of hepatocellular carcinoma development in viral hepatitis. Hepatol Res. 2011;41:965–70.
Jung KS, Kim SU, Ahn SH, et al. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan). Hepatology. 2011;53:885–94.
Wang HM, Hung CH, Lu SN, et al. Liver stiffness measurement as an alternative to fibrotic stage in risk assessment of hepatocellular carcinoma incidence for chronic hepatitis C patients. Liver Int. 2013;33:756–61.
Masuzaki R, Tateishi R, Yoshida H, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954–61.
Singh S, Fujii LL, Murad MH, et al. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1573–84.
Sporea I, Sirli R, Bota S, et al. Is ARFI elastography reliable for predicting fibrosis severity in chronic HCV hepatitis? World J Radiol. 2011;28(3):188–93.
Rizzo L, Calvaruso V, Cacopardo B, et al. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2011;106:2112–20.
Tsilidis KK, Kasimis JC, Lopez DS, et al. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607.
de Groot M, Anderson R, Freedland KE, et al. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–30.
Koh WP, Wang R, Jin A, et al. Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Br J Cancer. 2013;108:1182–8.
Aizawa N, Enomoto H, Imanishi H, et al. Elevation of the glycated albumin to glycated hemoglobin ratio during the progression of hepatitis C virus related liver fibrosis. World J Hepatol. 2012;4:11–7.
Hosaka T, Suzuki F, Kobayashi M, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107.
Kumada T, Toyoda H, Tada T, et al. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: a propensity score analysis. J Hepatol. 2013;58:427–33.
Kasahara A, Hayashi N, Mochizuki K, et al. Risk factors for hepatocellular carcinoma and its incidence after interferon treatment in patients with chronic hepatitis C. Osaka Liver Disease Study Group. Hepatology. 1998;27:1394–402.
Acknowledgments
We thank Masahiro Yoshida at Ultrasound Imaging Center, Hyogo College of Medicine, for his valuable help with obtaining ultrasound examinations. This work was supported by a Grant-in-Aid for Researchers, Hyogo College of Medicine, 2014, and a Grants-in-Aid for Scientific Research (C) 15K09029 (JSPS KAKENHI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Financial support
This work was supported by a Grant-in-Aid for Researchers, Hyogo College of Medicine, 2014, and a Grants-in-Aid for Scientific Research (C) 15K09029 (JSPS KAKENHI).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aoki, T., Iijima, H., Tada, T. et al. Prediction of development of hepatocellular carcinoma using a new scoring system involving virtual touch quantification in patients with chronic liver diseases. J Gastroenterol 52, 104–112 (2017). https://doi.org/10.1007/s00535-016-1228-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-016-1228-7