Abstract
Purpose
This study sought to compare ultrasound-guided measurements of the abductor pollicis brevis (APB) using the water bath technique (WBT) and the direct contact method (DM) and investigate whether the DM can reproduce the measurements that would be obtained with a non-contact method, such as the WBT.
Methods
The APB muscles of 80 hands (40 healthy adults) were measured. The WBT was performed in a plastic container filled with water. The probe was placed adjacent to the skin surface without contact. In the DM, sonographic images were obtained with the probe and skin separated by sufficient transmission gel. The muscle thickness and cross-sectional area (CSA) were calculated with both methods. All subjects were examined three times by two examiners to estimate the inter- and intra-observer reliability. Bland–Altman analysis was performed to examine the agreement between the methods.
Results
No significant differences in the thickness or CSA of the APB were found. The interclass correlation coefficients for the WBT and DM showed almost perfect intra- and inter-observer reliability (range 0.87–0.94). There was no systematic bias between the techniques in the Bland–Altman analysis.
Conclusion
Similar to the WBT, the DM provides measurements of the APB thickness and CSA without causing morphometric changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atrophy of the abductor pollicis brevis (APB) is frequently recognized in carpal tunnel syndrome (CTS) associated with median nerve neuropathy [1]. Because the APB plays a key role in thumb motion [2, 3], weakness of the APB can cause severe hand dysfunction. When determining the severity of CTS, it is important to evaluate muscle atrophy of the APB, but to date this has been mainly assessed by subjective visualization [4, 5].
As a result of advances in ultrasound imaging, several studies have shown the utility of sonographic evaluation of the APB [6,7,8]. However, muscles close to the surface of the skin, such as the APB, are convex and soft, and their morphometry tends to be altered when a probe is applied (direct contact method; DM). Previous reports have indicated that the curved surface of the hand results in suboptimal contact between the probe and the skin [9, 10]. Yet, the water bath technique (WBT) can provide images using a probe without touching the skin. Hence, some studies have indicated that the WBT better preserves the morphometry of the underlying musculature as compared with the DM [9, 10]. However, the DM is commonly used in the clinical setting, and it remains unclear whether the images and measurements of the underlying muscles accurately reflect the muscle, as would be determined using the WBT. Recent reports have indicated some of the technical tips associated with probe use in the sonographic examination of the APB thickness and cross-sectional area (CSA), ensuring that it is placed lightly on the skin [6] or placed onto the skin using sufficient transmission gel to avoid causing morphometric changes [7]. However, it remains unclear whether the DM can be used to measure the thickness and CSA of a muscle such as the APB with the same accuracy as those measurements taken with the WBT. Therefore, the purpose of this study was to investigate whether the DM can reproduce the measurements that would be obtained with a non-contact method, such as the WBT.
Materials and methods
Participants
Our Institutional Review Board approved this study, and all participants provided written informed consent before participating. Forty healthy adults (19 men and 21 women, mean age: 37.8 years, range: 23–56 years) participated in this study. Exclusion criteria were pregnancy, history of peripheral neuropathy, cervical radiculopathy, cerebral vascular disorder, hypothyroidism, diabetes mellitus, rheumatoid arthritis, and severe hand trauma.
Ultrasound evaluation
Ultrasound was conducted using B-mode US equipment (Aplio MX SSA-780A; Toshiba Medical Systems Corporation, Otawara, Japan) with a linear probe (6.2–12 MHz).
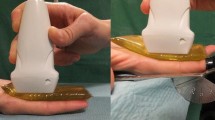
The WBT was carried out in a plastic container filled with water. The temperature of the water was measured and adjusted to approximately 32°C. The subject was placed in a relaxed sitting position with elbow in 90° flexion, forearm in 90° supination, the hand in neutral position, and the thumb held in maximum radial abduction. The thenar muscles were imaged inside the container. Before measuring, a line was marked between the radial sesamoid bone of the thumb and the scaphoid tuberosity. These bony landmarks could be easily identified by palpation as well as being confirmed by ultrasound. The line is between one of the origin and insertion of the APB [11]. An axial image was then acquired at the midpoint of the line (see Fig. 1). The probe was placed as perpendicular as possible to the flexor pollicis longus, according to the method of Kim et al. [12]. In ultrasound evaluation by WBT, the probe was covered with a transparent thin cover and placed adjacent to the skin surface without contact.
Ultrasound measuring method. a Water bath technique was carried out in a plastic container filled with water over the top of the thenar muscles. The Direct-contact method was carried out in the same container without water. b A line was marked between the radial sesamoid bone of the thumb and the scaphoid tuberosity (black dots) and a perpendicular bisector of the line. Axial imaging was acquired at the midpoint of the line
The DM was carried out with sufficient transmission gel, particularly at both ends of the probe, because of the convex surface of the APB. The gel temperature was approximately 32°C, and the probe was placed on the skin with minimal pressure (Fig. 2).
The thickness and CSA of the APB were calculated with the measurement function of the ultrasound device. In the transverse image, the APB thickness was calculated on the perpendicular line of the most volar point of the first metacarpal bone. The APB-CSA was measured simultaneously in the image (Fig. 3). All ultrasound measurements were conducted bilaterally.
Ultrasound image of the thickness and cross-sectional area (CSA) of abductor pollicis brevis (APB). The probe was adjusted so that the flexor pollicis longus (FPL) was seen in high brightness during axial imaging. APB thickness was calculated at the line of the most volar point of the first metacarpal bone (white arrow). CSA was calculated simultaneously (white outline)
Two examiners (KF and KO) performed ultrasonography examinations. Both examiners had a comprehensive understanding of hand anatomy (graduate student majoring in hand surgery and certified hand surgeon, respectively). Examinations were conducted three times per subject at a minimum interval of 1 week by both examiners. The inter- and intra-observer reliability were estimated.
To compare the distance between the probe and the skin surface, the shortest distance between the upper edge of the image and the skin surface was measured on the images for both methods.
Statistical analysis
Data were presented as the mean and standard deviation (SD) for continuous variables. SPSS version 22 (IBM Corporation, Armonk, NY) was used for all statistical analysis. The Pearson correlation coefficient (r) was evaluated to determine the correlation between WBT and DM measurements of APB thickness and CSA. Positive r values were classified as no correlation (0.00–0.19), weak correlation (0.20–0.39), moderate correlation (0.40–0.59), strong correlation (0.60–0.79), and very strong correlation (0.80–1.00) [13]. The interclass correlation coefficients (ICCs) were calculated to estimate the inter- and intra-observer reliability between the two methods in the measurements of APB thickness and CSA. ICC values were classified as poor (ICC ≦ 0.00), slight (0.01–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), and almost perfect (0.81–1.00) [14]. ICC values were considered to be significant when the upper and lower boundaries of the 95% confidence intervals did not overlap [15]. Bland–Altman plots were used to analyze the absolute agreement between the measurements of the WBT and the DM. The 95% limit of agreement was defined as the mean difference ± 1.96 × standard division [16].
Results
We found no significant difference in the mean ± SD of APB thickness and CSA between the WBT and the DM. There were very strong positive correlations between the two methods (r = 0.96–0.97, P < 0.01) (Table 1).
The inter- and intra-observer reliability of APB thickness and CSA by both methods was almost perfect reliability (ICC range: 0.87–0.94). There was no significant difference in the ICC values between the WBT and the DM (Tables 2, 3).
The Bland–Altman analysis showed that, relative to measurements taken using the DM, the WBT overestimated the thickness by an average of 0.05 mm, with 95% limits of agreement of − 0.33 to 0.43 mm. Likewise, the CSA was slightly larger with the WBT, with WBT overestimating CSA by an average of 0.01 cm2, with 95% limits of agreement of − 0.06 to 0.09 cm2 (Fig. 4). No systematic bias was found for either technique.
Bland–Altman plots of the agreement between the water bath technique (WBT) and direct contact method (DM) for the mean thickness (a) and cross-sectional area (CSA) (b) of abductor pollicis brevis. Plots show the difference between the WBT and DM (y-axis) relative to the mean of both measurements (x-axis). There was a total of 160 points in each analysis (80 hands × 2 observers). 1.96 × standard deviation (SD) of the difference between the WBT and DM was calculated as 95% limit of agreement
Finally, we measured the distance between the upper edge of the image and the skin surface using both methods. The distance with the DM was 2.03 ± 0.82 mm, whereas the distance with the WBT was 2.06 ± 0.85 mm, with no significant difference observed (P = 0.88).
Discussion
The purpose of this study was to investigate whether the DM can reproduce the measurements of APB thickness and CSA that would be obtained with the WBT. We performed a sonographic evaluation of the APB to compare the reliability and validity of the two methods in terms of measurements of the muscle. We found no significant difference in the measurements of APB thickness or CSA between the WBT and the DM, with a very strong positive correlation between the two techniques. The ICC was almost perfect for the inter- and intra-observer reliabilities. There was also no systematic bias, as analyzed using the Bland–Altman method.
Ultrasound studies of CTS often focus on the morphometry or stiffness of the median nerve [17, 18]. Only a few ultrasound studies have focused on changes in muscle thickness or the CSA in patients with CTS [6,7,8]. Approximately 95% of the APB is innervated by the median nerve [11], and, therefore, this muscle is the earliest muscle among the thenar muscles to be affected in patients with CTS [1]. Hence, a quantitative evaluation of the APB via ultrasound could provide meaningful information for patients with CTS. Some studies that have used ultrasound to evaluate APB thickness and/or CSA in healthy volunteers have shown almost perfect inter- and intra-observer reliability when using the DM [6, 7]. Here, we similarly showed almost perfect inter- and intra-observer reliability for measurements of the ABP with the DM.
Previous studies have emphasized the importance of paying attention to the probe as it contacts the skin, as the pressure causes morphometric changes to the underlying structures, including the muscle and its fascia. For example, Ishida et al. [19] reported that the pressure of the probe influenced the thickness of abdominal muscles evaluated by ultrasound. On the contrary, the probe in the WBT does not contact the skin, preserving the proper morphometry of the muscles. Indeed, Krishnamurthy et al. [10] reported that the WBT resulted in improved characterization of tissues in their native state, particularly when evaluating tissues in the extremities of children. Yet, the DM is most commonly used in the clinical setting to observe underlying structures. Given its widespread clinical use, it is important to clarify whether this technique provides the same structural information of the underlying structures or whether the contact made between the probe and the skin through the gel distorts the structures of interest. Indeed, specific to the APB, previous reports have recommended placing the probe lightly on the skin [6] or with sufficient transmission gel to avoid muscle compression [7]. Although these studies were concerned with the effects of probe contact on morphometry, they did not clarify whether the DM could reproduce the measurements that would be obtained with a non-contact method, such as the WBT. In our comparative analysis, we ensured complete separation of the probe and skin via transmission gel. As a result, we found that the DM could offer a comparable set of measurements as that obtained with the WBT if performed carefully. Our method is somewhat different to the so-called ‘direct method’ (i.e., applying pressure), but we believe that it can be regarded as a DM from the viewpoint of observing objects via transmission gel.
The reliability of the measurements depends on the examiner’s technique [7, 20]. König et al. [21] noted the reliability was more affected by probe positioning error than error arising from interpretation. Therefore, we defined a clear bony landmark for measurements. This positioning may have contributed to the almost-perfect reliability. It may also have contributed to the lack of difference in the measured distances between the probe and the skin surface between the two techniques.
This study has several limitations. First, the sample size could not be verified by power analysis. However, the number of samples was almost the same as that in previous reports (n = 62–77) [6, 7]. Second, the examiner carried out all evaluations for both methods (WBT and DM) sequentially. Therefore, we could not avoid recall bias. Third, the sonographic technique was performed by examiners who had a comprehensive understanding of hand anatomy. This limitation may have reduced the differences in the measurements between the two methods. Fourth, the enrolled subjects were all relatively young and healthy volunteers. Because the APB thickness or CSA of older patients or patients with CTS is smaller than that of our subjects [7, 8], this will likely affect the probe pressure and, possibly, affect the measurement error. Further study is needed to evaluate the morphometry of the APB by both methods in healthy older people and those with CTS who typically have smaller APB muscles. Fifth, we did not consider how the difference in the acoustic coupling medium would affect the measurements. The acoustic coupling medium facilitates the propagation of acoustic energy between the probe and the object. When ultrasonography is performed in water, there is no gap between the probe and the specimen, so that acoustic energy tends to propagate. Yet, Casarotto et al. previously reported that there was little difference in the acoustic characteristics between gel and water [22]. On the other hand, Balmaseda et al. reported that gel offered a smaller degree of acoustic attenuation and a better impedance match compared with water in their experimental set-up [23]. Due to the differences in experimental conditions, a consensus has not been reached. Furthermore, in phantom artery experiments using the WBT, Potter et al. measured the arterial lumen by separating the probe distance from 10 to 25 mm at an interval of 5 mm. As a result, they reported that a 5-mm increase in the distance between the probe and the artery reduced the measured lumen diameter by 0.04 mm [24]. In our study, the probe distance to the skin surface was less than 5 mm in WBT, with no statistically significant difference in the distance of the probe from the skin between the two methods. Hence, we regarded there to be little influence of the acoustic coupling medium in the evaluation of the two modes of ultrasound. Finally, we did not evaluate whether pressure changes, gravity, or the weight of the gel had an effect on the skin surface in DM or the effect of water pressure in WBT. However, our study showed that the measurements taken with the DM were not significantly different from those taken with the WBT, presumably suggesting little influence of these other outside factors.
The strength of this study was that the measurements of the APB obtained with the DM were comparable with those obtained with the WBT. We believe that it is important to pay particular attention to the position of the probe by maintaining sufficient gel between the probe and the object, and avoiding making contact between the probe and the skin. Even if sufficient transmission gel is used, there is a possibility that the probe will contact the skin, and this may cause deformation to the underlying connective tissues, fascia, and muscles. To avoid this, we ensured complete separation of the probe and the skin via transmission gel. In addition, the image is obtained while ensuring a space between the upper edge of the image and the skin surface. Although this method may not necessarily be the gold standard, we believe it is meaningful to show that the DM can reproduce the measurements that would be obtained with the WBT.
Conclusion
In conclusion, we showed that, similar to the WBT, the DM could be used to obtain measurements of APB thickness and CSA without causing morphometric changes through deformation to the shape of the muscle. This is important, as methods that can reliably identify muscles without affecting their morphology can be used to assess disease-related muscle changes, such as APB atrophy or weakness in patients with CTS. Further studies in CTS patients should be conducted to evaluate the severity or motor dysfunction that occurs due to APB atrophy in patients with CTS using the DM.
References
Phalen GS. The carpal-tunnel syndrome. J Bone Jt Surg Am. 1966;48:211–28.
Jan SVS, Rooze M. Anatomical variations of the intrinsic muscles of the thumb. Anat Rec. 1994;238:131–46.
Cooney WP, Linscheid RL, An KN. Opposition of the thumb: an anatomic and biomechanical study of tendon transfers. J Hand Surg Am. 1984;9:777–86.
Brown RA, Gelberman RH, Seiler JG, et al. Carpal tunnel release. A prospective, randomised assessment of open and endoscopic methods. J Bone Jt Surg Am. 1993;76:1265–75.
Nagaoka M, Nagao S, Matsuzaki H. Endoscopic release for carpal tunnel syndrome accompanied by thenar muscle atrophy. Arthroscopy. 2004;20:848–50.
Simon NG, Ralph JW, Lomen-Hoerth C, et al. Quantitative ultrasound of denervated hand muscles. Muscle Nerve. 2015;52:221–30.
Mohseny B, Nijhuis TH, Hundepool CA, et al. Ultrasonographic quantification of intrinsic hand muscle cross-sectional area; reliability and validity for predicting muscle strength. Arch Phys Med Rehabil. 2015;96:845–53.
Lee H, Jee S, Park SH, Ahn SC, et al. Quantitative muscle ultarasonography in carpal tunnel syndrome. Ann Rehabil Med. 2016;40:1048–56.
Javadzadeh HR, Davoudi A, Davoudi F, et al. Diagnostic value of "bedside ultrasonography" and the "water bath technique" in distal forearm, wrist, and hand bone fractures. Emerg Radiol. 2014;21:1–4.
Krishnamurthy R, Yoo JH, Thapa M, Callahan MJ. Water-bath method for sonographic evaluation of superficial structures of the extremities in children. Pediatr Radiol. 2013;43:S41–47.
Gupta S, Michelsen-Jost H. Anatomy and function of the thenar muscles. Hand Clin. 2012;28:1–7.
Kim JS, Seok HY, Kim BJ. The significance of muscle echo intensity on ultrasound for focal neuropathy: the median- to ulnar-innervated muscle echo intensity ratio in carpal tunnel syndrome. Clin Neurophysiol. 2016;127:880–5.
Zou KH, Tuncali K, Silverman SG. Correlation and simple lonear regression. Radiology. 2003;227:617–22.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Doornberg J, Lindenhovius A, Kloen P, et al. Two and three-dimensional computed tomography for the classification and management of distal humeral fractures. Evaluation of reliability and diagnostic accuracy. J Bone Jt Surg Am. 2006;88:1795–801.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Gruber L, Gruber H, Djurdjevic T, et al. Gender influence on clinical presentation and high-resolution ultrasound findings in primary carpal tunnel syndrome: do women only differ in incidence? J Med Ultrason. 2001;2016:413–20.
Arslan H, Yavuz A, İlgen F, et al. The efficiency of acoustic radiation force impulse (ARFI) elastography in the diagnosis and staging of carpal tunnel syndrome. J Med Ultrason. 2001;2018:453–9.
Ishida H, Watanabe S. Influence of inward pressure of the transducer on lateral abdominal muscle thickness during ultrasound imaging. J Orthop Sports Phys Ther. 2012;42:815–8.
Cartwright MS, Demar S, Griffin LP, et al. Validity and reliability of nerve and muscle ultrasound. Muscle Nerve. 2013;47:515–21.
König N, Cassel M, Intziegianni K, et al. Inter-rater reliability and measurement error of sonographic muscle architecture assessments. J Ultrasound Med. 2014;33:769–77.
Casarotto RA, Adamowski JC, Fallopa F, et al. Coupling agents in therapeutic ultrasound: acoustic and thermal behavior. Arch Phys Med Rehabil. 2004;85:162–5.
Balmaseda MT Jr, Fatehi MT, Koozekanani SH, et al. Ultrasound therapy: a comparative study of different coupling media. Arch Phys Med Rehabil. 1986;67:147.
Potter K, Reed CJ, Green DJ, Hankey GJ, et al. Ultrasound setting significantly alter arterial lumen and wall thickness measurement. Cardiovasc Ultrasound. 2008. https://doi.org/10.1186/1476-7120-6-6.
Acknowledgements
We thank Rebecca Jackson, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest with regard to the presented research.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Fujino, K., Ohno, K., Fujiwara, K. et al. Sonographic morphometry of abductor pollicis brevis: can direct contact yield images comparable with those obtained by the water bath technique?. J Med Ultrasonics 46, 489–495 (2019). https://doi.org/10.1007/s10396-019-00945-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-019-00945-3