Abstract
Pericardiocentesis is performed to treat cardiac tamponade or diagnose the cause of pericardial effusion. Cardiogenic shock with right ventricular (RV) dysfunction is a rare complication after pericardiocentesis. We report a case of an 82-year-old man who suddenly suffered cardiopulmonary arrest 12 h after pericardiocentesis. A transthoracic echocardiogram showed remarkable RV dysfunction and tricuspid valve dysfunction. Tricuspid valve closure was severely impaired, and the tricuspid regurgitation signal showed laminar flow with an early peak. However, after treatment with high-dose inotropic drugs, hemodynamic parameters gradually recovered. A transthoracic echocardiogram performed 24 h later showed improved motion of the RV and the tricuspid valve, resulting in a reduction in tricuspid regurgitation. RV and tricuspid valve dysfunction after pericardiocentesis needs to be recognized as a critical complication. Physicians also need to pay attention to not only the amount of drainage but also underlying RV dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pericardiocentesis is performed to treat cardiac tamponade or diagnose the cause of pericardial effusion, and it should be done carefully and safely. However, there are some complications associated with this procedure, such as bleeding, liver damage, and pneumothorax. Moreover, cardiogenic shock with significant right ventricular (RV) dysfunction after pericardiocentesis has been described as a rare complication [1–3], but tricuspid valve dysfunction has not been reported. We describe a case of a patient with cardiogenic shock due to severe RV and tricuspid valve dysfunction 12 h after pericardiocentesis.
Case report
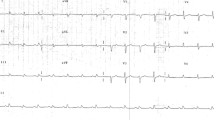
An 82-year-old man was referred to our hospital for exacerbation of dyspnea. He had a history of rheumatoid arthritis, systemic lupus erythematosus, and atrial fibrillation. On admission, his blood pressure was 106/44 mmHg, pulse rate was 76 beats/min, and oxygen saturation was 99 % (ambient air). Jugular venous distention was not obvious, and neither third heart sounds nor a cardiac murmur were audible. An electrocardiogram showed atrial fibrillation and inverted T waves and ST segment depressions in II, III, and aVF leads. A chest X-ray showed an enlarged cardiothoracic ratio of 70 % and left-sided small pleural effusion. Laboratory data were as follows: blood urea nitrogen level of 49.5 mg/dL, serum creatinine level of 1.2 mg/dL, white blood cell count of 8430/µL, C-reactive protein level less than 0.2 mg/dL, brain natriuretic peptide level of 310.9 pg/mL, troponin T level of 0.529 ng/mL, creatinine kinase level of 206 U/L, and creatinine kinase MB level of 22 U/L.
A transthoracic echocardiogram (TTE) showed normal left ventricular function with moderate pericardial effusion and mild tricuspid regurgitation (TR) (Fig. 1). TR peak velocity was 3.0 m/s, and dilatation of the inferior vena cava was not detected. The longitudinal strain of the RV free wall was decreased to −15.2 %.
Transthoracic echocardiograms of the right ventricle and tricuspid valve on admission in the mid-systolic phase (a, b) and mid-diastolic phase (c, d) (yellow arrow tricuspid valve). The motion of the tricuspid valve was normal. Tricuspid regurgitation was mild (e), and the peak velocity of tricuspid regurgitation was 3.0 m/s (f). Moderate pericardial effusion was shown in a short axis view (g). RA right atrium, RV right ventricle
Although findings of cardiac tamponade were not observed, the patient’s dyspnea may have been related to pericardial effusion. Pericardiocentesis was performed to relieve his symptoms and examine the cause of pericardial effusion. A volume of 430 mL of light yellow exudative pericardial effusion was removed in 10 min. The blood pressure and stroke volume were 120/44 mmHg and 35.62 mL, respectively, before pericardiocentesis, and 100/60 mmHg and 45.33 mL, respectively, just after pericardiocentesis. Microbial and cytological examinations of this fluid were negative. A tuberculosis polymerase chain reaction was also negative. However, anti-double strand DNA test was positive, and complement 3 and complement 4 levels were low. Therefore, we diagnosed the patient with pericarditis associated with systemic lupus erythematosus.
Twelve hours after the procedure, the patient suddenly suffered from cardiopulmonary arrest. After successful cardiopulmonary resuscitation, TTE performed immediately after resuscitation showed normal left ventricular wall motion, but markedly dilated RV and severe hypokinesis of the RV wall motion. The longitudinal strain of the RV free wall was reduced to −5.0 %. Moreover, the tricuspid valve closure was severely impaired, and the TR signal showed laminar flow with an early peak (Fig. 2). After treatment with high-dose inotropic drugs, the patient gradually recovered, and hemodynamic parameters were normalized. When TTE was performed 24 h later, it showed improved wall motion of the RV and the tricuspid valve, resulting in a reduction in TR (Fig. 3). The longitudinal strain of the RV free wall improved to −15.9 %. Chronological changes in echocardiographic indices of RV function are shown in Table 1.
Transthoracic echocardiograms of the right ventricle and tricuspid valve immediately after resuscitation in the mid-systolic phase (a, b) and mid-diastolic phase (c, d) (yellow arrow tricuspid valve). The right ventricle was enlarged, and the tricuspid valve closure was severely impaired. Tricuspid regurgitation showed laminar flow (e), and the velocity of tricuspid regurgitation showed an early peak (f). Pericardial effusion was removed completely (g). RA right atrium, RV right ventricle
Transthoracic echocardiograms in the right ventricle and tricuspid valve 24 h after cardiopulmonary arrest in the mid-systolic phase (a, b) and mid-diastolic phase (c, d) (yellow arrow tricuspid valve). The right ventricle remained enlarged, but the motion of the tricuspid valve was improved. Tricuspid regurgitation was reduced to mild (e), and the velocity of tricuspid regurgitation was also improved (f). Pericardial effusion did not increase (g). RA right atrium, RV right ventricle
Discussion
We report a rare case of cardiopulmonary arrest after pericardiocentesis with remarkable RV and tricuspid valve dysfunction. Some reports have described cases of cardiogenic shock with significant RV dysfunction after pericardiocentesis [1–3]. However, there are no reports on tricuspid valve dysfunction and the chronological recovery course after pericardiocentesis by echocardiography.
Armstrong et al. [4] reported three patients who showed an increase in the RV volume after pericardiocentesis, and they also showed the chronological improvement. An increase in RV volume was also observed by Dante et al. [5]. They showed that an increase in end-diastolic volume and hemodynamic changes after pericardiocentesis were more prominent in the RV than in the left ventricle. Venous return after pericardiocentesis increases temporarily, and this may lead to temporary RV enlargement.
In the present case, tricuspid valve closure was markedly impaired, resulting in severe TR and a decrease in pressure gradient between the RV and right atrium. As a result of reduced cardiac output, our patient progressed to cardiogenic shock and cardiopulmonary arrest. However, taking into consideration the fact that the motion of the tricuspid valve recovered completely 24 h after cardiopulmonary arrest, the valve itself was not impaired. Impaired tricuspid valve motion and severe TR may be caused by RV dysfunction. In our patient, RV dysfunction also improved 24 h after cardiopulmonary arrest; therefore, RV stunning occurred. The mechanism of the stunning is due to not only an abrupt increase of venous return and RV enlargement after pericardiocentesis but also the existence of underlying myocardial impairment in the RV [6]. In our case, the longitudinal strain of the RV free wall before pericardiocentesis was decreased and the troponin T level was high. Myocarditis due to systemic lupus erythematosus may lead to myocardial dysfunction in the RV. Therefore, measuring the longitudinal strain of the RV free wall may be useful for detecting the presence of myocardial damage before pericardiocentesis.
Whether the amount of drainage and speed affect hemodynamics after pericardiocentesis is unclear. Sunday et al. [1] reported a case of cardiogenic shock after 700 mL of fluid was drained. Armstrong et al. [4] described that the amount of drainage in three cases of RV volume overload after pericardiocentesis was 200, 900, and 1400 mL. Therefore, pericardiocentesis is not always safe even when the amount of drainage is small. Physicians need to pay attention to not only the amount of drainage but also underlying RV dysfunction to prevent this rare but potentially fatal complication.
References
Sunday R, Robinson LA, Bosek V. Low cardiac output complicating pericardiectomy for pericardial tamponade. Ann Thorac Surg. 1999;67:228–31.
Anguera I, Paré C, Perez-Villa F. Severe right ventricular dysfunction following pericardiocentesis for cardiac tamponade. Int J Cardiol. 1997;59:212–4.
Viola AR. The influence of pericardiectomy on the hemodynamics of chronic constrictive pericarditis. Circulation. 1973;48:1038–42.
Armstrong WF, Feigenbaum H, Dillon JC. Acute right ventricular dilation and echocardiographic volume overload following pericardiocentesis for relief of cardiac tamponade. Am Heart J. 1984;107:1266–70.
Manyari DE, Kostuk WJ, Purves P. Effect of pericardiocentesis on right and left ventricular function and volumes in pericardial effusion. Am J Cardiol. 1983;52:159–62.
Dosios T, Theakos N, Angouras D, et al. Risk factors affecting the survival of patients with pericardial effusion submitted to subxiphoid pericardiostomy. Chest. 2003;124:242–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical statements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from the patient included in the study.
About this article
Cite this article
Kuroda, M., Amano, M., Enomoto, S. et al. Severe right ventricular and tricuspid valve dysfunction after pericardiocentesis. J Med Ultrasonics 43, 533–536 (2016). https://doi.org/10.1007/s10396-016-0738-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-016-0738-5