Abstract
The changes in cardiac function that occur after pericardiocentesis are unclear. An understanding of the effect of pericardiocentesis on right ventricular (RV) and left ventricular (LV) function is clinically important. This study was performed to assess RV and LV function with echocardiography before and after pericardiocentesis. In total, 19 consecutive patients who underwent pericardiocentesis for more than moderate pericardial effusion were prospectively enrolled from August 2015 to October 2017. Comprehensive transthoracic echocardiography was performed before, immediately after (within 3 h), and 1 day after pericardiocentesis to investigate the changes in RV and LV function. The mean age of all patients was 72.6 ± 12.2 years. No pericardiocentesis-related complications occurred during the procedure, but one patient died of right heart failure 8 h after pericardiocentesis. After pericardiocentesis, RV inflow and outflow diameters increased (p < 0.05 versus values before pericardiocentesis), and the parameters of RV function (tricuspid annular plane systolic excursion, tricuspid lateral annular systolic velocity, fractional area change, and RV free wall longitudinal strain) significantly decreased (p < 0.001 versus values before pericardiocentesis). These abnormal values or RV dysfunction remained 1 day after pericardiocentesis (p > 0.05 versus values immediately after pericardiocentesis). Conversely, no parameters of LV function changed after pericardiocentesis. Of 19 patients, 13 patients showed RV dysfunction immediately after pericardiocentesis and 6 patients did not. RV free wall longitudinal strain before pericardiocentesis in patients with post-procedural RV dysfunction was reduced compared to those without post-procedural RV dysfunction ( − 18.9 ± 3.6 versus − 28.4 ± 6.3%; p = 0.005). The area under the curve values for prediction of post-procedural RV dysfunction was 0.910 for RV free wall longitudinal strain. The occurrence of RV dysfunction after pericardiocentesis should be given more attention, and pre-procedural RV free wall longitudinal strain may be a predictor of post-procedural RV dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pericardiocentesis is commonly performed to diagnose the cause of pericardial effusion or relieve cardiac tamponade in the clinical setting. However, the changes in cardiac function that occur after pericardiocentesis remain unclear. Several case reports have described cardiogenic shock with significant right ventricular (RV) dysfunction after pericardiocentesis [1, 2]. Moreover, we reported transient worsening of RV function after pericardiocentesis [3]. An understanding of the effect of pericardiocentesis on RV and left ventricular (LV) function is important for safe performance of this procedure. The present study was performed to assess RV and LV function with echocardiography before and after relief of pericardial effusion.
Materials and methods
Study population
In total, 19 consecutive patients who had dyspnea due to a large amount of pleural effusion or needed to diagnose their background disease were prospectively enrolled in this study. All patients underwent pericardiocentesis for more than moderate pericardial effusion from August 2015 to October 2017. Patients who underwent emergency pericardiocentesis for pericardial effusion due to aortic dissection with cardiac tamponade were excluded. The study protocol was approved by the institutional ethics committee at Tenri Hospital. All patients provided written informed consent to participate in this study.
Pericardiocentesis procedure
Patients underwent echocardiography-guided primary percutaneous pericardiocentesis for therapeutic or diagnostic purposes. Percutaneous pericardiocentesis was performed using the shortest distance to the pericardial cavity from the subxiphoid or intercostal space. After accessing the pericardial space, the needle was exchanged over a guide wire to a dilator, followed by a multi-hole pigtail catheter. The catheter was then sutured and affixed to the chest wall, where it was kept in place for a few days. A 150 mL sample of aspirated fluid was sent for pathologic, chemical, and microbiological testing. The catheter was removed earlier if the fluid drainage dropped to < 10 mL per hour with no residual effusion seen by echocardiography.
Echocardiographic examination and parameters
Comprehensive transthoracic echocardiography was performed before, immediately after (within 3 h), and 1 day after pericardiocentesis using a high-quality, commercially available ultrasound system (Vivid E9 ultrasonic unit; GE Healthcare, Tokyo, Japan). The follow-up echocardiography was performed at 6 months after pericardiocentesis. The frame rate was > 50 frames per second. Images were analyzed using EchoPAC PC version 113 (GE Healthcare). Measurements and recordings were obtained according to the American Society of Echocardiography recommendations; the measured parameters were tricuspid annular plane systolic excursion (TAPSE), tricuspid lateral annular systolic velocity (Sʹ), and fractional area change (FAC) [4]. The ejection fraction and stroke volume were calculated using the modified Simpson’s method. The early diastolic mitral annular tissue velocity (eʹ) was measured in the apical four-chamber view with the sample volume positioned at the lateral mitral annulus. Basal and mid-cavity RV linear dimensions in the basal one third and the middle third of RV inflow were measured at end diastole in the RV-focused view. Proximal RV outflow diameter was measured from the anterior RV wall to the interventricular septal-aortic junction at end diastole in the parasternal long-axis view. Peak longitudinal strain measurements were obtained from gray-scale images recorded in the apical four-chamber, two-chamber, and long-axis views. Global longitudinal strain was obtained by averaging all segmental peak strain values from the apical four-chamber, two-chamber, and long-axis views. The peak strain for the three RV free wall segments was averaged to produce the RV free wall longitudinal strain, with exclusion of the interventricular septum to avoid LV interaction. To certify their accuracy, all echocardiographic parameters were remeasured by experienced sonographers at our institution.
Predictors of RV dysfunction immediately after pericardiocentesis
To evaluate the predictors of RV dysfunction immediately after pericardiocentesis, 19 patients were divided into 2 groups: patients who showed RV dysfunction immediately after the procedure and patients without RV dysfunction. Pre-procedural parameters were compared between the 2 groups. RV dysfunction is defined as meeting at least three of the four criteria: a TAPSE of < 17 mm, an Sʹ of < 9.5 cm, an FAC of < 35%, and an RV free wall longitudinal strain > − 20%.
Statistical analysis
Statistical analyses were performed using SPSS Statistics for Windows (ver. 22.0; IBM, Armonk, NY). All data are expressed as mean ± standard deviation. Categorical variables were compared using the Chi-square test. Differences in continuous variables between the two groups were determined using the Mann–Whitney U test. One-way repeated-measures analysis of variance and a post hoc test (Turkey–Kramer test) were used to test for significance adjustment for multiple comparisons. Receiver operating characteristic analysis was performed to investigate the sensitivity and the specificity for predicting the occurrence of RV dysfunction after pericardiocentesis and to determine the best cutoff value. Intra- and inter-observer variability of RV free wall longitudinal strain were estimated using intraclass correlation coefficient (ICC). Statistical significance was set at a p value of 0.05.
Results
Patients’ baseline characteristics
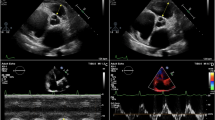
The demographic characteristics of all 19 patients are shown in Table 1. Atrial fibrillation was present in three patients, none of whom had a history of myocardial infarction or valvular heart disease. Pericardiocentesis was carried out for therapeutic reasons in 14 patients and for diagnostic purposes in five patients. Cardiac tamponade was present in five (26%) patients. No pericardiocentesis-related complications such as myocardial puncture or coronary laceration occurred during the procedure. However, one patient in whom pericardiocentesis was performed to relieve cardiac tamponade caused by massive pericardial effusion died 8 h after the procedure. The cause of death was right heart failure caused by severe RV dysfunction after pericardiocentesis (Fig. 1).
A patient died of right heart failure caused by severe RV dysfunction 8 h after pericardiocentesis. a End-diastolic and b end-systolic phase before pericardiocentesis: RV free wall longitudinal strain, − 15.9%; TAPSE, 9.3 mm; Sʹ, 8.0 cm/s; and FAC, 40.7%. c End-diastolic (red arrow) and d end-systolic (red arrow) phase immediately after pericardiocentesis: RV free wall longitudinal strain, − 3.0%; TAPSE, 5.2 mm; Sʹ, 2.7 cm/s; and FAC, 22.3%. RV right ventricular, TAPSE tricuspid annular plane systolic excursion, S’ tricuspid lateral annular systolic velocity, FAC fractional area change
Changes in both chamber size and function before and after pericardiocentesis
The changes in vital signs, volumes, and RV and LV function before, immediately after, and 1 day after pericardiocentesis are shown in Table 2 and Fig. 2. The mean values of the four indices of RV function before pericardiocentesis were normal. The number of patients who showed an abnormal value was small: three (16%) patients had a TAPSE of < 17 mm, three (16%) had an Sʹ of < 9.5 cm, none (0%) had an FAC of < 35%, and six (32%) had an RV free wall longitudinal strain > − 20% [4]. However, all of these parameters significantly decreased immediately after pericardiocentesis, showing abnormal values (p < 0.001 respectively); tricuspid regurgitation peak velocity was the only RV function parameter that remained normal. Those abnormal values remained 1 day after pericardiocentesis (Fig. 3). RV inflow and outflow diameters increased significantly immediately after pericardiocentesis. They decreased but remained large at one day after pericardiocentesis compared with those before pericardiocentesis. In contrast, no parameters of LV size and function changed immediately or one day after pericardiocentesis, although the E/A and E/eʹ values were slightly high and the LV global longitudinal strain was low during pre-procedural echocardiography. Intra- and inter-observer ICC in our institution were 0.971 and 0.953 for RV free wall longitudinal strain.
Changes in right ventricular and left ventricular function before, immediately after, and 1 day after pericardiocentesis. The right ventricular indices decreased significantly (p < 0.05) and showed abnormal values immediately after pericardiocentesis. These abnormal values remained 1 day after pericardiocentesis. In contrast, left ventricular function did not worsen immediately and 1 day after pericardiocentesis
A representative case of transition to RV dysfunction before and after pericardiocentesis. a End-diastolic and b end-systolic phase before pericardiocentesis: RV free wall longitudinal strain, − 20.9%; TAPSE, 20.4 mm; Sʹ, 17.1 cm/s; and FAC, 51.2%. c End-diastolic and d end-systolic phase immediately after pericardiocentesis: RV free wall longitudinal strain, − 14.3%; TAPSE, 7.2 mm; Sʹ, 6.6 cm/s; and FAC, 39.1%. e End-diastolic and f end-systolic phase 1 day after pericardiocentesis: RV free wall longitudinal strain, − 14.3%; TAPSE, 10.0 mm; Sʹ, 6.4 cm/s; and FAC, 43.3%. RV right ventricular, TAPSE tricuspid annular plane systolic excursion, Sʹ tricuspid lateral annular systolic velocity, FAC fractional area change
Only 10 patients could attend the follow-up echocardiography at 6 months after pericardiocentesis; six patients died of lung cancer, two patients transferred to different hospitals, and one patient died of right heart failure just after the procedure. Regarding echocardiographic LV parameters, LV size (LV end-diastolic diameter of 40.4 ± 7.5 mm, and LV end-systolic diameter of 25.8 ± 5.3 mm), ejection fraction (69.3 ± 6.5%), and LV longitudinal global strain ( − 14.8 ± 1.4%) remained unchanged. On the other hand, RV inflow and outflow diameters (basal RV inflow of 30.8 ± 4.6 mm, and mid-cavity RV inflow of 33.4 ± 4.8 mm, and RV outflow of 33.4 ± 4.8 mm) became smaller and RV function further improved (TAPSE of 17.8 ± 4.4%, Sʹ of 11.1 ± 3.2 cm/s, FAC of 41.8 ± 6.0%, and RV free wall longitudinal strain of − 20.6 ± 6.3%) compared with RV parameters 1 day after pericardiocentesis.
Predictors of RV dysfunction immediately after pericardiocentesis
Of the 19 patients, 13 patients showed RV dysfunction immediately after pericardiocentesis, and 6 patients did not. RV free wall longitudinal strain before pericardiocentesis in patients with post-procedural RV dysfunction was reduced compared to those without post-procedural RV dysfunction ( − 18.9 ± 3.6 vs. − 28.4 ± 6.3%; p = 0.005). The other pre-procedural parameters were not different between the 2 groups (Table 3). Receiver operating characteristic analysis revealed that a RV free wall longitudinal strain cutoff value of − 23.0 had a sensitivity of 100% and a specificity of 83.3% for predicting the occurrence of RV dysfunction after pericardiocentesis (area under the curve = 0.910).
Discussion
In the present study, we found that RV dysfunction with enlargement of RV size occurred immediately after pericardiocentesis and that in most patients, the RV dysfunction remained 1 day after the procedure. One patient developed severe RV dysfunction immediately after pericardiocentesis and eventually died of right heart failure. Conversely, no parameters of LV size and function showed significant changes before and after pericardiocentesis. Moreover, the value of RV free wall longitudinal strain before pericardiocentesis was an only predictor of post-procedural RV dysfunction.
Two possible factors may be related to the emergence of RV dysfunction after pericardiocentesis: (1) a rapid increase in preload to the right ventricle due to hemodynamic changes after pericardiocentesis and (2) pre-existing RV myocardial damage.
Hemodynamic changes before and after pericardiocentesis
Generally, a heart that has been decompressed by a large amount of pericardial effusion demonstrates marked hemodynamic improvement by pericardiocentesis as cardiac filling and stroke volume are increased. The right ventricle receives a large volume load caused by increasing venous return immediately after pericardiocentesis. Moreover, previous studies have shown larger volume changes in the right than left ventricle [5, 6]. RV dilatation with signs of volume overload after pericardiocentesis has also been reported [1, 7, 8]. Therefore, rapid and massive volume overload in the right ventricle is related to RV dysfunction and dilatation after pericardiocentesis. On the other hand, stroke volume and cardiac index before and after pericardiocentesis were unchanged. RV size increased after pericardiocentesis, but decrease in RV function resulted in unchanged stroke volume.
The effects of the drainage amount and speed on hemodynamics after pericardiocentesis are unclear. Armstrong et al. [7] reported that the amount of drainage in three patients with RV volume overload and RV dysfunction after pericardiocentesis was 200, 900, and 1400 mL. In another case, only 430 mL of pericardial effusion was removed from a patient with cardiogenic shock caused by improved motion of the RV and tricuspid valves [3]. Smiseth et al. [9] reported that the pericardial pressure significantly decreased regardless of the amount of pericardial fluid removed (range 120–1200 mL). No reports have described the relationship between the speed of drainage and hemodynamic changes after relief of pericardial effusion. Physicians’ only option is to avoid removing a large amount of pericardial effusion at high speed; however, prevention of RV dysfunction after pericardiocentesis by this technique alone is doubtful.
Pre-existing RV myocardial damage before pericardiocentesis and prolonged RV failure after pericardiocentesis
Pre-existing RV myocardial damage, which may be masked by reduced preload and afterload before pericardiocentesis, may also be considered a risk factor for RV dysfunction after pericardiocentesis.
First, most patients who undergo pericardiocentesis have pericarditis due to a malignant tumor, connective tissue disease, or radiation. Continued inflammation involving the epicardium results in reduced RV free wall motion and progressive myocardial injury [10, 11]. Second, these patients sometimes have direct myocardial involvement from a malignant tumor or concomitant myocarditis caused by connective tissue disease such as systemic lupus erythematosus, polymyositis, or dermatomyositis [12]. In the current study, pre-procedural RV free wall longitudinal strain was a predictor of RV dysfunction after pericardiocentesis, but the other conventional parameters of RV function were not. The masked pre-existing RV dysfunction may be identified by RV free wall longitudinal strain. Finally, Geffroy et al. [8] reported that a rapid increase in RV wall tension immediately after pericardiocentesis impaired coronary flow despite a normal coronary angiogram. This abnormal coronary flow pattern sometimes induces RV ischemic lesions. Therefore, in addition to a rapidly increasing preload induced by hemodynamic changes, these potential RV myocardial damages may affect the stunned myocardium immediately after pericardiocentesis.
The masked pre-existing RV dysfunction is actualized by the rapid increase in venous return, pressure elevation, and impaired coronary flow immediately after pericardiocentesis, and severe RV dysfunction subsequently occurs. The right ventricle is more strongly influenced by the pericardium and loading condition than the left ventricle. As a result, an interventricular mismatch is permitted following relief of pericardial effusion [13], and RV stunning may often occur although LV function does not worsen after pericardiocentesis.
Changes in LV function before and after pericardiocentesis
LV systolic failure after removal of a large volume of fluid by pericardiocentesis has been reported even in the absence of a history of LV dysfunction [6], but such cases are very rare compared with RV dysfunction. Grose et al. [14] reported that the LV pump function was well preserved in patients with cardiac tamponade and that the ejection fraction was unchanged by pericardiocentesis. Manyari et al. [5] also reported that the LV volume did not increase after pericardiocentesis and that the LV ejection fraction was not significantly affected. In the present study, the LV systolic and diastolic function, including the LV longitudinal strain and ejection fraction, were not significantly different before and after pericardiocentesis.
However, the LV longitudinal strain before pericardiocentesis was low and the E/eʹ was slightly high in most patients. Therefore, pre-existing myocardial damage affected not only the right ventricle but also the left, and the longitudinal systolic function and diastolic function were impaired despite a normal ejection fraction in patients with a large volume of pericardial effusion. However, the hemodynamic changes caused by the reduction of external compression and rapid increase in preload after pericardiocentesis might not significantly affect LV function compared with RV function.
Limitations
The main limitation of this study is that it was a single-center study with a small sample size. Additionally, the first follow-up echocardiography was performed within 3 h after pericardiocentesis, and placement of a right heart catheter immediately after the procedure was not performed in all patients. Thus, the hemodynamic changes that occurred during or immediately after this procedure were not observed. This may be another reason why stroke volume was unchanged immediately after pericardiocentesis. Finally, the follow-up echocardiography after discharged was planned at 6 months after, but one-third of the enrolled patients died of cancer within 6 months. Therefore, sufficient long-term follow-up data after discharge were not obtained.
Conclusions
RV dysfunction occurred immediately after pericardiocentesis and remained 1 day after the procedure. However, LV function was not significantly affected by the hemodynamic changes compared with RV function. The occurrence of RV dysfunction after pericardiocentesis should be given more attention, and pre-procedural RV free wall longitudinal strain may be a predictor of post-procedural RV dysfunction.
References
Sunday R, Robinson LA, Bosek V (1999) Low cardiac output complicating pericardiectomy for pericardial tamponade. Ann Thorac Surg 67:228–231
Anguera I, Paré C, Perez-Villa F (1997) Severe right ventricular dysfunction following pericardiocentesis for cardiac tamponade. Int J Cardiol 59:212–214
Kuroda M, Amano M, Enomoto S, Miyake M, Kondo H, Tamura T, Kaitani K, Izumi C, Nakagawa Y (2016) Severe right ventricular and tricuspid valve dysfunction after pericardiocentesis. J Med Ultrason (2001) 43:533–536
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1–39.e14
Manyari DE, Kostuk WJ, Purves P (1983) Effect of pericardiocentesis on right and left ventricular function and volumes in pericardial effusion. Am J cardiol 52:159–162
Chamoun A, Cenz R, Mager A, Rahman A, Champion C, Ahmad M, Birnbaum Y (2003) Acute left ventricular failure after large volume pericardiocentesis. Clin Cardiol 26:588–590
Armstrong WF, Feigenbaum H, Dillon JC (1984) Acute right ventricular dilation and echocardiographic volume overload following pericardiocentesis for relief of cardiac tamponade. Am Heart J 107:1266–1270
Geffroy A, Beloeil H, Bouvier E, Chaumeil A, Albaladejo P, Marty J (2004) Prolonged right ventricular failure after relief of cardiac tamponade. Can J Anaesth 51:482–485
Smiseth OA, Frais MA, Kingma I, White AV, Knudtson ML, Cohen JM, Manyari DE, Smith ER, Tyberg JV (1986) Assessment of pericardial constraint: the relation between right ventricular filling pressure and pericardial pressure measured after pericardiocentesis. J Am Coll Cardiol 7:307–314
Homsi M, Mahenthiran J, Vaz D, Sawada SG (2007) Reduced right ventricular systolic function in constrictive pericarditis indicates myocardial involvement and persistent right ventricular dysfunction and symptoms after pericardiectomy. J Am Soc Echocardiogr 20:1417.e1–7
Levine HD (1973) Myocardial fibrosis in constrictive pericarditis. Electrocardiographic and pathologic observations. Circulation 48:1268–1281
Dosios T, Theakos N, Angouras D, Asimacopoulos P (2003) Risk factors affecting the survival of patients with pericardial effusion submitted to subxiphoid pericardiostomy. Chest 124:242–246
Ditchey R, Engler R, LeWinter M, Pavelec R, Bhargava V, Covell J, Moores W, Shabetai R (1981) The role of the right heart in acute cardiac tamponade in dogs. Circ Res 48:701–710
Grose R, Greenberg M, Steingart R, Cohen MV (1982) Left ventricular volume and function during relief of cardiac tamponade in man. Circulation 66:149–155
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amano, M., Izumi, C., Baba, M. et al. Occurrence of right ventricular dysfunction immediately after pericardiocentesis. Heart Vessels 35, 69–77 (2020). https://doi.org/10.1007/s00380-019-01456-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-019-01456-4