Abstract
Phlegmonous esophagitis is a rare and sometimes fatal condition. Cases surgically treated have been reported previously; however, surgical approaches may be risky in the elderly. An 86-year-old woman presented with a sore throat and high fever after eating fish. She was first diagnosed with a deep cervical abscess, and conservative treatment was initially selected. However, her respiratory failure worsened, so emergent tracheostomy and surgical drainage were performed. Computed tomography showed intramural low-density lesions along the entire length of the esophagus and she was diagnosed with phlegmonous esophagitis. To avoid surgical intervention, endoscopic drainage was first attempted. Mucosal incision was made on the lower esophagus guided by endoscopic ultrasonography, using the insulated-tip electrosurgical knife. After the endoscopic drainage, her general health status improved, and the esophageal wall thickness was reduced. While some cases have recovered with conservative treatment, endoscopic drainage may be useful in certain patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Phlegmonitis of the gastrointestinal tract is a condition caused by a suppurative bacterial infection focusing on the submucosal layer. Phlegmonous esophagitis (PE) is very rare, and it can sometimes be a fatal disease due to the severe intrathoracic inflammation and accompanying unstable respiratory status [1]. Both conservative and surgical approaches to treatment have been reported previously. In recent years, some cases have been treated effectively with a conservative approach using effective antibiotic therapy, and the mortality rate has been decreasing. Herein we describe our experience with an elderly woman with acute PE treated with endoscopic ultrasonography (EUS)-guided internal drainage.
Case presentation
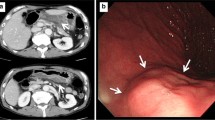
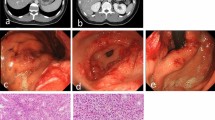
An 86-year-old woman with a history of hypertension experienced a high fever and sore throat after eating horse mackerel. Her physical examination showed redness, swelling on the left submandibular region. The vital signs showed systemic inflammatory response syndrome. The serum examination results showed a marked inflammatory reaction (WBC 11670/µl, CRP 34.1 mg/dl). Computed tomography (CT) from the neck to the upper mediastinal area showed a low-density area around the submental region and upper esophagus (Fig. 1). She was diagnosed with a deep neck abscess, and conservative treatment was administered. The next day, her respiratory status worsened, therefore, emergent tracheostomy and surgical cervical drainage were performed under general anesthesia. The bacteriological culture of the pus showed oral anaerobes. On the fourth day, the thoraco-abdominal CT scan showed a markedly thickened esophageal wall and an intramural low-density area along the entire length of the esophagus (Fig. 2a, b). Upper endoscopic examination showed reddish and edematous mucosal layer of the esophagus. There was an ulcer, fistula, and a small amount of pus was discharged (Fig. 3a, b). Based on the clinical course and images, she was diagnosed with PE. Because of her poor general condition, endoscopic drainage was planned first. The EUS image demonstrated diffuse esophageal wall thickening showing low-echoic lesion in the submucosal layer (the third layer). Mucosal incision was made and a little pus was discharged and bacterial culture showed oral anaerobes. A guide-wire was inserted through the incision and 5-cm longitudinal mucosal incision was made toward the esophago-gastric junction using an insulated-tip (IT) diathermic knife guided by the wire for internal drainage (Fig. 3c). After the drainage, CT findings showed a slight reduction of the low-density area along the esophagus (Fig. 2c, d). The endoscopy findings one month after the drainage showed mild esophageal stenosis and longitudinal ulceration (Fig. 3d). The inflammatory reaction got gradually resolved, but respiratory status remained unstable and the mechanical ventilation had been required for 5 months after the drainage (Fig. 4). For the nutritional therapy, enteral feeding per jejunostomy which was made 2 weeks after the drainage was unable to continue because of intractable diarrhea. Confirming the cure of the esophageal fistula endoscopically, oral intake was attempted with liquid diet 2 month after the drainage, but food was discharged from the tracheostomy orifice because of severe aspiration. Before the discharge, CT exams demonstrated the wall thickness and the intramural low-density area were reduced (Fig. 2e, f). She was transferred to another hospital for rehabilitation 6 months after the treatment.
Cervical and upper mediastinal CT scan findings on the day of admission (Fig. 1, arrow shows left submental abscess, arrowhead indicates low-density lesion along the upper esophagus)
Upper endoscopy findings; reddish and edematous mucosa during the internal drainage using guide-wire (Fig. 3a), the fistula evacuating small amounts of pus (Fig. 3b, arrow: mucosal fistula), the longitudinal mucosal incision was made on the lower esophagus using an insulated-tip (IT) diathermic knife guided by the wire inserted into the submucosal layer (Fig. 3c), one month after the drainage showed esophageal stenosis and edematous mucosa was resolved (Fig. 3d)
Discussion
Phlegmonous esophagitis is a very rare condition characterized by a suppurative bacterial infection of the esophagus focusing on the submucosal and muscular layers. In the past series, Karimata et al. reported their fulminant PE case and a review of 13 cases. The pathogenesis of acute PE has previously been linked to uncontrolled diabetes mellitus, immunosuppressive status, heavy alcohol use, poor nutritional status, and aging. The CT findings are definitive diagnostic modalities that generally show diffuse thickening of the esophageal wall, edematous enlargement of the posterior mediastinum [2]. Upper endoscopy often demonstrates diffuse mucosal erythema, pseudo-lumen, and ulcer or circular stricture of the esophagus. Recently, some cases have been resolved conservatively [1, 3,4,5]. As surgical interventions, seromyotomy of the gastric wall or total gastrectomy with trans-hiatal lower esophagectomy or bypass operation for chronic stenosis or persistent chest pain have been reported [1, 2, 6, 7].
Only 3 cases of phlegmonous gastritis in which EUS imaging was performed were found in the English literature. EUS examination was beneficial for diagnosis and assessment for the effectiveness of treatment [8,9,10]. The characteristic EUS imaging demonstrates a diffuse wall thickening and obscuration of layers, especially the submucosal layer (the third layer). EUS imaging after recovery showed improvement of the wall thickening and reduced obscuration of layer construction. Gordon et al. reviewed phlegmonous gastritis cases and discussed the usefulness of EUS imaging [8, 9].
Takeno et al. discussed the usefulness of the endoscopic drainage with mucosal incision for the esophageal submucosal abscess, and they suggested that injure of the muscular layer should be prevented for spreading of inflammation to the thoracic cavity by drainage. Therefore, EUS imaging during the drainage is effective in checking the mucosal layer and recognizing the appropriate drainage layer.
Takatori et al. [10] reported a case of acute phlegmonous gastritis treated by EUS-guided drainage, just as in an endoscopic mucosal resection (EMR), with an IT diathermic knife. A large amount of pus was discharged by several mucosal incisions guided by EUS viewing, and conservative treatment succeeded. Therefore, EUS imaging might provide insight for optimal drainage methods and for response evaluations of the inflammation. Moreover, Chang et al. reported accidental internal drainage of PE by causing intramural dissection with a mucosal rupture upon nasogastric tube insertion [11].
In our case, we speculated the pathogenesis of PE from her clinical history and the bacterial cultures of the pus showing the oral anaerobes. The damage to the laryngopharyngeal mucosa due to fish bone caused the inflammation around this area; consequently the inflammation spread along the esophagus, and progressed to PE. Considering the patient’s age and poor general health condition in our case, we estimated that transthoracic surgical intervention under general anesthesia was too invasive, so we initially selected endoscopic drainage. Follow-up CT examination demonstrated the reduction of wall thickness and the intramural low-density area, which showed the effectiveness of this method. With regard to the possibility of recovery by conservative treatment for PE or phlegmonous esophagogastritis, EUS-guided internal drainage should be performed first to avoid surgical stress, which may be intolerable for patients with a poor general health condition.
If we experience patients diagnosed as phlegmonous esophagitis (PE) again, at first we would treat conservatively, because recently PE with sepsis or SIRS successfully treated conservatively with broad-spectrum antibiotics and intensive care have been reported. However, for the intractable cases, we should consider the drainage management endoscopically or surgically depending on available treatment modalities, especially for the patients with intramural abscess formation on CT.
After the EUS-guided internal drainage, esophageal stenosis was observed. In the past series, stenosis or chronic pain has been reported as complications of PE itself. Additionally, drainage with EMR or submucosal dissection technique might include stenosis as a late complication as well. Therefore, we should consider the occurrence of stenosis as a complication of PE or endoscopic internal drainage.
Conclusions
We reported the case of an elderly woman with acute PE treated with EUS-guided endoscopic drainage. Endoscopic internal drainage is useful treatment modality and should be performed first in cases of poor general health condition of the patient.
References
Karimata H, Nishimaki T, Oshita A, et al. Acute phlegmonous esophagitis as a rare but threatening complication of chemoradiotherapy: report of a case. Surg Today. 2014;44:1147–51.
Kim HS, Hwang JH, Hong SS, et al. Acute diffuse phlegmonous esophagogastritis: a case report. J Korean Med Sci. 2010;25:1532–5.
Hu DCH, McGrath KM, Jowell PS, et al. Phlegmonous gastritis: successful treatment with antibiotics and resolution documented by EUS. Gastrointest Endosc. 2000;52:793–5.
Iwakiri Y, Kabemura T, Yasuda D, et al. A case of acute phlegmonous gastritis successfully treated with antibiotics. J Clin Gastroenterol. 1999;26:175–7.
Takeno S, Moroga T, Ono K, et al. Endoscopic mucosal incision for successful treatment of submucosal abscess extending the full length of the esophagus due to fish bone: report of a case. Esophagus. 2015;12:199–202.
Wakayama T, Watanabe H, Ishizaki Y, et al. A case of phlegmonous esophagitis associated with diffuse phlegmonous gastritis. Am J Gastroenterol. 1994;89:804–6.
Hsu CY, Liu JS, Chen DF, et al. Acute diffuse phlegmonous esophagogastritis: report of a survived case. Hepatogastroenterology. 1996;43:1347–52.
Itonaga M, Ueda K, Ichinose M. Phlegmonous gastritis caused by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). Dig Endosc. 2012;24:488.
Kim GY, Ward J, Henessey B, et al. Phlegmonous gastritis: case report and review. Gastrointest Endosc. 2005;61:168–74.
Takatori K, Yamashita Y, Inoue H, et al. A case of phlegmonous gastritis successfully treated by endoscopic drainage therapy using IT-knife and medical therapy. Gastroenterol Endosc. 2005;48:2772–9.
Chang PC, Wang WL, Hwang TZ, et al. Intramural dissection with mucosal rupture alleviating phlegmonous esophagitis. Eur J Cardiothorac Surg. 2012;41:442–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of interest
All the authors declare that they have no conflict of interests.
Informed consent
Additional informed consent was obtained from the patient for whom identifying information is included in this article.
Rights and permissions
About this article
Cite this article
Tonouchi, A., Kuwabara, S., Furukawa, K. et al. Phlegmonous esophagitis treated by endoscopic drainage. Esophagus 14, 183–187 (2017). https://doi.org/10.1007/s10388-016-0562-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-016-0562-4