Abstract
Purpose
To examine the effect of switching from a prostanoid FP receptor agonists to EP2 receptor agonist (omidenepag isopropyl) on the deepening of the upper eyelid sulcus (DUES) and intraocular pressure (IOP) in Japanese glaucoma patients over 3 months post treatment.
Study design
Prospective observational study.
Methods
Patients with glaucoma who received FP receptor agonists treatment and had complained of DUES-related reduction in quality of life were included. Their FP receptor agonists was switched to omidenepag isopropyl without a drug holiday. At baseline and 1 and 3 months post-switch, photographs were taken and the changes in DUES were assessed by three independent observers. IOP and adverse events were also assessed.
Results
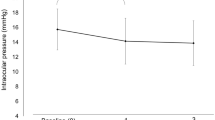
The study included 23 eyes of 23 patients (6 men, 17 women; average age, 60.6 years). After switching, DUES improved in 12 eyes at 1 month and in 16 eyes at 3 months; eyes in the remaining patients showed no worsening of the condition. The mean IOP before switching was 15.3 ± 3.3 mmHg (95% confidence interval 13.9–16.7 mmHg). Following the switch, the mean IOP values were 15.6 ± 3.3 mmHg (14.1–17.0 mmHg) at 1 month and 15.5 ± 3.3 mmHg (14.1–16.9 mmHg) at 3 months (P = 1.0 at 1 month, P = 1.0 at 3 months; both adjusted by Bonferroni correction). No adverse effects were observed.
Conclusion
Omidenepag isopropyl improved DUES while maintaining IOP in over 70% of Japanese patients with glaucoma who exhibited DUES caused by FP receptor agonists; the improvement was observed within 3 months after switching from FP receptor agonists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the Japan Glaucoma Society Guidelines for Glaucoma (4th Edition), the first-line treatment for primary open-angle glaucoma (POAG) are intraocular pressure (IOP)-lowering eye drops [1]. Prostaglandin FP receptor agonists (FP receptor agonists) eye drops have an excellent IOP-lowering effect, with good tolerability that facilitates adherence to treatment [2]. As such, they are widely used as first-line drugs both for normal pressure glaucoma and in patients with high IOP [3, 4].
However, the local side effects of FP receptor agonists reported thus far include conjunctival hyperemia, skin pigmentation, and long thickened dark lashes [5]. In addition to these side effects, prostaglandin-associated periorbitopathy syndrome (PAPS) has become a major problem [6]. PAPS is a general term that refers to the deepening of the upper eyelid sulcus (DUES) [7], flattening of the lower eyelid, and retraction of the eyeball; it is a local ocular phenomenon that affects the periorbital muscles and fat [8, 9]. Many patients consider DUES a cosmetic problem because it is directly related to appearance. Fat production is suppressed via FP receptors an event confirmed by in vitro experiments [10]. The suppression of fat production in the eyelids and orbits causes characteristic periocular changes. Once DUES develops, the eyelids harden, a sunken eye manifests, and the palpebral fissure narrows. Daily medical care (e.g., IOP measurements with an applanation tonometer) is affected in clinical practice [11] and DUES can worsen postoperative trabeculectomy outcomes [12].
Four FP receptor agonists available in Japan (latanoprost, travoprost, bimatoprost, and tafluprost) are known to cause DUES at varying frequency [13]. Because glaucoma is a chronic disease, lifelong IOP management (including the general use of FP receptor agonist treatment) is inevitable. DUES could considerably change a patient’s facial appearance; consequently, it affects the quality of life (QOL), especially in young people, women, and patients receiving unilateral eye treatment [14].
Omidenepag isopropyl ophthalmic solution 0.002% (Eybelis® ophthalmic solution 0.002%) is a selective prostanoid EP2 receptor agonist [15]. This is the first eye medication found to be non-inferior to latanoprost ophthalmic solution, the oldest FP receptor agonist, in lowering IOP in Japanese OAG patients [16]. DUES has not been reported with the use of omidenepag isopropyl in clinical trials or basic experiments [17]. This is because the active form of omidenepag does not bind to FP receptors, which are thought to be the cause of suppressed fat production. Therefore, omidenepag isopropyl could lower IOP while avoiding the onset of periorbital changes, such as DUES.
In a previous study, patients who developed DUES with the use of bimatoprost had the changes in appearance improved after switching to latanoprost (85%) [18]. Among the four FP receptor agonists, latanoprost is the least likely to cause DUES [13]. However, this data is a frequency comparison among FP receptor agonists (i.e., latanoprost, travoprost, tafluprost and bimatoprost), all of which can induce DUES. There is a need to evaluate clinical changes in DUES using a method that does not involve FP receptor agonists.
In this study, we prospectively investigated the effects of omidenepag isopropyl treatment on DUES caused by FP receptor agonists in Japanese patients with glaucoma over 3 months. We also assessed changes in IOP and the occurrence of adverse effects during the observation period.
Patients and methods
This prospective study included data from the clinical records of Japanese patients with glaucoma who were recruited between May 2019 and March 2020 from Yotsuya Shirato Eye Clinic (Tokyo, Japan). All protocols and methods adhered to the tenets of the Declaration of Helsinki; the protocol was approved by the Medical Corporation TOUKEIKAI Kitamachi Clinic IRB (number: STS06405). Written informed consent was obtained from all patients. The registered UMIN ID is UMIN000036705.
This report provides interim 3-month follow-up results. The enrolled patients will continue to be followed for a total of 7 months (the conclusion of the study) with the prescribed protocol.
Patients
The inclusion criteria were: (1) Japanese adult patients (aged ≥ 20 years) with glaucoma or ocular hypertension receiving FP receptor agonists treatment (including concomitant drugs); (2) patients who wished to change their treatment due to DUES-induced QOL decline (did not feel good about their appearance); and (3) both genders were eligible.
The exclusion criteria were an aphakic eye or intraocular lens in either eye.
Medication protocol
After patients had provided written informed consent, only FP receptor agonists treatment was switched to omidenepag isopropyl without a drug holiday. Patients were asked to apply one drop once daily. Patients using multiple drugs were advised to continue their drugs’ routine, except for the FP receptor agonists. Adverse events were assessed over a 3-month follow-up period.
IOP measurement
IOP was checked three times [day 0 (baseline), 1 month, and 3 months]. At each visit to the clinic (at approximately similar times on examination days), topical anesthesia was applied and two measurements were taken by Goldmann applanation tonometry (Haag Streit) by a well-trained examiner. Whenever the difference between the two measurements exceeded 2 mmHg, a third measurement was taken. The average value of the two measurements within 2 mmHg was used in the analysis.
Visual field test
Humphrey visual field (VF) Swedish Interactive Testing Algorithm (SITA) 30-2 (Humphrey visual field analyzer, Carl Zeiss Meditec) was used for the baseline VF (fix loss < 15%, false-positive < 15%, and false-negative < 15%) within the 6 months before enrollment.
Frontal photography
A frontal face photograph without makeup on the eyebrows or lower eyelids was taken without the flash (Sony Cyber-Shot: DSC-WX300, Sony Inc.) but with the same settings (Auto photo mode). The initial photograph served as baseline; all successive photos were taken using the same method.
Sample size and power analysis
In a previous study, 85% of DUES patients who had switched from bimatoprost 0.03% to latanoprost 0.005% showed a reduction in DUES at 6 months [18]. In the present study, we did not specify the FP receptor agonists used before the switch and there was no information on a reduction in DUES after switching from an FP receptor agonists to omidenepag isopropyl ophthalmic solution 0.002%. Assuming an 80% disappearance rate of DUES, we set up 30 cases in this study to estimate the 95% confidence interval in the range of 63–90%.
Statistical analysis
The primary outcome in this study was the objective change in DUES (photographic evaluation). Secondary outcomes were the changes in IOP and the presence or absence of adverse events. Both eyes were switched to EP2 receptor agonist, but the analysis was limited to one eye per patient. The selection criteria were: (1) the eye with stronger objective symptom of DUES was selected, (2) whenever the degree of DUES was the same, the eye with higher IOP at the switching was selected, (3) whenever the IOP was the same, the right eye was selected.
The evaluation method of DUES followed previous reports [7, 13, 18, 19]. Three independent co-investigators compared the 1 and 3-month anterior frontal photographs with the baseline photographs; investigators were blinded to the chronologies of the photograph sequences. Changes in DUES at the two time points were evaluated on a three-point scale (improved, no change, or worsened). When the assessment was not unanimous, the final evaluation was decided through discussion and determined final consensus among the co-investigators. The numbers of patients judged to exhibit improvement/no change/worsening at each time point and the relevant ratios (95% confidence intervals, CI) were calculated. The physician in charge of examining a particular patient did not participate in the evaluation of that patient’s photographs. Comparison of patient backgrounds between the improvement group and the rest of the group at 1 month and 3 months was examined by unpaired t-test or Fisher's exact test. Baseline IOP measurements were compared to IOP values at 1 and 3 months using a mixed model [20]. The structure of the model was specified to include time for fixed effects and repeated measures, and the covariance structure for repeated measures was adopted compound symmetry. In the present study, IOP data were measured repeatedly at different times, and since it was necessary to make comparisons at each time, analysis of variance was performed and the Bonferroni method was adopted to correct for the significance. JMP (version 15.0, SAS Institute) and IBM SPSS Statistics (version 23.0, IBM Corp.) software were used. The significance level was set to 5%.
Results
Twenty-three patients with glaucoma were enrolled (Table 1); 22 patients were followed up for 3 months (one patient did not come to the clinic after the switch). In total, 22 eyes of 22 patients were analyzed. Before switching, nine patients were using bimatoprost, nine travoprost, four latanoprost, and one tafluprost. Eye drop adherence (self-report) after switching to omidenepag isopropyl was 100% for all patients. By 3 months, there were no clinical adverse effects.
After switching to omidenepag isopropyl, DUES had improved in 12 eyes (57%; 95% CI 37–76%) by 1 month and in 16 eyes (73%; 52–87%) by 3 months. None of the remaining eight eyes exhibited worsening at 1 or 3 months (Table 2).
A comparison of the patients’ background in the DUES’ improvement group and the DUES’ unchanged group showed that the number of eye drops used at 3 months was significantly lower in the unchanged group (Table 3).
A representative patient who exhibited improvement is shown in Fig. 1. A 66-year-old woman had used travoprost OU for 8 years. After switching to omidenepag isopropyl, DUES decreased slightly after 1 month, with further noticeable improvement after 3 months. The patient reported that her dimpled eyes felt softened. In contrast, some patients did not improve, as shown in Fig. 2. A 57-year-old man had used bimatoprost OU for 10 years. It is slightly difficult to see in the photo, but DUES and upper eyelid sclerosis are observed OU. Despite switching to omidenepag isopropyl, his DUES did not show any clinically significant improvement at either 1 or 3 months.
Representative patient in whom DUES was alleviated by switching treatment to omidenepag isopropyl ophthalmic solution. This 66-year-old woman showed DUES OU with the use of travoprost ophthalmic solution (photograph a, white arrow). DUES improved after switching to omidenepag isopropyl ophthalmic solution. Photographs a–c indicate 0 months (starting point), 1 and 3 months after starting omidenepag isopropyl, respectively. DUES deepening of upper eyelid sulcus
Representative patient in whom DUES was not alleviated by switching to omidenepag isopropyl ophthalmic solution. This 57-year-old man showed DUES OU with the use of bimatoprost ophthalmic solution (photograph a, white arrow). DUES did not change after switching to omidenepag isopropyl ophthalmic solution. Photographs a–c indicate 0 months (starting point), 1 and 3 months after starting omidenepag isopropyl, respectively
The mean IOP before switching was 15.3 ± 3.3 mmHg (95% range 13.9–16.7 mmHg). After the switch, the mean IOP values were 15.6 ± 3.3 mmHg (14.1–17.0 mmHg) at 1 month and 15.5 ± 3.3 mmHg (14.1–16.9 mmHg) at 3 months (P = 1.0 at 1 month, P = 1.0 at 3 months; both adjusted by Bonferroni correction). IOP changes in each case were shown in Table 4.
Discussion
This study was a 3-month analysis of the changes in DUES after switching from an FP receptor agonists to EP2 receptor agonist (omidenepag isopropyl). The cohort included Japanese patients with glaucoma who had reported a DUES-induced decline in QOL. DUES improved in over 70% of patients at the 3-month follow-up. No adverse effects were observed, and there were no changes in IOP during the study course. The use of omidenepag isopropyl in patients suffering from DUES caused by an FP receptor agonists could be considered a therapeutic drug of choice.
DUES is associated with loss of fat in the eyelid and orbital adipose tissue, as indicated by histological [9] and orbital [21] examinations. The fat reduction is due to FP receptor-driven inhibition of adipogenesis [10]. The frequency of DUES depends on the type of FP receptor agonists [13]. When DUES becomes prominent, the skin of the upper eyelid loses its extensibility, causing difficulty in full opening. Thus, performing routine medical eye care, such as IOP measurements, becomes challenging. In such instances, a recoil tonometer (e.g., Icare® tonometer) is useful [11]. However, measurement errors with Icare® compared with Goldmann tonometry are reported [22], it is, therefore, necessary to fully understand this point before using this instrument. Furthermore, the outcomes of filtration surgery are poor in patients with DUES [12]. Overall, glaucoma management is likely to become difficult in these patients. Although some of the patients enrolled in this study had difficulty with IOP measurements when using the Goldmann applanation tonometer, the error was minimized by taking two or three measurements.
In this study, photographs were used as an objective evaluation method as adopted by previous studies [7, 13, 18, 19]. After switching to omidenepag isopropyl, 57% of eyes showed an improvement of DUES at 1 month; increasing to 73% at 3 months. A previous study demonstrates that 85% of eyes that developed DUES through the use of bimatoprost improved after 2 months when bimatoprost was changed to latanoprost [18]. Many parameters, including differences in patient background, the degree of DUES, and the number of patients may have contributed to the difference in the improvement ratio observed in the present study. Nevertheless, over 70% cases showed objective improvement.
In the comparison of patient background between the improved and unchanged groups, there were no significant items at 1 month, but the number of eye drops applied in the unchanged group was significantly lower at 3 months. This might indicate that DUES can be expected to improve regardless of the number of eye drops used. The duration of use of FP receptor agonists tended to be longer in the DUES improvement group, but the results were limited due to its small sample size.
The mean IOP before the switch to omidenepag isopropyl was 15.3 mmHg, whereas these values were 15.6 mmHg at 1 month and 15.5 mmHg at 3 months after the switch (P = 1.0). Switching from an FP receptor agonists to omidenepag isopropyl produced similar trends in patients receiving single-agent treatment and those receiving the combined treatment (data not shown). These data show that the IOP did not elevate after switching to omidenepag isopropyl. This is the most important aspect of glaucoma management and patients would be concerned about this. A previous study that observed DUES switching from bimatoprost to latanoprost revealed that the mean IOP before (13.9 ± 0.7 mmHg) and after (14.4 ± 0.6 mmHg) the switch to latanoprost did not differ significantly [18]. Taken together, these results show that changing to a prostanoid receptor agonist that does not or is unlikely to cause DUES does not change the level of IOP.
Although the study period was only 3 months, no patient discontinued treatment, which would have led to treatment dropout with ocular side effects. For example, conjunctival hyperemia, a common side effect with new administration of omidenepag isopropyl, was scarcely observed as the previous FP receptor agonists had been instilled for at least 6 months and switched without any drug holiday. Only slight hyperemia can be evident after the switch [23], when a FP receptor agonists is newly administered after previous use of another FP receptor agonists. As both eye drops are prostanoid receptor agonists, they may have a similar mechanism.
This study had several limitations. First, the number of patients recruited was relatively small. It is difficult to collect a large number of patients in a single-center for a prospective study; multicenter studies would allow a larger number of patients, but high-quality photographs presumably could not be collected by the same photographer. Furthermore, DUES does not always develop in patients receiving FP receptor agonists treatment; affected patients may not always experience a reduction of QOL. A second important limitation was that the relationship between the onset of DUES and FP receptor agonists use was not determined, because no photographs were taken when the FP receptor agonists treatment was initiated. However, the patients reported clear subjective awareness of DUES. They had complained of a related reduction in QOL, and their routine medical care (i.e., IOP measurements) had become difficult. Thus, DUES was clinically confirmed by a physician and the patient at the start of the study.
The third limitation involves the subjective nature of photographic judgment. The objective presence of DUES was clear to many patients; however, the three glaucoma specialists made judgments based on their qualitative criteria. These decisions may have varied, particularly concerning patients in whom DUES was not prominent before and after the switch. However, there is no preferred method to make quantitative decisions in the examination room; thus, we presumed that the comparison between baseline and follow-up photographs provided a more sensitive means of detecting subtle changes in the eyelid. We examined and photographed patients at approximately the same time and under identical conditions. However, a variety of factors could have affected the appearance of the upper eyelid. This represents a limitation of the current semi-quantitative measurement methods available to evaluate DUES.
Additionally, patients’ subjective experience regarding changes in DUES would be valuable but have not been gathered. Patients will be interviewed at 7 months (i.e., the conclusion of the study) and the results will be published in a second report. The final limitation concerns the duration of FP receptor agonists treatment and the glaucoma subtypes. Patients in whom DUES improved relatively rapidly may have only been using the FP receptor agonists eye drops for a short period. Similarly, patients who had used an FP receptor agonists for a long time may have exhibited smaller changes and no improvement. The duration of FP receptor agonists use was not recorded for some patients in this study. Future studies should only include patients for whom the duration of FP receptor agonists use is known. Additionally, this study consisted of Japanese patients with glaucoma; thus, the conclusions cannot be generalized to other populations.
In conclusion, changing treatment from an FP receptor agonists to omidenepag isopropyl improved DUES in 73% of Japanese glaucoma patients within 3 months. During this same period, there were no adverse effects and no changes in IOP. Given these results, patients with FP receptor agonists-induced DUES that affects patients’ QOL and/or daily medical care may benefit from switching to omidenepag isopropyl to alleviate DUES, while maintaining IOP.
References
The Japan Glaucoma Society. Glaucoma Practice Guidelines (4th edition). Nippon Ganka Gakkai Zasshi. 2018;122:5–53 (in Japanese).
Kashiwagi K, Furuya T. Persistence with topical glaucoma therapy among newly diagnosed Japanese patients. Jpn J Ophthalmol. 2014;58:68–74.
Li T, Lindsley K, Rouse B, Hong H, Shi Q, Friedman DS, et al. Comparative effectiveness of first-line medications for primary open-angle glaucoma: a systematic review and network meta-analysis. Ophthalmology. 2016;123:129–40.
Cheng JW, Cai JP, Wei RL. Meta-analysis of medical intervention for normal tension glaucoma. Ophthalmology. 2009;116:1243–9.
Alm A, Grierson I, Shields MB. Side effects associated with prostaglandin analog therapy. Surv Ophthalmol. 2008;53(Suppl 1):S93-105.
Tan P, Malhotra R. Oculoplastic considerations in patients with glaucoma. Surv Ophthalmol. 2016;61:718–25.
Aihara M, Shirato S, Sakata R. Incidence of deepening of the upper eyelid sulcus after switching from latanoprost to bimatoprost. Jpn J Ophthalmol. 2011;55:600–4.
Kucukevcilioglu M, Bayer A, Uysal Y, Altinsoy HI. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126–31.
Park J, Cho HK, Moon JI. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn J Ophthalmol. 2011;55:22–7.
Taketani Y, Yamagishi R, Fujishiro T, Igarashi M, Sakata R, Aihara M. Activation of the prostanoid FP receptor inhibits adipogenesis leading to deepening of the upper eyelid sulcus in prostaglandin-associated periorbitopathy. Invest Ophthalmol Vis Sci. 2014;55:1269–76.
Lee YK, Lee JY, Moon JI, Park MH. Effectiveness of the ICare rebound tonometer in patients with overestimated intraocular pressure due to tight orbit syndrome. Jpn J Ophthalmol. 2014;58:496–502.
Miki T, Naito T, Fujiwara M, Araki R, Kiyoi R, Shiode Y, et al. Effects of pre-surgical administration of prostaglandin analogs on the outcome of trabeculectomy. PLoS ONE. 2017;12:e0181550.
Sakata R, Shirato S, Miyata K, Aihara M. Incidence of deepening of the upper eyelid sulcus in prostaglandin-associated periorbitopathy with a latanoprost ophthalmic solution. Eye (Lond). 2014;28:1446–51.
Jayaram A, Wladis E, Akkara JD, Yen MT, Seibold LK, Burkat CN. Prostaglandin associated periorbitopathy. In: EyeWiki. American Academy of Ophthalmology. https://eyewiki.aao.org/Prostaglandin_Associated_Periorbitopathy.
Kirihara T, Taniguchi T, Yamamura K, Iwamura R, Yoneda K, Odani-Kawabata N, et al. Pharmacologic characterization of omidenepag isopropyl, a novel selective EP2 receptor agonist, as an ocular hypotensive agent. Invest Ophthalmol Vis Sci. 2018;59:145–53.
Aihara M, Lu F, Kawata H, Iwata A, Odani-Kawabata N, Shams NK. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: the phase 3 AYAME study. Am J Ophthalmol. 2020;220:53–63.
Yamamoto Y, Taniguchi T, Inazumi T, Iwamura R, Yoneda K, Odani-Kawabata N, et al. Effects of the selective EP2 receptor agonist omidenepag on adipocyte differentiation in 3T3-L1 cells. J Ocul Pharmacol Ther. 2020. https://doi.org/10.1089/jop.2019.0079.
Sakata R, Shirato S, Miyata K, Aihara M. Recovery from deepening of the upper eyelid sulcus after switching from bimatoprost to latanoprost. Jpn J Ophthalmol. 2013;57:179–84.
Sakata R, Shirato S, Miyata K, Aihara M. Incidence of deepening of the upper eyelid sulcus on treatment with a tafluprost ophthalmic solution. Jpn J Ophthalmol. 2014;58:212–7.
Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88:9–25.
Jayaprakasam A, Ghazi-Nouri S. Periorbital fat atrophy—an unfamiliar side effect of prostaglandin analogues. Orbit. 2010;29:357–9.
Nakakura S. Icare((R)) rebound tonometers: review of their characteristics and ease of use. Clin Ophthalmol. 2018;12:1245–53.
Whitson JT, Trattler WB, Matossian C, Williams J, Hollander DA. Ocular surface tolerability of prostaglandin analogs in patients with glaucoma or ocular hypertension. J Ocul Pharmacol Ther. 2010;26:287–92.
Acknowledgements
This study was funded by Santen Pharmaceutical Co., Ltd, Osaka.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
R. Sakata, None; T. Fujishiro, None; H. Saito, None; N. Nakamura, None; M. Honjo, None; S. Shirato, None; E. Miyamoto, Employee (Santen); Y. Yamada, Employee (Santen); M. Aihara, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Rei Sakata
About this article
Cite this article
Sakata, R., Fujishiro, T., Saito, H. et al. Recovery of deepening of the upper eyelid sulcus after switching from prostaglandin FP receptor agonists to EP2 receptor agonist: a 3-month prospective analysis. Jpn J Ophthalmol 65, 591–597 (2021). https://doi.org/10.1007/s10384-021-00855-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-021-00855-3