Abstract
Purpose
To clarify the efficacy of aflibercept for treating exudative age-related macular degeneration (AMD).
Methods
We prospectively studied 47 eyes with AMD. Forty-seven patients (mean age 72.2 years) received three consecutive monthly intravitreal aflibercept injections followed by an injection every 2 months until 12 months. The primary outcome was the 12-month visual results compared with baseline; the secondary outcomes were the prevalence of geography atrophy (GA), a dry macula at month 12, and anatomic changes on optical coherence tomography.
Results
The mean logarithm of the minimum angle of resolution best-corrected visual acuity (BCVA) in 27 eyes with typical AMD and 20 eyes with polypoidal choroidal vasculopathy (PCV) significantly (p < 0.0001, p < 0.05, respectively) improved from 0.60 to 0.32 at baseline to 0.29 and 0.21 at month 12. At month 12, 22 (81.5 %) eyes with typical AMD and 17 (85 %) eyes with PCV had dry macula. The subfoveal choroidal thicknesses in typical AMD and PCV decreased significantly (p < 0.0001 for both comparisons) from 241 ± 118 and 294 ± 76 μ at baseline to 198 ± 104 and 244 ± 84 μ at month 12. Progressing or new GA was seen in three eyes with typical AMD and one eye with PCV; the mean change in the BCVA was significantly (p = 0.0026) worse at month 12. No other complications developed.
Conclusion
Intravitreal aflibercept significantly improved VA and anatomic changes in typical AMD and PCV over 12 months. Development of GA might be a risk for declining VA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Exudative age-related macular degeneration (AMD) associated with choroidal neovascularization (CNV) is responsible for significant visual loss [1, 2]. Gass classified CNV into types 1 and 2 [3], and this classification is commonly used in treating patients with AMD. Major clinical trials report the superior efficacy of anti-vascular endothelial growth factor (VEGF) drugs such as ranibizumab (Lucentis, Genentech, Inc., South San Francisco, CA, USA) [4, 5], and the equally efficacious aflibercept (Eylea, Regeneron, Tarrytown, NY, USA, and Bayer, Berlin, Germany) [6].
Polypoidal choroidal vasculopathy (PCV) is known for its characteristic indocyanine green angiography (ICGA) findings [7, 8], which are highly prevalent in Asian and Caucasian patients with AMD [8–10]. In these patients, photodynamic therapy (PDT) with verteporfin (Visudyne, Novartis Pharma AG, Basel, Switzerland) seems to be more effective than anti-VEGF monotherapy using bevacizumab (Avastin, Genentech, Inc.) or ranibizumab, especially for occluding polypoidal lesions [11]. Several recent studies report the efficacy of intravitreal aflibercept injections for occluding polypoidal lesions and causing subfoveal choroidal thinning (SFCT) [12–15]. The subfoveal choroidal thickness (SFCT) might be useful in determining treatment choices. However, most of those reports are either short-term or retrospective studies.
A recent multicenter cohort study evaluates the 7-year outcomes in three types of CNV for patients with AMD, treated with Ranibizumab and labeled as ANCHOR, MARINA, and HORIZON. The study reports an overall mean decrease of 8.6 letters in the Early Treatment Diabetic Retinopathy Study (ETDRS) scores (the SEVEN-UP STUDY) [16]. The SEVEN-UP study found that macular atrophy is correlated significantly with poor visual outcomes. Moreover, the Comparison of Age-related Macular Degeneration Treatments Trials also shows that geographic atrophy (GA) is a concern in patients with AMD after treatment with anti-VEGF therapy, because GA could directly cause a decline in VA [17]. Only a Few reports deal with GA after injections of aflibercept. A prospective study of the prevalence of GA after anti-VEGF monotherapy is needed.
The purpose of the current study was to prospectively study the efficiency of aflibercept for treating patients with AMD including PCV.
Methods
The institutional review board/ethics committee of Fukushima Medical University approved this prospective nonrandomized study (Umin ID000010997, Jun 19/2013). The primary outcome was the 12-month visual outcomes compared with baseline; the secondary outcomes included the prevalence rates of GA and dry macula at month 12. After the potential risks and benefits were explained in detail, all patients provided written informed consent. Aflibercept became available for medical use in Japan in December 2012. Patients were recruited from June 2013 to December 2013.
The inclusion criteria were the presence of treatment-naive neovascular AMD with CNV under the fovea detected by fluorescein angiography (FA), patients aged older than 50 years, and informed consent for inclusion in the study. There were no limitations on the visual acuity (VA) levels or greatest linear dimensions (GLDs) of the lesions at baseline.
The exclusion criteria included previous laser photocoagulation or submacular surgery and retinal angiomatous proliferation, an axial length exceeding 26.5 mm, uncontrolled glaucoma, retinal pigment epithelial tears, GA exceeding 175 mm of linear dimension, diabetic maculopathy, retinal vascular occlusion, idiopathic macular telangiectasia, and previous treatments with PDT or intravitreal injections of ranibizumab, bevacizumab, or pegaptanib sodium (Macugen, Pfizer Canada Inc., Saint Laurent, Quebec, Canada).
All patients were treated with three consecutive monthly intravitreal injections of aflibercept followed by injections every 2 months until 12 months. We classified the patients based on the subtype of neovascular AMD, i.e., PCV or typical AMD. A clinical diagnosis of PCV was established based on the findings of the polypoidal lesions on ICGA.
We used the best-corrected VA (BCVA) measured with a Japanese standard decimal VA chart and calculated the mean BCVA using the logarithm of the minimum angle of resolution (logMAR) scale. In the discussion, we converted the decimal VA into the ETDRS VA letter scores with a mathematical method as reported previously according to the retreatment guidelines for ranibizumab in Japan [18, 19].
All patients underwent a standardized examination including slit-lamp biomicroscopy with a contact lens, fundus color photography, FA, ICGA with a fundus camera (TRC-50 FA/IA/IMAGEnet H1024 system, Topcon, Tokyo, Japan) and confocal scanning laser ophthalmoscopy [Heidelberg Retina Angiograph 2 (HRA2), Heidelberg Engineering, Heidelberg, Germany]. The central retinal thickness (CRT) and SFCT were measured at baseline and 1, 2, 3, 4, 6, 8, 10, and 12 months after treatment using spectral-domain optical coherence tomography (OCT) (Heidelberg Spectralis OCT, Heidelberg Engineering). FA was performed to determine the lesion type, location, CNV activity, and GLD of typical AMD. ICGA was performed to determine the presence and location of polypoidal lesions and branching vascular network vessels and the GLD of PCV. FA and ICGA were performed at baseline and 3 and 12 months after treatment. A dry macula was defined as the absence of subretinal and intraretinal spaces by OCT and investigated at month 12.
GA was defined as the area with increased visibility of choroidal vasculature on fundus photographs and short-wavelength autofluorescence (SW-AF) images obtained by HRA2 with hypoautofluorescence exceeding a linear dimension of 175 mm. SW-AF was performed at baseline and 3, 6, and 12 months after treatment.
Intravitreal aflibercept was injected 3.5–4.0 mm posterior to the corneal limbus into the vitreous cavity using a 30-gauge needle after topical anesthesia was applied. After three consecutive monthly intravitreal injections of aflibercept, the drug was injected every 2 months until 12 months with follow-up examinations including OCT.
Statistical analysis was performed using the Student’s t test for visual outcomes. p < 0.05 was considered significant.
Results
Forty-seven eyes of 47 Japanese patients (40 men, 7 women; age range 59–93 years; mean ± standard deviation 72.2 ± 6.4 years) with AMD were included. All patients completed 12 months of follow-up and received seven injections. Table 1 shows the baseline characteristics of and clinical data from the 47 patients (47 eyes). PCV was present on ICGA in 20 (42.6 %) eyes. The other 27 eyes were diagnosed with typical AMD, i.e., predominantly classic CNV in eight (29.6 %) eyes, minimally classic CNV in six (22.2 %) eyes, and occult with no classic CNV in 13 (48.1 %) eyes using FA. The mean GLDs of typical AMD measured by FA was 3999 μm and PCV measured by ICGA 2912 μm.
Eyes with PCV
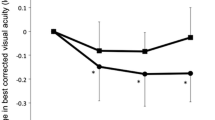
In the 20 eyes with PCV, the mean logMAR BCVA (Snellen equivalent) levels at baseline and month 12 were 0.32 (20/42) and 0.21 (20/32), indicating significant (p < 0.05) improvement after treatment (Fig. 1). At month 12, the BCVA increased in two (10 %) of the 20 eyes by three lines or more, and the VA was stable in 18 (90 %) eyes (defined as a loss of fewer than three lines of vision). The BCVA did not decrease by three or more lines in any patients during the 12 months of the study.
The changes in the mean best-corrected visual acuity (BCVA) in 27 eyes with typical age-related macular degeneration (tAMD) and 20 eyes with polypoidal choroidal vasculopathy (PCV) treated with aflibercept during the 12 months of the study. A significant improvement in the mean BCVA is seen in eyes with tAMD at months 1, 2, 3, 4, 6, 8, 10, and 12 (p = 0.021, p = 0.0061, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, respectively, paired t test) and PCV at months 3, 6, 8, 10, and 12 (p = 0.0057, p = 0.011, p = 0.017, p = 0.011, p = 0.020, respectively, paired t test). The vertical bars indicate ± 1 standard error of the mean. Months 1, 2, 3, 4, 6, 8, 10, and 12 are equivalent to 1, 2, 3, 4, 6, 8, 10, and 12 months after the initial treatment
ICGA showed complete regression of polypoidal lesions in 12 (60 %) eyes and fewer polypoidal lesions in eight (40 %) eyes at month 3. At month 12; 14 (70 %) eyes had complete regression of the polypoidal lesions and six (30 %) eyes had decreased polypoidal lesions by ICGA. No polypoidal lesions recurred during follow-up.
In the eyes with PCV, the CRT decreased significantly (p < 0.0001) from 310 ± 128 μm at baseline to 156 ± 35 μm at month 12 (Fig. 2). Nineteen of the 20 eyes had a serous retinal detachment (SRD) and one eye had both an SRD and edema at baseline. A pigment epithelial detachment (PED) was seen in seven (35 %) eyes on OCT (a serous PED in 2 eyes; a hemorrhagic PED in 5 eyes). OCT showed dry macula in 17 (85 %) of 20 eyes at month 12. In the remaining three eyes, the SRDs recurred in two eyes and there was a residual SRD in one eye. Of the three eyes without a dry macula, one eye had complete regression and two eyes had incomplete regression of the polypoidal lesions by ICGA. OCT showed that the PED resolved in five of the seven eyes, and two eyes had a persistent hemorrhagic PED at month 12. The SFCT decreased significantly (p < 0.0001) from 294 ± 76 μ at baseline to 244 ± 84 μm at month 12 (Fig. 3). Figures 4 and 5 show a typical case of PCV treated with aflibercept.
The changes in the central retinal thickness (CRT) in 27 eyes with typical age-related macular degeneration (tAMD) and 20 eyes with polypoidal choroidal vasculopathy (PCV) treated with aflibercept during the 12 months of the study. A significant decrease in the CRT is seen in eyes with tAMD at months 1, 2, 3, 4, 6, 8, 10, and 12 (p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, respectively, paired t test) and PCV at months 1, 2, 3, 4, 6, 8, 10, and 12 (p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, respectively, paired t test). The vertical bars indicate ± 1 standard error of the mean. Months 1, 2, 3, 4, 6, 8, 10, and 12 are equivalent to 1, 2, 3, 4, 6, 8, 10, and 12 months after the initial treatment
The changes in the subfoveal choroidal thickness (SFCT) in 27 eyes with typical age-related macular degeneration (tAMD) and 20 eyes with polypoidal choroidal vasculopathy (PCV) treated with aflibercept during the 12 months of the study. Significant decreases in the SFCT are seen in eyes with tAMD at months 1, 2, 3, 4, 6, 8, 10, and 12 (p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.001, p < 0.0001, respectively, paired t test) and PCV at months 1, 2, 3, 4, 6, 8, 10, and 12 (p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.0001, p < 0.001, p < 0.0001, respectively, paired t test). The vertical bars indicate ± 1 standard error of the mean. Months 1, 2, 3, 4, 6, 8, 10, and 12 are equivalent to 1, 2, 3, 4, 6, 8, 10, and 12 months after the initial treatment
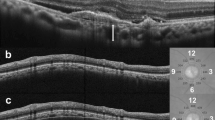
A 70-year-old man with polypoidal choroidal vasculopathy treated with aflibercept. At baseline, the best-corrected visual acuity (VA) is 0.70 logarithm of the minimum angle of resolution VA (Snellen equivalent; 20/100). a A color fundus photograph shows a serous retinal detachment (SRD), grayish-white subretinal fibrinous exudate with subretinal hemorrhage and lipid. b Fluorescein angiography shows occult with no classic choroidal neovascularization. c Early-phase and d late-phase indocyanine green angiography images clearly show polypoidal lesions (arrows). Horizontal (e) and vertical (f) optical coherence tomography images show an SRD, protrusion of the highly reflective retinal pigment epithelium line corresponding to the polypoidal lesions, and highly reflective area corresponding to the fibrinous exudate
Twelve months after intravitreal aflibercept injections in the same case described in Fig. 4 Seven injections of aflibercept were administered due to proactive treatment with three consecutive monthly followed by intravitreal injections every 2 months. a At month 12, the best-corrected visual acuity (VA) improved to 0.05 70 logarithm of the minimum angle of resolution VA (Snellen equivalent; 20/22). A color fundus photograph shows resolution of a serous retinal detachment (SRD), fibrinous exudate, subretinal hemorrhage, and lipid. b A fluorescein angiography image shows regression of leakage at the macular area. c An early-phase indocyanine green angiography image shows complete regression of the polypoidal lesions. d A fundus autofluorescence image shows no geographic atrophy. Horizontal (e) and vertical (f) optical coherence tomography images show resolution of the SRD
Eyes with typical AMD
In the 27 eyes with typical AMD, the mean logMAR BCVA level (Snellen equivalent) improved significantly (p < 0.0001) from 0.60 (20/80) at baseline to 0.29 (20/39) at month 12 (Fig. 1). Fourteen (51.9 %) eyes had a three-line or greater increase in BCVA, and 13 (48.1 %) eyes had stable VA (defined as a loss or gain within three lines of vision) at month 12. The BCVA did not decrease by three or more lines in any patient over the 12 months of the study.
The CRT decreased significantly (p < 0.0001) from 385 ± 158 μm at baseline to 161 ± 84 μm at month 12 (Fig. 2). At baseline, an SRD developed in 19 of the 27 eyes, cystoid macular edema in two eyes, and both SRD and edema in six eyes. OCT showed a fibrovascular PED in four (14.8 %) eyes. At month 12, 22 (81.5 %) eyes had a dry macula by OCT. In the remaining five eyes, three had a recurrent SRD, one eye had recurrent macular edema, and one eye had both a recurrent SRD and residual macular edema. OCT showed resolved fibrovascular PEDs in two eyes and residual PEDs in two eyes at month 12. The SFCT at month 12 was 198 ± 104 μm, which was significantly (p < 0.0001) thinner compared with the baseline value of 241 ± 118 μm (Fig. 3). Figures 6 and 7 show a case of typical AMD treated with aflibercept.
An 87-year-old woman with typical age-related macular degeneration treated with aflibercept. At baseline, the best-corrected visual acuity (VA) is 0.82 70 logarithm of the minimum angle of resolution VA (Snellen equivalent; 20/133). a A color fundus photograph shows a subretinal hemorrhage, edema, and drusen at the macular area. b A fluorescein angiography image shows minimally classic choroidal neovascularization. c An early-phase indocyanine green angiography image shows no polypoidal lesions. d A fundus autofluorescence image shows no geographic atrophy. Horizontal (e) and vertical (f) optical coherence tomography images show a large area of cystoid macular edema
Twelve months after intravitreal aflibercept injections in the same case described in Fig. 6. Seven injections of aflibercept were administered with three consecutive monthly followed by intravitreal injections every 2 months. a At month 12, the best-corrected visual acuity (VA) declined to 1.05 70 logarithm of the minimum angle of resolution (logMAR) VA (Snellen equivalent; 20/222). A color fundus photograph shows geographic atrophy (GA) involving the fovea (arrows). b A fluorescein angiography image shows decreased leakage at the macular area. c An early-phase indocyanine green angiography image shows no polypoidal lesions. d A fundus autofluorescence image shows GA as hypoautofluorescence (arrows). Horizontal (e) and vertical (f) optical coherence tomography images show resolution of the cystoid macular edema
GA detected by SW-AF was seen in three eyes with typical AMD and one eye with PCV at baseline. At month 12, progressing or new GA was seen in three (11.1 %) eyes with typical AMD (Fig. 7) and one (5 %) eye with PCV. In these four eyes, the mean change in the BCVA at month 12 (−1.31 lines) was significantly (p = 0.0026, Mann–Whitney U test) worse compared with the 43 eyes without progressing or developing GA (2.62 lines).
No complications developed, such as unexpected increasing subretinal hemorrhages (>1 disc diameter), RPE tears, ocular inflammation, increased intraocular pressure over 21 mmHg, severe visual loss, endophthalmitis, progression of cataract, or systemic adverse events.
Discussion
The current study showed that intravitreal aflibercept injections significantly improved the VA and there were positive anatomic changes in patients with both typical AMD and PCV during the 12 months of the study. However, ophthalmologists should be aware that there is a risk of declining VA with progressing or new GA.
The VEGF Trap-Eye: investigation of efficacy and safety in Wet AMD (VIEW 1 and VIEW 2) studies report equal efficaciousness of ranibizumab [6]. However, no major studies report the efficacy for patients with PCV. Oishi et al. report the efficacy of aflibercept in 98 patients with AMD and 42 patients with PCV during 12 months who received injections once monthly for 3 months followed by once every 2 months for 8 months [20]. In that study, 27 (73 %) of 37 patients had complete regression of polypoidal lesions with improvement of VA at month 12. In the current study, ICGA showed that the polypoidal lesions completely regressed in 14 (70 %) of 20 eyes and decreased in six (30 %) eyes with BCVA improvement of 1.49 lines, which was equivalent to 7.5 letters on the ETDRS chart. The Efficacy and Safety of Verteporfin Photodynamic Therapy in Combination with Ranibizumab or Alone Versus Ranibizumab Monotherapy in Patients with Symptomatic Macular Polypoidal Choroidal Vasculopathy (EVEREST) study reports that ranibizumab monotherapy, PDT monotherapy, and ranibizumab plus PDT groups improved 9.2, 7.5, and 10.9 letters, respectively, at month 6 [11]. Although the EVEREST study had a 6-month follow-up, aflibercept monotherapy for PCV in the current study had almost equivalent efficacy for VA as the EVEREST study. The EVEREST study also showed that the polypoidal lesions regressed completely in six (28.6 %) eyes in the ranibizumab monotherapy group [11]. Aflibercept might have a high probability of achieving complete regression of polypoidal lesions compared with the previously used anti-VEGF drugs [12–15, 21]. The mechanism of aflibercept’s high success in occluding polypoidal lesions compared with previously used anti-VEGF agents such as bevacizumab or ranibizumab is still in question. We reported a significant decrease in the SFCT with complete regression of polyps (50 and 56.5 %) in PCV eyes refractory to ranibizumab with 3 and 6 months of follow-up [13, 21]. In our previous study we speculated that that might have resulted from the powerful VEGF-binding affinity of aflibercept and the promotion of thrombus formation [13]. A third possible reason is that aflibercept may curb the CNV near or under the retinal pigment epithelium (RPE). One study claims that in monkey eyes aflibercept was being taken up by the RPE [22]; if that is correct, the same medication may generate greater occlusion of polypoidal lesions compared with ranibizumab [21]. Moreover, complications or systemic adverse events did not occur in the current study. Anti-VEGF therapy tends to cause fewer adverse events such as unexpected increases in subretinal hemorrhages compared with PDT monotherapy, indicating that aflibercept monotherapy might be the first treatment option for patients with PCV.
Of the 27 current eyes with typical AMD, the BCVA 12 months from baseline improved a mean of 3.14 lines, which was equivalent to 15.5 letters on the ETDRS chart. The VIEW 1 and 2 studies report that the group treated with injections every 8 weeks gained 7.9 and 8.9 letters, respectively, at week 52 [6]. Although the exact reason why the current study found more gains in ETDRS letters than the VIEW studies is unknown, it might depend on the characteristics’ differences of Japanese and Caucasian patients.
The presence of a serous PED in eyes with AMD is a risk factor for severe and irreversible visual loss [23]. In addition, we reported that PDT with verteporfin was less successful for treating occult CNV associated with a serous PED [24]. Recently, Cho et al. reported that serous PEDs had a significantly (p = 0.022) higher chance of resolution compared with fibrovascular PEDs after anti-VEGF treatment during 12 months [25]. In the current study, four eyes had a fibrovascular PED at baseline that resolved in two eyes; in the other two eyes, the PED was unchanged at month 12 with improvement in the VA by a mean of 1.52 lines. Intravitreal aflibercept may be effective for such intractable cases that have undergone previous treatments.
GA is reported to increase significantly with age and was present in 3.5 % of people aged 75 years and older in the US [26]. Although the GA enlarges annually in eyes with AMD during the natural course [27], monthly or frequent injections of anti-VEGF agents could cause enlargement of the GA. Kuroda et al. report that RPE atrophy developed in 5.4 % of eyes with neovascular AMD during 26.7 months of ranibizumab treatment [28]. In the current study, the GA progressed or newly developed at month 12 in three (11.1 %) eyes with typical AMD and one (5 %) eye with PCV. The mean change in the BCVA of the four eyes with progressing or new GA at month 12 was a decrease of 1.31 lines, which was significantly worse compared with the 2.62-line improvement in the remaining 43 eyes without progressing or new GA. Therefore, ophthalmologists should be alert to the progression or development of GA during follow-up because of the risk for decreasing VA. Long-term, prospective, randomized studies are needed to compare ranibizumab and aflibercept for progression of GA.
The limitation in the current study was the small sample size. Large, long-term, prospective, randomized studies are needed to confirm the current results.
In conclusion, the current study showed that intravitreal aflibercept injections significantly improved the VA and the anatomic changes in patients with both typical AMD and PCV during the 12 months of the study. However, it is important to be aware of the presence of progressing or new GA because of the risk of declining VA.
References
Ferris FL 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–2.
Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1220–31.
Gass JD. Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am J Ophthalmol. 1994;118:285–98.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, MARINA Study Group, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, et al. ANCHOR Study Group Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, VIEW 1 and VIEW 2 Study Groups, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48.
Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10:1–8.
Imamura Y, Engelbert M, Iida T, Freund KB, Yannuzzi LA. Polypoidal choroidal vasculopathy: a review. Surv Ophthalmol. 2010;55:501–15.
Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol. 2007;144:15–22.
Lafaut BA, Leys AM, Snyers B, Rasquin F, De Laey JJ. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000;238:752–9.
Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, et al. EVEREST Study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–64.
Inoue M, Arakawa A, Yamane S, Kadonosono K. Short-term efficacy of intravitreal aflibercept in treatment-naive patients with polypoidal choroidal vasculopathy. Retina. 2014;34:2178–84.
Saito M, Kano M, Itagaki K, Oguchi Y, Sekiryu T. Switching to intravitreal aflibercept injection for polypoidal choroidal vasculopathy refractory to ranibizumab. Retina. 2014;34:2192–201.
Koizumi H, Kano M, Yamamoto A, Saito M, Maruko I, Sekiryu T, et al. Aflibercept therapy for polypoidal choroidal vasculopathy: short-term results of a multicentre study. Br J Ophthalmol. 2015;99:1284–8.
Yamamoto A, Okada AA, Kano M, Koizumi H, Saito M, Maruko I, et al. Intravitreal aflibercept for polypoidal choroidal vasculopathy. Ophthalmology. 2015;122:1866–72.
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120:2292–9.
Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG, Maguire MG, Fine SL, Ying GS, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98.
Tano Y, Ohji M, Ishibashi T, Shiraga F, Tokoro T, Yuzawa M, et al. Re-treatment guideline of ranibizumab (genetical recombination) in the maintenance phase. Nippon Ganka Gakkai Zasshi. 2009;113:1098–103 (In Japanese).
Saito M, Iida T, Kano M. Intravitreal ranibizumab for exudative age-related macular degeneration with good baseline visual acuity. Retina. 2012;32:1250–9.
Oishi A, Tsujikawa A, Yamashiro K, Ooto S, Tamura H, Nakanishi H, et al. One year result of aflibercept treatment on age-related macular degeneration and predictive factors for visual outcome. Am J Ophthalmol. 2015;159:853–60.
Saito M, Kano M, Itagaki K, Ise S, Imaizumi K, Sekiryu T. subfoveal choroidal thickness in polypoidal choroidal vasculopathy after switching to intravitreal aflibercept injection. Jpn J Ophthalmol. 2016;60:35–41.
Julien S, Biesemeier A, Taubitz T, Schraermeyer U. Different effects of intravitreally injected ranibizumab and aflibercept on retinal and choroidal tissues of monkey eyes. Br J Ophthalmol. 2014;98:813–25.
Elman MJ, Fine SL, Murphy RP, Patz A, Auer C. The natural history of serous retinal pigment epithelium detachment in patients with age-related macular degeneration. Ophthalmology. 1986;93:224–30.
Saito M, Iida T, Nagayama D. Photodynamic therapy with verteporfin for age-related macular degeneration or polypoidal choroidal vasculopathy: comparison of the presence of serous retinal pigment epithelial detachment. Br J Ophthalmol. 2008;92:1642–7.
Cho HJ, Kim KM, Kim HS, Lee DW, Kim CG, Kim JW. Response of pigment epithelial detachment to anti-vascular endothelial growth factor treatment in age-related macular degeneration. Am J Ophthalmol. 2016;166:112–9 (Epub ahead of print).
Klein R, Klein BE, Franke T. The relationship of cardiovascular disease and its risk factors to age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1993;100:406–14.
Sunness JS, Margalit E, Srikumaran D, Applegate CA, Tian Y, Perry D, et al. The long-term natural history of geographic atrophy from age-related macular degeneration: enlargement of atrophy and implications for interventional clinical trials. Ophthalmology. 2007;114:271–7.
Kuroda Y, Yamashiro K, Tsujikawa A, Ooto S, Tamura H, Oishi A, et al. Retinal pigment epithelial atrophy in neovascular age-related macular degeneration after ranibizumab treatment. Am J Ophthalmol. 2016;161(94–103):e1.
Conflicts of interest
M. Saito, Lecture fees (Bayer, HOYA, Novartis, Santen, Senju); M. Kano, None; K. Itagaki, None; T. Sekiryu, Grant (Bayer, Novartis).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Saito, M., Kano, M., Itagaki, K. et al. Efficacy of intravitreal aflibercept in Japanese patients with exudative age-related macular degeneration. Jpn J Ophthalmol 61, 74–83 (2017). https://doi.org/10.1007/s10384-016-0478-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-016-0478-5