Abstract
Purpose
We aimed to compare changes in subfoveal and peripapillary choroidal thickness (CT) after intravitreal aflibercept or ranibizumab injections for neovascular age-related macular degeneration (AMD).
Methods
Medical records of 54 treatment-naïve, consecutive patients (54 eyes) who were diagnosed with neovascular AMD and received three monthly injections of aflibercept (21 eyes) or ranibizumab (33 eyes) were reviewed. Subfoveal and peripapillary CT were measured with images obtained using spectral domain optical coherence tomography at baseline and at three months.
Results
Subfoveal CT decreased from 232.2 ± 94.4 μm at baseline to 207.1 ± 89.3 μm at three months in the aflibercept group (p < 0.001) and from 231.5 ± 102.9 μm to 220.0 ± 98.0 μm in the ranibizumab group (p = 0.006). The reduction was greater in the aflibercept group than in the ranibizumab group (p = 0.024). Peripapillary CT decreased from 157.2 ± 62.2 μm at baseline to 147.4 ± 62.2 μm at three months in the aflibercept group (p < 0.001). However, the change in peripapillary CT from 154.9 ± 46.5 μm at baseline to 152.3 ± 50.0 μm at three months was not significant in the ranibizumab group (p = 0.123).
Conclusions
Intravitreally injected aflibercept significantly decreased subfoveal CT more than ranibizumab. Choroidal thinning after aflibercept injection was not limited to the subfoveal area, but extended beyond the macula as well.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neovascular age-related macular degeneration (AMD) is a leading cause of visual impairment worldwide [1]. It is associated with choroidal neovascularization (CNV), and many kinds of treatments have been used to inhibit exudation associated with CNV [1–5]. Recently, intravitreal injections of anti-vascular endothelial growth factor (VEGF) agents such as ranibizumab (Lucentis; Genentech, South San Francisco, CA) and aflibercept (Eylea; Regeneron, Tarrytown, New York, USA; and Bayer HealthCare, Berlin, Germany) have been widely used to treat neovascular AMD [2–5]. Both drugs efficiently resolve the exudation associated with CNV and improve visual acuity in eyes with neovascular AMD [2–5]. Despite their similar clinical effects, both drugs have different structures, half-lives, binding affinities and biological activities [4, 6, 7].
VEGF has been suggested to be an important regulator of the pathologic angiogenesis and neovascularization associated with neovascular AMD [8]. Thus, targeted inhibition of VEGF has been attempted through the use of anti-VEGF agents; however, in the choroid, a highly vascular tissue that supplies blood to the retinal pigment epithelium (RPE) and outer retina, VEGF is associated with maintenance of choroidal capillaries and blood flow [9–11]. For these reasons, there have been concerns about the effect of intravitreally injected anti-VEGF drugs on the choroid [12, 13]. Recent studies reported decreased subfoveal choroidal thickness (CT) after intravitreal injection of ranibizumab or aflibercept in eyes with neovascular AMD [14–19]. It was suggested that intravitreally injected anti-VEGF drugs have a pharmacologic effect not only on neovascular tissue, but also on the underlying choroid [17, 19]. However, these studies only analyzed the choroid of the macular region, and it was unclear whether normal choroid outside of the macula without overlying retinal pathology is affected by intravitreal anti-VEGF injection.
Peripapillary choroid, which surrounds the optic disc, has a different blood supply from the macula because the choroid is supplied by various ciliary arteries and has separate, segmental distributions without anastomoses between the supplying arteries [20]. Recently, peripapillary choroid has been investigated in the context of several retinal diseases [21–23]. In these studies, the nasal peripapillary choroid was defined as the choroid outside of the macula [22, 23]. In the current study, we defined the nasal peripapillary choroid as the choroid outside of the macula, and we investigated short-term changes of subfoveal and peripapillary CT after three consecutive monthly intravitreal ranibizumab or aflibercept injections [24].
Materials and methods
This study was approved by the institutional review board of Korea University Medical Centre. All research and data collection followed the tenets of the Declaration of Helsinki. Medical records of patients who were diagnosed with neovascular AMD between January 2013 and June 2015 were reviewed retrospectively. We included patients who underwent three monthly intravitreal injections of aflibercept or ranibizumab for neovascular AMD. After the introduction of aflibercept in Korea, all treatment-naïve patients in our clinic received aflibercept as a first line treatment for neovascular AMD from July 2014 and these patients were categorized as the aflibercept group. The ranibizumab group consisted of the patients who underwent intravitreal injections of ranibizumab before July 2014.
This study included only treatment-naïve neovascular AMD patients 50 years of age or older who showed exudative changes from CNV on fluorescein angiography and spectral domain-optical coherence tomography (SD-OCT). This study excluded cases of polypoidal choroidal vasculopathy (PCV), which was diagnosed if the patient showed CNV on fluorescein angiography and if any one of the following criteria were met: 1) a branching network visible underneath the RPE or focal hyperfluorescent polyps visible in areas of the branching network with indocyanine green angiography, 2) the presence of an elevated, reddish-orange lesion protruding from the choroid on fundoscopic examination or 3) the existence of recurrent hemorrhagic or serous pigment epithelial detachment [25–28]. We classified neovascular lesion types into three categories, type 1 (sub-RPE), type 2 (subretinal) and type 3 (intraretinal), according to the macular photocoagulation study group and the guidelines proposed by Freund based on angiographic and OCT features [29–31]. Classification was performed by two retinal specialists (C.Y. and J.A.). In cases with disagreement, the case was reviewed by the two observers and a final decision was made by consensus.
Cases of high myopia (axial length ≥ 26.0 mm or refractive errors ≥ -6.0 diopters), glaucoma, optic nerve disorder and a history of refractive surgery or cataract surgery within the past six months were excluded from this study. Patients with a history of vitreoretinal surgery, retinal and choroidal disorders, including vascular disease or uveitis and previous treatment with photodynamic therapy or other intravitreal anti-VEGF injections were excluded from this study. Data were collected at baseline and three months after monthly intravitreal injections of aflibercept or ranibizumab.
Spectral domain optical coherence tomography
We used SD-OCT (3D OCT-1000 Mark II, software version 6.21;Topcon Corp., Tokyo, Japan) with a wavelength of 840 nm, a horizontal resolution ≤20 μm and an axial resolution of up to 5 μm. We obtained 6-mm horizontal line-scan images centered on the fovea and 3.4-mm circle scan images centered on the optic disc. In our retina clinic, we usually perform OCT with a 6-mm-zone macula scan and an additional 3.4-mm-diameter circle scan of the optic disc to detect comorbid diseases of optic nerve such as glaucoma [32]. The choroidal mode was used to obtain choroidal layer images. The line scan and circle scan consisted of 1024 A-scans, and circle scan images were scanned four times. All images were averaged to improve the signal-to-noise ratio. Images were excluded if they were low quality with a signal strength indicator (Q-factor) value < 45 or if the choroidal layer was not visible due to media opacities or severe subfoveal hemorrhages.
Measurement of subfoveal and peripapillary choroidal thickness
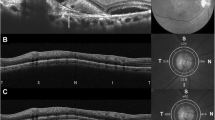
Line-scan images obtained using the choroidal mode of the OCT were used to measure subfoveal CT (Fig. 1). Using a caliper tool in the OCT image viewer program, the CT was measured manually. The CT was defined as the perpendicular distance between the RPE and the inner margin of the chorioscleral junction [33]. If a hyporeflective layer representing a suprachoroidal layer was observed on OCT, it was not included in CT [34].
Measurement of subfoveal choroidal thickness (CT) and peripapillary CT. (a) Subfoveal CT was measured perpendicularly at the fovea from the Bruch’s membrane to the chorioretinal interface. (b) Peripapillary CT was calculated via retinal nerve fiber layer (RNFL) scan around the optic disc. Retinal thicknesses (RT) between the internal limiting membrane and retinal pigment epithelium RPE) at all 12 sectors around the optic disc were obtained. (c) Using the software modification tool, the segmentation line indicating the RPE was moved to the chorioscleral junction. Then, the chorioretinal thickness at all 12 sectors was obtained. Peripapillary CT at each sector was obtained by subtracting the RT from chorioretinal thickness. Each sector was numbered clockwise from 1 to 12 o’clock, in the right eye and counter clockwise in the left eye. The 9 o’clock sector in both eyes corresponded to the temporal peripapillary area. Nasal and temporal peripapillary choroids were defined from sectors 1 to 5 and from sectors 7 to 11, respectively
A 360° 3.4-mm-diameter circle scan obtained using the standard protocol for retinal nerve fiber layer (RNFL) assessment was used to analyze the peripapillary choroid. Circle scans provide values of 12 sectors surrounding the optic disc (Fig. 1). Peripapillary CT was measured by a previously reported method [22, 23, 35] First, the retinal thickness of each sector was obtained, and by using a modification tool in the OCT image viewer program, the segmentation line indicating RPE was modified and moved to the chorioscleral junction by the examiner. With this modification, we obtained chorioretinal thickness. Peripapillary CT was subsequently obtained by subtracting retinal thickness from chorioretinal thickness. Nasal and temporal peripapillary choroids were defined from sectors 1 to 5 and from sectors 7 to 11, respectively. All measurements were performed by two independent examiners (C.Y. and J.A.) who were blinded to patient information. The mean of values from two examiners are used for analysis, and peripapillary CT was measured without access to information about macula.
Statistical methods
Normal distribution of all continuous parameters was verified using the Kolmogorov-Smirnov test. The parameters of the aflibercept group and the ranibizumab group were compared using chi-square tests for categorical variables and independent t-tests for continuous variables. Paired t-test was used to analyze the change in peripapillary and macular CT at each visit. The proportional change in CT was calculated by comparing CT before and after intravitreal injection, and it was defined as the difference in CT (baseline CT – CT at three months) divided by baseline CT. The best-corrected visual acuity (BCVA) was converted to the logarithm of the minimum angle of resolution (logMAR). Statistical analyses were performed using SPSS software version 20.0 for Windows (IBM Corp., Armonk, NY, USA). Results with p values < 0.05 were considered statistically significant. If the patients had neovascular AMD in both eyes, the right eye was chosen for analysis. If the right eye did not meet the inclusion criteria or was inadequate for analysis, the left eye was chosen.
Results
Fifty-four eyes of 54 patients with neovascular AMD were included in this study. Twenty-one and 33 patients received intravitreal injections of aflibercept and ranibizumab, respectively. The mean age of the aflibercept group was 72.1 ± 8.1 and did not differ significantly from that of the ranibizumab group (70.0 ± 7.4, p = 0.331). The aflibercept group consisted of 11 males and 10 females, and the ranibizumab group consisted of 15 males and 18 females. There was no statistically significant difference in group composition (p = 0.414). The ranibizumab group included 18 patients with hypertension (54.5 %) and seven patients (26.9 %) with diabetes. The aflibercept group included 13 patients with hypertension (61.9 %) and four patients (23.5 %) with diabetes. There were no differences in the prevalence of hypertension or diabetes between the two groups (p = 0.778 and p = 1.000, respectively). Neovascular lesions were identified in 19 eyes with type 2 lesions and two eyes with type 3 lesions in the aflibercept group and 30 eyes with type 2 lesions and three eyes with type 3 lesions in the ranibizumab group. There were no differences in composition between the two groups (p = 1.000). The mean BCVA (LogMAR) of the aflibercept and ranibizumab groups was 0.41 ± 0.29 and 0.66 ± 0.37 at baseline, respectively, and the mean BCVA of the aflibercept group was significantly better than that of the ranibizumab group (p = 0.017). The mean BCVA (LogMAR) of the aflibercept and ranibizumab groups improved to 0.23 ± 0.22 and 0.49 ± 0.39, respectively, after three monthly intravitreal injections (p = 0.001 and p = 0.004, respectively). The median disease duration of the aflibercept group (median, 30 days, interquartile range, 10 to 150 days) did not vary significantly from that of ranibizumab group (median, 30 days; interquartile range, 14.5 to 90 days, p = 0.924).
Subfoveal choroidal thickness after intravitreal aflibercept and ranibizumab injections
The mean subfoveal CTs of the aflibercept and ranibizumab groups were 232.2 ± 94.4 μm and 231.5 ± 102.9 μm, respectively, at baseline, and they were not significantly different (p = 0.981). After three monthly doses, the subfoveal CT of the aflibercept group decreased to 207.1 ± 89.3 μm (p < 0.001), and that of the ranibizumab group decreased to 220.0 ± 98.0 μm (p = 0.006) (Fig. 2) (Additional data for the results on changes in CT are given in Online Resource 1). The decrease in both the amount and proportion of subfoveal CT was greater in the aflibercept group than in the ranibizumab group (Table 1). Changes in subfoveal CT after intravitreal injection in the two groups did not vary according to the presence of hypertension and diabetes (all p > 0.05).
Changes in subfoveal choroidal thickness and peripapillary choroidal thickness before and after intravitreal aflibercept and ranibizumab injections. (a) The aflibercept group showed decreased CT at both the subfoveal and peripapillary areas. (b) The ranibizumab group showed decreased CT at the subfovea, but no statistically significant changes in peripapillary areas were observed
Peripapillary choroidal thickness after intravitreal aflibercept and ranibizumab injections
The mean peripapillary CTs of the aflibercept and ranibizumab groups at baseline were 157.2 ± 62.2 μm and 154.9 ± 46.5 μm, respectively, and they were not significantly different (p = 0.883). The mean nasal and temporal peripapillary CTs of the aflibercept group were 152.4 ± 59.4 μm and 168.6 ± 69.4 μm, respectively, and they were not significantly different from those of the ranibizumab group (150.4 ± 44.1 μm 165.3 ± 53.0 μm, p = 0.895 and p = 0.850, respectively). After three monthly intravitreal injections, the mean peripapillary CT of the aflibercept group decreased to 147.4 ± 62.2 μm (p < 0.001), and that of the ranibizumab group decreased to 152.3 ± 50.0 μm. However, the change in the ranibizumab group was not statistically significant (p = 0.123) (Fig. 2) (Additional data for the results on changes in CT are given in Online Resource 1). The decrease in both the amount and proportion of mean peripapillary CT in the aflibercept group was greater than in the ranibizumab group (Table 1). Changes in mean peripapillary CT after intravitreal injection did not vary according to the presence of hypertension or diabetes in either group (all p > 0.05).
The mean nasal and temporal peripapillary CTs of the aflibercept group at baseline decreased to 144.0 ± 60.6 μm and 157.1 ± 67.9 μm, respectively, after treatment (p = 0.002 and p < 0.001, respectively). In addition, the mean nasal and temporal peripapillary CTs of the ranibizumab group at baseline also decreased to 149.7 ± 48.3 μm and 161.1 ± 55.4 μm, respectively, after treatment; however, these changes were not statistically significant (p = 0.660 and p = 0.066, respectively) (Fig. 2) (Additional data for the results on changes in CT are given in Online Resource 1). The decrease in both the amount and proportion of mean nasal peripapillary CT in the aflibercept group was greater than in the ranibizumab group (p = 0.011 and p = 0.021, respectively) (Table 1).
All sectors of peripapillary CT decreased in the aflibercept group (all, p < 0.05), but peripapillary CT decreased only at 8 and 9 o’clock in the ranibizumab group (p = 0.037 and p = 0.038, respectively) (Fig. 3) (Additional data for the results on changes in peripapillary CT are given in Online Resource 2). Representative cases are shown in Figs. 4 and 5.
Changes in peripapillary choroidal thickness (CT) at each sector. (a) Peripapillary CT at all sectors decreased significantly after three monthly injections of aflibercept. (b) Nasal peripapillary CT did not change after three monthly injections of ranibizumab, while the 8 and 9 o’clock areas decreased in thickness. *p < 0.05 with paired t-test
Representative case of a 67-year-old female patient with neovascular AMD treated with aflibercept. (a and b) Subfoveal choroidal thickness (CT) at baseline was 184 μm and decreased to 153 μm after three monthly injections. (c and d) Mean peripapillary CT at baseline was 200 μm and decreased to 180 μm after treatment. T, temporal; S, superior; I, inferior; N, nasal
Representative case of a 74-year-old female patient with neovascular AMD treated with ranibizumab. (a and b) Subfoveal choroidal thickness (CT) at baseline was 222 μm and decreased to 207 μm after three monthly injections. (c and d) Mean peripapillary CT at baseline was 170 μm and 164 μm at three months. T, temporal; S, superior; I, inferior; N, nasal
Interobserver reliability
Intraclass correlation coefficients were analyzed to assess interobserver reliability (Additional data for the results on interobserver reliability measurements are given in Online Resource 3). The intraclass correlation coefficients ranged from 0.911 to 0.981 with good agreement.
Discussion
In this study, we demonstrated that subfoveal CT decreased in eyes with neovascular AMD after intravitreal aflibercept or ranibizumab injections. However, mean peripapillary CT and nasal peripapillary CT, which is the choroid outside of the macula, only decreased in the aflibercept group. To our knowledge, changes in CT outside of the macula following intravitreal injections of anti-VEGF drugs have not been reported.
Subfoveal CT decreased in both the aflibercept and ranibizumab groups. The aflibercept group showed a greater decrease in both the amount and proportion of CT after treatment than the ranibizumab group. Some previous studies reported subfoveal CT changes after intravitreal injection of aflibercept or ranibizumab in eyes with neovascular AMD, and this treatment using anti-VEGF drugs was suggested to affect not only on the CNV, but also the underlying choroid [16–19]. According to the studies of Yamazaki and Koizumi, the mean subfoveal CT after three monthly injections of ranibizumab or aflibercept decreased about 18 μm (7.4 %) and 34.1 μm (13.5 %), respectively, when compared to the baseline CT [17, 19]. Subfoveal CT decreased by a greater amount after treatment with aflibercept compared to ranibizumab. In addition, Hata et al. compared the change in CT after ranibizumab and aflibercept treatments, and they also reported that aflibercept-treated eyes showed a greater decrease in subfoveal CT (25.1 ± 25.6 μm) than the ranibizumab-treated eyes (5.2 ± 16.3 μm) [16]. In the current study, the subfoveal CT of the aflibercept group decreased by 25.1 μm and 12.1 %, while the subfoveal CT of the ranibizumab group decreased by 11.6 μm and 5.4 %. These results were similar to those of previous studies, and it seems that aflibercept had a greater effect on subfoveal CT than ranibizumab.
Unlike subfoveal CT, peripapillary CT showed different changes according to the treatment drug. After three monthly doses, the aflibercept group showed a significantly decreased mean peripapillary CT, while the ranibizumab group did not. On subanalysis, nasal peripapillary CT decreased significantly in the aflibercept group, but the ranibizumab group did not show any changes in nasal peripapillary CT. Temporal peripapillary CT also showed a similar tendency. The aflibercet group showed a significant decrease in temporal peripapillary CT, but the ranibizumab group did not. The ranibizumab group also showed a slight decreasing trend in temporal peripapillary CT with borderline statistical significance. This might be because the temporal peripapillary choroid is located near the fovea, and it might also undergo choroidal change with the macular choroid after treatment with anti-VEGF drugs. Subanalysis of peripapillary CT also revealed these patterns. The aflibercept group showed decreased peripapillary CT in all 12 sectors, while the ranibizumab group showed decreased peripapillary CT only at the 8 and 9 o’clock sectors. This might suggest that aflibercept affects not only the macular choroid, but also the choroid outside of the macula.
Previous studies suggest that CT changes might be associated with the decreased permeability of the choroid or with the vasoconstrictive effect of anti-VEGF drugs [17, 19]. This might be a common effect of both drugs on the macular choroid. However, results of nasal peripapillary CT in this study, which is outside of the macula, were different between the two groups. These differing results might come from the different characteristics of the two drugs. Ranibizumab works by blocking the receptor-binding domain of all isoforms of VEGF-A, while aflibercept binds to all VEGF-A isoforms, VEGF-B and placental growth factor (PlGF) [3, 36]. Additionally, aflibercept is known to have a higher affinity for VEGF, a longer half-life and a greater lowering effect on systemic VEGF than ranibizumab [6, 7, 36, 37]. VEGF is associated with vascular hyperpermeability and exudates from CNV in pathologic conditions [5, 6, 38]. Eyes with neovascular AMD have increased intraocular VEGF levels, and increased VEGF might affect the overall choroid [39, 40]. Intravitreally injected anti-VEGF drugs might block the effect of VEGF and decrease the overall choroidal hyperpermeability. However, due to the high affinity and longer half-life of aflibercept, it can block various members of the VEGF family efficiently [6, 7, 36, 37]. This might result in different patterns of changes in CT after treatment between the two groups. In addition, the differing results of this study might come from the vasoconstrictive effect of anti-VEGF drugs [41, 42]. The vasoconstrictive effect of both drugs remains controversial, and the potency of this effect has not yet been investigated. Considering results of studies that have reported VEGF to be a potent vasodilator in vascular beds, the drugs’ different blocking effects and half-lives might have affected the results [41, 42].
Different effects of two drugs on the choroidal tissue might affect the results. In addition to the previously mentioned effects of VEGF, VEGF plays a physiologic role in maintaining choriocapillaries, which allows for vasodilation and survival of vascular endothelial cells [11, 40]. Considering the physiologic role of VEGF, aflibercept, which have higher affinity to various kinds of VEGF and a longer half-life, could also have a greater effect on the underlying choroid [6, 7, 36, 37]. Regarding the effect of anti-VEGF agents on the choroid, a recent experimental study investigated the effects of ranibizumab and aflibercept on the choroid of monkey eyes and showed different results between the two drugs [12]. It reported that stasis and hemolysis of the choriocapillaris and choroidal vessels were more frequently found after aflibercept treatment compared with ranibizumab treatment. In addition, the reduction in endothelium thickness, the number of fenestrations and the areas of hemolysis were more pronounced in choriocapillaries after aflibercept treatment [12]. It was suggested that these different effects on the choroid might result from the structure of aflibercept, which has a fragmented crystallizable region that ranibizumab lacks [12].
However, the clinical significance of the results of the current study remains limited because the long-term effect of decreased CT on the disease prognosis after anti-VEGF treatments has not been elucidated. Also, from a clinical point of view, both aflibercept and ranibizumab treatments clearly improve visual acuity and exudative retinal changes in eyes with neovascular AMD [2, 4, 5]. Aflibercept can be effective in cases of neovascular AMD that are resistant to ranibizumab therapy [43, 44]. In addition, a previous study reported that aflibercept had a good therapeutic effect in neovascular AMD eyes both with and without choroidal hyperpermeability, while ranibizumab was less effective in eyes with choroidal vascular hyperpermeability [16]. In that study, the aflibercept-treated group showed greater changes in CT and pigment epithelial detachment than the ranibizumab-treated group [16]. This suggest that aflibercept might be beneficial even given its effect on the choroid.
It remains unclear whether the greater decrease in CT observed in eyes treated with aflibercept compared to those treated with ranibizumab is associated with disease prognosis in this study. However, it was recently reported that macular CT in eyes with neovascular AMD decreased after intravitreal injection of ranibizumab, and this decrease was thought to be associated with decreased choroidal exudation from choroidal hyperpermeability [45]. Greater reductions in CT after treatment were associated with visual acuity gains, while increased CT in eyes with neovascular AMD was suggested to reflect choroidal hyperpermeability [45]. Thus, increased CT in eyes with AMD might reflect disease activity, and CT could be a useful diagnostic tool for identifying eyes that would benefit from aggressive therapy. However, because the relationship between decreased CT in eyes with neovascular AMD and long-term visual prognosis and recurrence remains unclear, further studies are needed to elucidate the role of CT in eyes with AMD.
RPE or geographic atrophy may develop in eyes with neovascular AMD and anti-VEGF treatment has been suggested to cause development or exacerbation of RPE and geographic atrophy [46–48]. Because ranibizumab and aflibercept have different characteristics and these drugs might have different effects on the choroid, the incidence of RPE and geographic atrophy may vary based on anti-VEGF drug type [4, 6, 7, 12]. In addition, types of neovascular AMD had been reported to be associated with the development of geographic atrophy. Type 1 neovascularization, which is associated with thicker choroid than types 2 or 3, has a lower geographic atrophy incidence than types 2 and 3 [29, 49]. In eyes with type 3 neovascularization (retinal angiomatous proliferation) with thinner choroid than typical neovascular AMD, the incidence of geographic atrophy was reportedly higher than that of typical neovascular AMD; thinner choroid was therefore reported to be a risk factor for geographic atrophy after anti-VEGF therapy [50]. Thus, greater changes in subfoveal and peripapillary CT from aflibercept treatment might not be beneficial in neovascular AMD with thin choroid. However, because this study was a short-term follow-up study with three monthly injections, we cannot draw conclusions on this topic. It might be necessary to decide the proper limit of CT that we need to apply careful intravitreal injections considering the balance of risk and benefits according to the kinds of anti-VEGF drugs and proper treatment regimen. Further long-term follow-up studies will be needed to elucidate the role of CT and to compare the potential risks and benefits of the two drugs.
In this study, it is unclear whether the decreased subfoveal or peripapillary CT observed after intravitreal injection is within the physiologically normal range of CT. Because we could not evaluate age-matched controls, we could not draw further conclusions. However, previous studies have reported normal subfoveal CT in older Asian persons with a mean age over 50 years to be from 209.2 to 253.8 μm [51–53]. In our previous study, mean subfoveal CT of normal controls with a mean age of 59.75 was 241.97 ± 66.37 μm [53]. In another study, normal peripapillary CT in older Asian persons with a mean age of over 50 years was from 135.59 to 165.03 μm [35, 54, 55]. It seems that, in the current study, subfoveal and mean peripapillary CT in both groups before and after intravitreal injection were similar to the previously reported range. However, because CT can vary according to age, sex, refractive error, type of OCT, and other factors, direct comparison of these CT values may not be appropriate [35, 52, 55, 56]. Further studies with age-matched normal controls are needed to determine the normal range of subfoveal and peripapillary CT, and whether the CT changes observed in this study fall within the physiologically normal range.
This study has several limitations. First, this study has a retrospective design with a small number of cases and a short-term follow-up period. Thus, it is not clear whether the observed differences are transient or long-term. Further prospective studies with long-term follow-up are needed to elucidate this matter. In addition, differences in baseline BCVA, which might be indicative of longer disease duration, could affect the results of this study. However, there were no differences in disease duration between the two groups. Second, because there was a lack of software with automated analysis, the measurements were performed manually by two examiners. Measurement errors may exist despite good interobserver reliability between the two examiners. Third, because of its retrospective design, we could not measure CT at multiple areas. The nasal peripapillary choroid only represents part of the choroid outside of the macula, and it does not sufficiently represent all choroid outside of the macula. The measurement of nasal choroid, which is far from the optic disc might provide stronger conclusion. Recently, swept-source OCT (SS-OCT) with the advantages of longer wavelength, deeper penetration, visualization of the entire choroid, wide scan range and provision of topographic information was introduced [45, 57]. Thus, studies using SS-OCT might provide more accurate results about changes in CT after intravitreal injection of anti-VEGF drugs when it compared with SD-OCT. Fourth, this study only included patients with age-related macular degenerations. Because we treat eyes with PCV using not only anti-VEGF drugs, but also with photodynamic therapy or direct focal laser if needed, treatments varied according to the patients. Thus cases with PCV were excluded in this study. Further studies about cases of PCV and other diseases with CNV might provide further information. Fifth, because of the retrospective study design, we could not analyze the diurnal variation in CT. Sixth, because bevacizumab is an off-label drug in South Korea, we could not investigate the effects of bevacizumab on CT in treatment-naïve neovascular AMD patients. Further studies including cases treated with bevacizumab will be needed to elucidate the effects of various anti-VEGF drugs on CT.
In conclusion, CT of the macula and outside of the macula decreased significantly in eyes with neovascular AMD after three months of aflibercept treatment, while only macular CT showed significant changes after ranibizumab treatment. Further studies with prospective design, large numbers and long-term follow-up will be needed to elucidate the long-term effect of anti-VEGF drugs on the choroid and disease prognosis.
References
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY (2012) Age-related macular degeneration. Lancet 379:1728–1738. doi:10.1016/S0140-6736(12)60282-7
Campbell JP, Bressler SB, Bressler NM (2012) Impact of availability of anti-vascular endothelial growth factor therapy on visual impairment and blindness due to neovascular age-related macular degeneration. Arch Ophthalmol 130:794–795. doi:10.1001/archophthalmol.2011.2480
Ferrara N, Damico L, Shams N, Lowman H, Kim R (2006) Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26:859–870. doi:10.1097/01.iae.0000242842.14624.e7
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U, View GVS (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119:2537–2548. doi:10.1016/j.ophtha.2012.09.006
Mitchell P, Korobelnik JF, Lanzetta P, Holz FG, Prunte C, Schmidt-Erfurth U, Tano Y, Wolf S (2010) Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol 94:2–13. doi:10.1136/bjo.2009.159160
Stewart MW (2012) Clinical and differential utility of VEGF inhibitors in wet age-related macular degeneration: focus on aflibercept. Clin Ophthalmol 6:1175–1186. doi:10.2147/OPTH.S33372
Stewart MW, Rosenfeld PJ (2008) Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol 92:667–668. doi:10.1136/bjo.2007.134874
Kvanta A, Algvere PV, Berglin L, Seregard S (1996) Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci 37:1929–1934
Blaauwgeers HG, Holtkamp GM, Rutten H, Witmer AN, Koolwijk P, Partanen TA, Alitalo K, Kroon ME, Kijlstra A, van Hinsbergh VW, Schlingemann RO (1999) Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol 155:421–428. doi:10.1016/S0002-9440(10)65138-3
Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E (2008) Regulation of retinal blood flow in health and disease. Prog Retin Eye Res 27:284–330. doi:10.1016/j.preteyeres.2008.02.002
Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA (2009) An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A 106:18751–18756. doi:10.1073/pnas.0905010106
Julien S, Biesemeier A, Taubitz T, Schraermeyer U (2014) Different effects of intravitreally injected ranibizumab and aflibercept on retinal and choroidal tissues of monkey eyes. Br J Ophthalmol 98:813–825. doi:10.1136/bjophthalmol-2013-304019
Peters S, Heiduschka P, Julien S, Ziemssen F, Fietz H, Bartz-Schmidt KU, Tubingen Bevacizumab Study G, Schraermeyer U (2007) Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol 143:995–1002. doi:10.1016/j.ajo.2007.03.007
Branchini L, Regatieri C, Adhi M, Flores-Moreno I, Manjunath V, Fujimoto JG, Duker JS (2013) Effect of intravitreous anti-vascular endothelial growth factor therapy on choroidal thickness in neovascular age-related macular degeneration using spectral-domain optical coherence tomography. JAMA Ophthalmol 131:693–694. doi:10.1001/jamaophthalmol.2013.692
Gharbiya M, Cruciani F, Mariotti C, Grandinetti F, Marenco M, Cacace V (2015) Choroidal thickness changes after Intravitreal antivascular endothelial growth factor therapy for age-related macular degeneration: ranibizumab versus aflibercept. J Ocul Pharmacol Ther 31:357–362. doi:10.1089/jop.2014.0160
Hata M, Oishi A, Tsujikawa A, Yamashiro K, Miyake M, Ooto S, Tamura H, Nakanishi H, Takahashi A, Yoshikawa M, Yoshimura N (2014) Efficacy of intravitreal injection of aflibercept in neovascular age-related macular degeneration with or without choroidal vascular hyperpermeability. Invest Ophthalmol Vis Sci 55:7874–7880. doi:10.1167/iovs.14-14610
Koizumi H, Kano M, Yamamoto A, Saito M, Maruko I, Kawasaki R, Sekiryu T, Okada AA, Iida T (2015) Short-term changes in choroidal thickness after aflibercept therapy for neovascular age-related macular degeneration. Am J Ophthalmol 159:627–633. doi:10.1016/j.ajo.2014.12.025
Mazaraki K, Fassnacht-Riederle H, Blum R, Becker M, Michels S (2015) Change in choroidal thickness after intravitreal aflibercept in pretreated and treatment-naive eyes for neovascular age-related macular degeneration. Br J Ophthalmol. doi:10.1136/bjophthalmol-2015-306636
Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S (2012) Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology 119:1621–1627. doi:10.1016/j.ophtha.2012.02.022
Hayreh SS (2004) Posterior ciliary artery circulation in health and disease: the Weisenfeld lecture. Invest Ophthalmol Vis Sci 45:749–757, 748
Vujosevic S, Martini F, Cavarzeran F, Pilotto E, Midena E (2012) Macular and peripapillary choroidal thickness in diabetic patients. Retina 32:1781–1790. doi:10.1097/IAE.0b013e31825db73d
Yun C, Oh J, Ahn SE, Hwang SY, Kim SW, Huh K (2015) Peripapillary choroidal thickness in patients with early age-related macular degeneration and reticular pseudodrusen. Graefes Arch Clin Exp Ophthalmol. doi:10.1007/s00417-015-3054-7
Yun C, Oh J, Han JY, Hwang SY, Moon SW, Huh K (2015) Peripapillary choroidal thickness in central serous chorioretinopathy: is choroid outside the macula also thick? Retina. doi:10.1097/IAE.0000000000000539
Lengyel I, Csutak A, Florea D, Leung I, Bird AC, Jonasson F, Peto T (2015) A population-based ultra-Widefield digital image grading study for age-related macular degeneration-like lesions at the peripheral retina. Ophthalmology 122:1340–1347. doi:10.1016/j.ophtha.2015.03.005
Koh AH, Expert PCVP, Chen LJ, Chen SJ, Chen Y, Giridhar A, Iida T, Kim H, Yuk Yau Lai T, Lee WK, Li X, Han Lim T, Ruamviboonsuk P, Sharma T, Tang S, Yuzawa M (2013) Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina 33:686–716. doi:10.1097/IAE.0b013e3182852446
Ahuja RM, Stanga PE, Vingerling JR, Reck AC, Bird AC (2000) Polypoidal choroidal vasculopathy in exudative and haemorrhagic pigment epithelial detachments. Br J Ophthalmol 84:479–484
Perkovich BT, Zakov ZN, Berlin LA, Weidenthal D, Avins LR (1990) An update on multiple recurrent serosanguineous retinal pigment epithelial detachments in black women. Retina 10:18–26
De Salvo G, Vaz-Pereira S, Keane PA, Tufail A, Liew G (2015) Sensitivity and specificity of spectral-domain optical coherence tomography in detecting idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol 158:1228–1238.e1. doi:10.1016/j.ajo.2014.08.025
Freund KB, Zweifel SA, Engelbert M (2010) Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina 30:1333–1349. doi:10.1097/IAE.0b013e3181e7976b
Macular Photocoagulation Study Group (1991) Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Arch Ophthalmol 109:1242–1257
Freund KB, Ho IV, Barbazetto IA, Koizumi H, Laud K, Ferrara D, Matsumoto Y, Sorenson JA, Yannuzzi L (2008) Type 3 neovascularization: the expanded spectrum of retinal angiomatous proliferation. Retina 28:201–211. doi:10.1097/IAE.0b013e3181669504
Griffith JF, Goldberg JL (2015) Prevalence of comorbid retinal disease in patients with glaucoma at an academic medical center. Clin Ophthalmol 9:1275–1284. doi:10.2147/OPTH.S85851. eCollection 2015
Spaide RF, Koizumi H, Pozzoni MC (2008) Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 146:496–500. doi:10.1016/j.ajo.2008.05.032
Yiu G, Pecen P, Sarin N, Chiu SJ, Farsiu S, Mruthyunjaya P, Toth CA (2014) Characterization of the choroid-scleral junction and suprachoroidal layer in healthy individuals on enhanced-depth imaging optical coherence tomography. JAMA Ophthalmol 132:174–181. doi:10.1001/jamaophthalmol.2013.7288
Oh J, Yoo C, Yun CM, Yang KS, Kim SW, Huh K (2013) Simplified method to measure the peripapillary choroidal thickness using three-dimensional optical coherence tomography. Korean J Ophthalmol 27:172–177. doi:10.3341/kjo.2013.27.3.172
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15:171–185. doi:10.1007/s10456-011-9249-6
Zehetner C, Kralinger MT, Modi YS, Waltl I, Ulmer H, Kirchmair R, Bechrakis NE, Kieselbach GF (2015) Systemic levels of vascular endothelial growth factor before and after intravitreal injection of aflibercept or ranibizumab in patients with age-related macular degeneration: a randomised, prospective trial. Acta Ophthalmol 93:e154–e159. doi:10.1111/aos.12604
Lowe J, Araujo J, Yang J, Reich M, Oldendorp A, Shiu V, Quarmby V, Lowman H, Lien S, Gaudreault J, Maia M (2007) Ranibizumab inhibits multiple forms of biologically active vascular endothelial growth factor in vitro and in vivo. Exp Eye Res 85:425–430. doi:10.1016/j.exer.2007.05.008
dell’Omo R, Cassetta M, dell’Omo E, di Salvatore A, Hughes JM, Aceto F, Porcellini A, Costagliola C (2012) Aqueous humor levels of vascular endothelial growth factor before and after intravitreal bevacizumab in type 3 versus type 1 and 2 neovascularization. A prospective, case-control study. Am J Ophthalmol 153:155–161.e152. doi:10.1016/j.ajo.2011.06.001
Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO (2003) Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 22:1–29
Papadopoulou DN, Mendrinos E, Mangioris G, Donati G, Pournaras CJ (2009) Intravitreal ranibizumab may induce retinal arteriolar vasoconstriction in patients with neovascular age-related macular degeneration. Ophthalmology 116:1755–1761. doi:10.1016/j.ophtha.2009.03.017
Sacu S, Pemp B, Weigert G, Matt G, Garhofer G, Pruente C, Schmetterer L, Schmidt-Erfurth U (2011) Response of retinal vessels and retrobulbar hemodynamics to intravitreal anti-VEGF treatment in eyes with branch retinal vein occlusion. Invest Ophthalmol Vis Sci 52:3046–3050. doi:10.1167/iovs.10-5842
Kawashima Y, Oishi A, Tsujikawa A, Yamashiro K, Miyake M, Ueda-Arakawa N, Yoshikawa M, Takahashi A, Yoshimura N (2014) Effects of aflibercept for ranibizumab-resistant neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. doi:10.1007/s00417-014-2838-5
Pinheiro-Costa J, Costa JM, Beato JN, Freitas-da-Costa P, Brandao E, Falcao MS, Falcao-Reis F, Carneiro AM (2015) Switch to aflibercept in the treatment of neovascular AMD: one-year results in clinical practice. Ophthalmologica 233:155–161. doi:10.1159/000381221
Razavi S, Souied EH, Darvizeh F, Querques G (2015) Assessment of choroidal topographic changes by swept-source optical coherence tomography after Intravitreal Ranibizumab for exudative age-related macular degeneration. Am J Ophthalmol 160:1006–1013. doi:10.1016/j.ajo.2015.08.009
Grunwald JE, Daniel E, Huang J, Ying GS, Maguire MG, Toth CA, Jaffe GJ, Fine SL, Blodi B, Klein ML, Martin AA, Hagstrom SA, Martin DF, CATT Research Group (2014) Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 121:150–161. doi:10.1016/j.ophtha.2013.08.015
Grunwald JE, Pistilli M, Ying GS, Maguire MG, Daniel E, Martin DF, Comparison of Age-related Macular Degeneration Treatments Trials Research Group (2015) Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 122:809–816. doi:10.1016/j.ophtha.2014.11.007
Young M, Chui L, Fallah N, Or C, Merkur AB, Kirker AW, Albiani DA, Forooghian F (2014) Exacerbation of choroidal and retinal pigment epithelial atrophy after anti-vascular endothelial growth factor treatment in neovascular age-related macular degeneration. Retina 34:1308–1315. doi:10.1097/IAE.0000000000000081
Xu L, Mrejen S, Jung JJ, Gallego-Pinazo R, Thompson D, Marsiglia M, Freund KB (2015) Geographic atrophy in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Retina 35:176–186. doi:10.1097/IAE.0000000000000374
Cho HJ, Lee TG, Han SY, Kim HS, Kim JH, Han JI, Lew YJ, Kim JW (2015) Long-term visual outcome and prognostic factors of Intravitreal anti-vascular endothelial growth factor treatment for retinal angiomatous proliferation. Graefes Arch Clin Exp Ophthalmol. doi:10.1007/s00417-015-2993-3
Fujiwara A, Shiragami C, Shirakata Y, Manabe S, Izumibata S, Shiraga F (2012) Enhanced depth imaging spectral-domain optical coherence tomography of subfoveal choroidal thickness in normal Japanese eyes. Jpn J Ophthalmol 56:230–235. doi:10.1007/s10384-012-0128-5
Wei WB, Xu L, Jonas JB, Shao L, Du KF, Wang S, Chen CX, Xu J, Wang YX, Zhou JQ, You QS (2013) Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology 120:175–180. doi:10.1016/j.ophtha.2012.07.048
Kim SW, Oh J, Kwon SS, Yoo J, Huh K (2011) Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina 31:1904–1911. doi:10.1097/IAE.0b013e31821801c5
Gupta P, Cheung CY, Baskaran M, Tian J, Marziliano P, Lamoureux EL, Cheung CM, Aung T, Wong TY, Cheng CY (2015) Relationship between peripapillary choroid and retinal nerve fiber layer thickness in a population-based sample of nonglaucomatous eyes. Am J Ophthalmol. doi:10.1016/j.ajo.2015.09.018
Huang W, Wang W, Zhou M, Chen S, Gao X, Fan Q, Ding X, Zhang X (2013) Peripapillary choroidal thickness in healthy Chinese subjects. BMC Ophthalmol 13:23. doi:10.1186/1471-2415-13-23
Laviers H, Zambarakji H (2014) Enhanced depth imaging-OCT of the choroid: a review of the current literature. Graefes Arch Clin Exp Ophthalmol 252:1871–1883. doi:10.1007/s00417-014-2840-y
Adhi M, Duker JS (2013) Optical coherence tomography—current and future applications. Curr Opin Ophthalmol 24:213–221. doi:10.1097/ICU.0b013e32835f8bf8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Korea University provided financial support in the form of research grant (grant number K1421461). The sponsor had no role in the design or conduct of this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic Supplementary Material 1
(PDF 10 kb)
Electronic Supplementary Material 2
(PDF 14 kb)
Electronic Supplementary Material 3
(PDF 69 kb)
Rights and permissions
About this article
Cite this article
Yun, C., Oh, J., Ahn, J. et al. Comparison of intravitreal aflibercept and ranibizumab injections on subfoveal and peripapillary choroidal thickness in eyes with neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 254, 1693–1702 (2016). https://doi.org/10.1007/s00417-015-3260-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3260-3