Abstract
Purpose
The enhanced S-cone syndrome (ESCS) is a rare hereditary retinal degeneration that has enhanced short wavelength-sensitive cone (S-cone) functions. The longitudinal clinical course of this disease has been rarely reported, and the genetic aspects of ESCS have not been well investigated in the Japanese population. In this report, we present our clinical and genetic findings for 2 patients with ESCS.
Patients and methods
The patients were 2 unrelated Japanese men. Standard ophthalmic examinations and mutation screening for the NR2E3 gene were performed.
Results
Patient 1 was a 36-year-old man, and his clinical findings were typical of ESCS. His decimal best-corrected visual acuity (BCVA) was 1.0 OD and 0.5 OS after removal of cataracts. Genetic investigations revealed a homozygous truncation frameshift, the p.I307LfsX33 mutation. Patient 2 was an 11-year-old boy when he was first examined by us. His clinical findings were typical of ESCS except for uveitis in the left eye. His decimal BCVA at the age of 39 years was maintained at 1.5 in each eye, although the retinal degeneration and visual field impairments had progressed during the follow-up period. The genetic investigations revealed homozygous mutations of p.R104Q in the NR2E3 gene.
Conclusions
The frameshift mutation, p.I307LfsX33, in the NR2E3 gene is a new causative mutation for ESCS. The clinical observations for patient 2 are the longest ever reported. The retinal degeneration caused by this mutation is slowly progressive, and these patients maintained good vision with maintenance of the foveal structure until their late thirties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As best we know, there is only one type of retinal dystrophy associated with enhanced retinal functions; the other dystrophies have reduced retinal functions. This disease, the enhanced S-cone syndrome (ESCS), is a rare hereditary retinal dystrophy that has an autosomal recessive inheritance pattern (OMIM #268100 [1]). ESCS was so named because patients with this disease have a markedly increased sensitivity to blue light [2, 3].
Patients with ESCS complain of night blindness from birth. Their vision gradually decreases, and visual field defects, typically a ring scotoma, also gradually worsen [4–6]. Patients with ESCS have retinal and retinal pigment epithelial (RPE) degeneration that extends from the vascular arcades to the mid-periphery [2, 5–17], but the fundus can be normal in children [16, 18]. The degeneration is accompanied by white-yellowish flecks in younger patients and clumped pigmentations in older patients [2, 5–17]. Cystic macular changes and foveal retinoschisis are frequently observed [2, 6, 7, 10, 13]. The degenerations slowly progress to diffuse and nonspecific degeneration at the end stage of the disease [6].
Recent clinical studies have shown that the clinical findings in patients with ESCS were varied: choroidal neovascularization (CNV) [19, 20], peripheral retinoschisis [4], large retinoschisis in the posterior pole [18, 21] that could resolve spontaneously [17], torpedo-like lesions along the vascular arcades with circumferential macular fibrosis [22], macular hole [23], and preserved foveal function [15]. There is also a mild form of ESCS [9, 20].
Electroretinography (ERG) is an important method for diagnosing ESCS because the fundus appearance varies widely among patients with ESCS. This disease has pathognomonic ERG findings in eyes with both normal and abnormal fundi [2–4, 6–8, 10, 16, 18, 24–27]: (1) flash ERGs (dark-adapted 3.0 or 10 ERG [28]) are supernormal or subnormal, but always very delayed; (2) cone ERGs recorded with a background light (light-adapted 3.0 ERG [28]) have an identical waveform to that of the flash ERG, except for the size, although they show different waveforms in healthy individuals; (3) rod ERGs (dark-adapted 0.01 ERG [28]) are non-recordable; and (4) light-adapted 30 Hz flicker ERGs [28] are delayed and severely reduced. These unique ERG findings were unexplainable until the pathology of ESCS was confirmed.
In 2002, a histopathologic study of the retina of eyes with ESCS revealed that most of the photoreceptors in eyes with ESCS were short wavelength-sensitive cones (S-cones), which are in the minority in healthy retinas [29]. In addition, rods were not present, and smaller numbers of middle wavelength- and long wavelength-sensitive (M- and L-) cones were present in the ESCS retina [29].
Thus, the high sensitivity to blue light and the unique ERG findings in this disease are now understood to be due to the abnormally large population of S-cones. The entire S-cone system in retinas with ESCS may be normal [30] or abnormal [10, 24, 25].
In 2000, the gene responsible for ESCS was identified [31]. This gene, the nuclear receptor subfamily 2, group E, member 3 gene (NR2E3, OMIM #604485) on chromosome 15q23, plays an important role in photoreceptor development and differentiation [32–34]. The NR2E3 protein, a photoreceptor-specific nuclear receptor (PNR), is a member of the nuclear hormone receptor superfamily of ligand-modulated transcription factors [32]. This protein is involved in proper development of cone and rod photoreceptors in concert with other transcription factors such as NRL and CRX [33, 34]. Although the details of these mechanisms are not well known, dysfunction of NR2E3 causes an abnormal differentiation of the progenitor cells, which results in an abnormally laminated retina with an extremely large population of S-cones and absence of rods in human retinas [29, 35].
Subsequently, pathogenic NR2E3 mutations were found in patients with Goldmann-Favre syndrome and clumped pigmentary retinal degeneration [36], autosomal recessive retinitis pigmentosa [37], and dominant retinitis pigmentosa [38]. These discoveries expanded the phenotypic variations caused by NR2E3 gene mutations (NR2E3-linked retinal dystrophy). At present, ESCS and Goldmann-Favre syndrome are considered to be members of the same disease [11, 12] because both show similar electrophysiologic [39], psychophysical [40], and histopathologic findings [41].
No effective therapy for ESCS/Goldmann-Favre syndrome is known other than carbonic anhydrase inhibitors for improvement of the foveal schisis [13].
Up to March 2016, the findings in almost 200 patients with ESCS had been reported in 45 publications. Among them, only 7 patients in 8 articles were reported from Japan [6–9, 17, 19, 21, 26]. Although the clinical and genetic features of ESCS have been collected by these research studies, the longitudinal clinical course of this disease has rarely been reported [4–6].
The purpose of this report was to present our findings in 2 Japanese patients with ESCS; one was found to have a new truncation mutation in the NR2E3 gene, and the other had homozygous mutations of p.R104Q in the NR2E3 gene. The latter patient was followed for 28 years and maintains good visual acuity at the age of 39 years.
Patients and methods
The research protocol was approved by the ethics review board of the Kinki University Faculty of Medicine on February 2, 2011 (approved #22-132) and of the Jikei University School of Medicine on December 11, 2012 (approved #24-231 6997). The research protocol conformed to the tenets of the Declaration of Helsinki of the World Medical Association. Written informed consent was obtained from all patients after the procedures had been explained in detail.
The patients were 2 unrelated Japanese men. Clinical examinations including visual acuity measurements, dilated ophthalmoscopy, fluorescein fundus angiography, Goldmann kinetic perimetry, Goldmann-Weekers dark adaptometry, the Farnsworth-Munsell 100-hue test, and International Society for Clinical Electrophysiology of Vision (ISCEV)-standard full-field ERGs [28] were performed.
The ISCEV-standard full-field ERGs were elicited by a white light-emitting diode (LED) embedded in a contact lens electrode with a driver (EW-102 with WLS-20; Mayo Corporation, Inazawa, Japan). The ERG responses were amplified and stored in a bio-amplifier [MEB-5504 (Neuropack Σ); Nihon Kohden, Tokyo, Japan]. ERG recordings with chromatic stimuli (chromatic ERGs) were also recorded to examine the S-cone function [42]. A contact lens electrode embedded with red, green, and blue LEDs (Mayo Corporation, not commercialized) was used for the chromatic ERG recordings [42, 43]. The luminance of each color LED was independently controlled by a driver (CLS-10; Mayo Corporation). The elicited ERGs were amplified and stored in the bio-amplifier.
Optical coherence tomographic (OCT) images were obtained using CIRRUS HD-OCT version 5.1 (Carl Zeiss Meditec, Dublin, CA, USA). These clinical studies were performed at Kinki University Hospital.
Genetic investigation of the NR2E3 gene was performed by polymerase chain reaction and direct sequencing at the Jikei University School of Medicine. Details of the genetic investigative procedures have been previously published [14]. In brief, DNA was extracted from the patient’s blood samples using the Gentra Puregene Blood Kit (Qiagen, Hilden, Germany). For mutation screening of the NR2E3 gene, all coding exons including exon/intron boundaries were amplified by polymerase chain reaction (PCR) with primer pairs (Sigma-Aldrich Japan, Tokyo, Japan) followed by sequencing. One hundred healthy Japanese individuals served as controls and were screened using PCR-restriction fragment length polymorphism analyses. We used pathogenicity prediction methods available online, e.g., PolyPhen [44], PolyPhen-2 [45], SIFT [46], and Align GVGD [47]. Mutation databases available in the Leiden Open Variation Database (LOVD v.3.0) [48] and the National Center for Biotechnology Information (NCBI) [49] were used to search for mutations of the amino acids in the patients.
Results
The clinical and genetic findings are shown in Figs. 1, 2, 3, 4, and 5.
Clinical findings for patient 1 with enhanced S-cone syndrome (ESCS). The phenotype of this patient is typical of ESCS, although this patient has a homozygous truncation mutation of p.I307LfsX33 in the NR2E3 gene (Fig. 5). FA fluorescein fundus angiography, OCT optical coherence tomography
Clinical findings for patient 2 with enhanced S-cone syndrome (ESCS). The patient had maintained a decimal visual acuity of 1.5 in both eyes until the age of 39 years, although retinal impairments were developing gradually and his left eye had intraocular inflammation of unknown cause. This patient has homozygous p.R104Q mutations in the NR2E3 gene (Fig. 5)
Electroretinography (ERG) results. International Society for Clinical Electrophysiology of Vision (ISCEV)-standard full-field ERGs [28] and chromatic ERGs [42] are shown. Responses from both eyes are superimposed. Chromatic ERGs were elicited by blue, green, and red stimuli on a yellow background. The luminances of the chromatic stimulations were 50, 159, and 224 cd/m2 for the blue, green, and red stimuli, respectively. The duration of the stimuli was 2 ms. A yellow background light was produced by a mixture of red (355 cd/m2) and green (316 cd/m2) light. The blue-on-yellow ERG had a double-peaked b-wave in a healthy individual: the rapid b-wave (L, M) is elicited by long- and middle-wavelength sensitive-cone systems, and the slow b-wave (S), by a short-wavelength sensitive-cone system [42]. The intensity of each chromatic photostimulus was adjusted to elicit the rapid b-wave (L, M) with almost the same amplitude in healthy individuals. Rod dark-adapted 0.01 ERG, flash dark-adapted 30 ERG, cone light-adapted 3.0 ERG, flicker light-adapted 30 Hz flicker ERG [28]
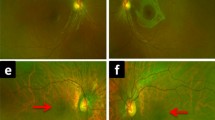
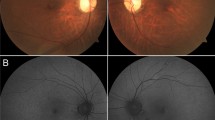
Patient 1 (ESCS5-20120816) was a 36-year-old man referred to our clinic because of retinal degeneration and posterior subcapsular cataracts in both eyes. He stated that he had had night blindness from infancy and that his parents were consanguineous (Fig. 1). He had retinal degeneration that extended from the vascular arcades to the mid-periphery with some clumped pigmentations at the level of the RPE (Fig. 2). He also had vitreous opacities in both eyes. Fluorescein fundus angiography showed extensive leakage of the dye in both eyes (Fig. 2).
His cataracts were removed, and his decimal best-corrected visual acuity (BCVA) improved from 0.5 to 1.0 OD and from 0.15 to 0.5 OS. He had mild hyperopia of +1.5 diopters sphere (DS) in each eye before the cataract surgery.
Goldmann kinetic perimetry showed a decrease in sensitivity in the mid-periphery in both eyes, and the right eye had a ring scotoma (Fig. 2). The dark-adaptation curve was monophasic with only the cone limb, which supported his complaint of complete night blindness. Color vision tests showed mild abnormalities: the total error score on the Farnsworth-Munsell 100-hue test was 164 OD and 184 OS (Fig. 2).
Full-field ISCEV-standard flash, cone, and flicker ERGs were reduced and delayed. Rod ERGs were non-recordable (Fig. 4). The chromatic ERGs showed normal S-cone responses and non-recordable L, M-cone responses (Fig. 4). From these ERG findings, ESCS was diagnosed.
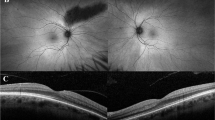
The OCT images showed that each retinal layer was thin, although the thickness of the nerve fiber layer was normal. The ellipsoid zone was absent except at the fovea (Fig. 2).
The patient is now 39 years old, and his decimal BCVA remains at 1.0 OD and 0.5 OS.
Genetic analysis showed a novel homozygous mutation of a 2-bp deletion (c.919_920del2 bp) in exon 6 of the NR2E3 gene, resulting in a truncation mutation by a frameshift (p.I307LfsX33; Fig. 5).
His parents, who did not report any night blindness, had no retinal degeneration and had normal retinal vessels. However, his father had age-related macular degeneration in the right eye, and his mother had branch retinal vein occlusion in the right eye and glaucoma in both eyes.
Patient 2 (ESCS3-20120414-2) was an 11-year-old boy who first visited our clinic because of inflammation in his left eye. He reported that he had had night blindness from infancy. His parents were consanguineous (Fig. 1). He had mild RPE depigmentation along the vascular arcades with a few white flecks in both eyes (Fig. 3). Mild RPE depigmentation was seen also in the macular area; however, no cystic changes were observed. His left eye had vitreous opacities, disc edema, and some retinal hemorrhages. Fluorescein fundus angiography showed hyperfluorescence due to the RPE impairments in both eyes, and dye leakage from the optic disc and retinal vessels in the left eye (Fig. 3).
His decimal BCVA was 1.0 OD with +0.25 DS combined with −1.35 D cylinder (DC) ax 90° and 1.0 OS with +2.5 DS combined with −1.75 DC ax 80°. Full-field, bright flash ERGs at age 11 showed abnormally large and delayed responses in both eyes (Fig. 4); however, ESCS was not suspected because this disease had not been reported at that time.
His uveitis was treated with steroids until the age of 22, when he was referred to another hospital. He returned to our hospital at the age of 36 when he was diagnosed as having ESCS owing to his unique ERG findings, namely delayed flash cone ERGs, decreased flicker ERGs, and non-recordable rod ERGs (Fig. 4). The flash ERG amplitude at age 38 was less than that at age 11 years (Fig. 4), although the recording systems and conditions differed between these recordings. The chromatic ERGs had supernormal S-cone responses and non-recordable L, M-cone responses (Fig. 4).
The retinal degeneration had slightly worsened with the appearance of small, white punctate lesions and faint pigmentations at the level of the RPE (Fig. 3). Goldmann kinetic perimetry showed a ring scotoma in both eyes that had increased during the 28 years that he had been undergoing follow-up examinations. The dark-adaptation curve was monophasic, with only the cone limb indicating no rod function (Fig. 3).
His BCVA at age 39 years was maintained at 1.5 in each eye, and the results of the Farnsworth-Munsell 100-hue test were almost perfect. His OCT images showed almost normal foveal structures except for a slight foveal schisis in the right eye and an epiretinal membrane in the left eye. The ellipsoid zone and external limiting membrane were indistinct away from the macula (Fig. 3).
Genetic analysis revealed a homozygous nucleotide substitution (c.311G > A) in the NR2E3 gene resulting in an arginine-to-glutamine mutation at amino acid position 104, p.R104Q (Fig. 5).
Discussion
Clinical course of ESCS
The longitudinal clinical course of patients with ESCS has been reported in several publications [4–6]. The findings suggested that the visual acuity decreases gradually with increasing age. However, the presence of severe macular schisis or CNV can cause a rapid decrease in the BCVA [18–21]. Surprisingly, ESCS patients with CNV have been children or adolescents [19, 20]; therefore, CNV is an important cause of the severe decrease in the visual acuity of younger patients with ESCS.
Our 2 patients did not have cystic macular changes or severe foveal schisis, which probably explains why they maintained good visual acuity and good color discrimination into their late thirties.
The BCVAs of the 214 eyes of the 109 patients with ESCS who have been reported are summarized in Fig. 6. No significant correlation was found between BCVA and age. Three groups are shown in Fig. 6 (blue-shaded areas A–C): the first group consists of the young patients aged around 10 years and with good visual acuity (A in Fig. 6); the second group consists of the patients in their twenties with reduced vision of about 1.0 logMAR units (decimal 0.1) (B in Fig. 6); and the third group consists of the patients in their thirties to forties with relatively good visual acuity (C in Fig. 6).
These 3 groups raise the hypothesis that patients with ESCS have 2 major clinical courses: one patient group whose vision is reduced when they are young, probably due to maculopathy including macular schisis or CNV (blue-shaded areas A–B in Fig. 6), and another patient group who maintain good vision until their fourth or fifth decade of life, probably owing to mild macular changes similar to those seen in the patients presented in this study and our earlier study [6] (blue-shaded areas A–C in Fig. 6).
Interestingly, the older patients shown in Fig. 6 appeared to have relatively good vision among all the patients with ESCS. Advanced ESCS with low vision in older patients is probably difficult to diagnose because of the nonspecific retinal degeneration with reduced ERG responses.
Genotype-phenotype correlations in ESCS
Up to March 2016, 63 kinds of pathogenic NR2E3 mutations have been reported in the Human Gene Mutation Database (HGMD) [50]. We investigated whether there was a significant correlation between the locus or type of mutations in NR2E3 and the phenotype, including the clinical course and visual acuity. However, no significant correlation was found except for mutation p.G56R, which was specific for autosomal dominant retinitis pigmentosa (RP37, OMIM #611131) [38].
The frameshift mutation found in patient 1, p.I307LfsX33, is a new causative mutation for ESCS; it is located in exon 6. This mutation truncates the 410 amino acids in the normal NR2E3 protein into 306 amino acids and causes the synthesis of a protein lacking more than half of the ligand-binding domain. Therefore, the phenotype of patient 1 would be expected to exhibit a severe form of ESCS. However, the phenotype of patient 1 was not so severe, but typical of ESCS. The extensive dye leakage shown on fluorescein fundus angiography (Fig. 2) was reported in both ESCS [26] and Goldmann-Favre syndrome [39]. To explain this discrepancy or the phenotypic variations of NR2E3-linked retinal dystrophy, further studies are required to determine whether there are additional modifying factors to the NR2E3 mutations in the spectrum of these diseases.
Patient 2, on the other hand, had a homozygous p.R104Q mutation, which was also reported earlier in 2 other Japanese patients by our group [6, 9]. This patient had a long-standing inflammation in the left eye of undetermined cause. We cannot conclude that there is a relationship between ESCS and the patient’s unilateral uveitis, although Udar et al. [27] reported a patient with ESCS and unilateral uveitis.
In the Japanese population, 7 kinds of pathogenic mutations in the NR2E3 gene have been reported: p.R48C [6], p.G51R [6], p.R104Q [6, 9, present study], p.I307LfsX33 (present study), p.R311Q [19], p.R334G [9], and p.Q350X [7, 17, 21]. All of these mutations other than p.R311Q [19] were first found in Japanese individuals. This suggests that the NR2E3 mutations in the Japanese population are different from those in the Caucasian population.
Our group found a mutation, p.R104Q, in 3 families from different regions of Japan. This indicates that the allelic frequency of p.R104Q in Japanese patients with ESCS may be high, although this mutation is very rare in the Japanese population [51]. Additional genetic investigations are necessary in a larger Asian cohort with ESCS/NR2E3-linked retinal dystrophy.
In conclusion, the frameshift mutation, p.I307LfsX33, in the NR2E3 gene is a new causative mutation for ESCS. This report is, as best we know, the longest clinical observation of a patient with ESCS. The retinal degeneration in ESCS is slowly progressive, and our patients maintained good vision and preserved foveal structure until at least the fourth decade of life.
References
Online Mendelian inheritance in man (OMIM). In: National Center for Biotechnology Information (NCBI), U.S. National Library of Medicine. 2016. http://www.ncbi.nlm.nih.gov/omim. Accessed 29 Apr 2016.
Marmor MF, Jacobson SG, Foerster MH, Kellner U, Weleber RG. Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and enhanced S cone sensitivity. Am J Ophthalmol. 1990;110:124–34.
Jacobson SG, Marmor MF, Kemp CM, Knighton RW. SWS (blue) cone hypersensitivity in a newly identified retinal degeneration. Invest Ophthalmol Vis Sci. 1990;31:827–38.
Kellner U, Zrenner E, Sadowski B, Foerster MH. Enhanced S cone sensitivity syndrome: long-term follow-up, electrophysiological and psychophysical findings. Clin Vis Sci. 1993;8:425–34.
Pachydaki SI, Bhatnagar PA, Barbazetto IA, Klaver CC, Freund BK, Yannuzzi LA. Long-term follow-up in enhanced S-cone syndrome. Retin Cases Brief Rep. 2009;3:118–20.
Kuniyoshi K, Hayashi T, Sakuramoto H, Nakao A, Sato T, Utsumi T, et al. Novel mutations in enhanced S-cone syndrome. Ophthalmology. 2013;120:431–431e.6.
Nakamura Y, Hayashi T, Kozaki K, Kubo A, Omoto S, Watanabe A, et al. Enhanced S-cone syndrome in a Japanese family with a nonsense NR2E3 mutation (Q350X). Acta Ophthalmol Scand. 2004;82:616–22.
Usui T, Ichibe M, Tanimoto N, Ueki S, Takagi M, Hasegawa S, et al. Ocular fundus images by scanning laser ophthalmoscopy in a patient with enhanced S-cone syndrome. Retina. 2004;24:946–52.
Hayashi T, Gekka T, Goto-Omoto S, Takeuchi T, Kubo A, Kitahara K. Novel NR2E3 mutations (R104Q, R334G) associated with a mild form of enhanced S-cone syndrome demonstrate compound heterozygosity. Ophthalmology. 2005;112:2115–22.
Audo I, Michaelides M, Robson AG, Hawlina M, Vaclavik V, Sandbach JM, et al. Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci. 2008;49:2082–93.
Pachydaki SI, Klaver CC, Barbazetto IA, Roy MS, Gouras P, Allikmets R, et al. Phenotypic features of patients with NR2E3 mutations. Arch Ophthalmol. 2009;127:71–5.
Bandah D, Merin S, Ashhab M, Banin E, Sharon D. The spectrum of retinal diseases caused by NR2E3 mutations in Israeli and Palestinian patients. Arch Ophthalmol. 2009;127:297–302.
Genead MA, Fishman GA, McAnany JJ. Efficacy of topical dorzolamide for treatment of cystic macular lesions in a patient with enhanced S-cone syndrome. Doc Ophthalmol. 2010;121:231–40.
Rocha-Sousa A, Hayashi T, Gomes NL, Penas S, Brandão E, Rocha P, et al. A novel mutation (Cys83Tyr) in the second zinc finger of NR2E3 in enhanced S-cone syndrome. Graefes Arch Clin Exp Ophthalmol. 2011;249:201–8.
Cima I, Brecelj J, Sustar M, Coppieters F, Leroy BP, De Baere E, et al. Enhanced S-cone syndrome with preserved macular structure and severely depressed retinal function. Doc Ophthalmol. 2012;125:161–8.
Hull S, Arno G, Sergouniotis PI, Tiffin P, Borman AD, Chandra A, et al. Clinical and molecular characterization of enhanced S-cone syndrome in children. JAMA Ophthalmol. 2014;132:1341–9.
Hayashi T, Gekka T, Tsuneoka H. Spontaneous resolution of large macular retinoschisis in enhanced S-cone syndrome. Ophthalmic Surg Lasers Imaging Retina. 2016;47:187–90.
Vaclavik V, Chakarova C, Bhattacharya SS, Robson AG, Holder GE, Bird AC, et al. Bilateral giant macular schisis in a patient with enhanced S-cone syndrome from a family showing pseudo-dominant inheritance. Br J Ophthalmol. 2008;92:299–300.
Nakamura M, Hotta Y, Piao CH, Kondo M, Terasaki H, Miyake Y. Enhanced S-cone syndrome with subfoveal neovascularization. Am J Ophthalmol. 2002;133:575–7.
Lam BL, Goldberg JL, Hartley KL, Stone EM, Liu M. Atypical mild enhanced S-cone syndrome with novel compound heterozygosity of the NR2E3 gene. Am J Ophthalmol. 2007;144:157–9.
Hayashi T, Kitahara K. Optical coherence tomography in enhanced S-cone syndrome: large macular retinoschisis with disorganized retinal lamination. Eur J Ophthalmol. 2005;15:643–6.
Yzer S, Barbazetto I, Allikmets R, van Schooneveld MJ, Bergen A, Tsang SH, et al. Expanded clinical spectrum of enhanced S-cone syndrome. JAMA Ophthalmol. 2013;131:1324–30.
Arevalo JF, Kozak I. Enhanced S-cone syndrome and macular hole. JAMA Ophthalmol. 2015;133:e15108.
Greenstein VC, Zaidi Q, Hood DC, Spehar B, Cideciyan AV, Jacobson SG. The enhanced S cone syndrome: an analysis of receptoral and post-receptoral changes. Vis Res. 1996;36:3711–22.
Marmor MF, Tan F, Sutter EE, Bearse MA Jr. Topography of cone electrophysiology in the enhanced S cone syndrome. Invest Ophthalmol Vis Sci. 1999;40:1866–73.
Yamamoto S, Hayashi M, Takeuchi S. Electroretinograms and visual evoked potentials elicited by spectral stimuli in a patient with enhanced S-cone syndrome. Jpn J Ophthalmol. 1999;43:433–7.
Udar N, Small K, Chalukya M, Silva-Garcia R, Marmor M. Developmental or degenerative-NR2E3 gene mutations in two patients with enhanced S cone syndrome. Mol Vis. 2011;17:519–25.
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, et al. ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015;130:1–12.
Milam AH, Rose L, Cideciyan AV, Barakat MR, Tang WX, Gupta N, et al. The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc Natl Acad Sci USA. 2002;99:473–8.
Ripamonti C, Aboshiha J, Henning GB, Sergouniotis PI, Michaelides M, Moore AT, et al. Vision in observers with enhanced S-cone syndrome: an excess of S-cones but connected mainly to conventional S-cone pathways. Invest Ophthalmol Vis Sci. 2014;55:963–76.
Haider NB, Jacobson SG, Cideciyan AV, Swiderski R, Streb LM, Searby C, et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000;24:127–31.
Kobayashi M, Takezawa S, Hara K, Yu RT, Umesono Y, Agata K, et al. Identification of a photoreceptor cell-specific nuclear receptor. Proc Natl Acad Sci USA. 1999;96:4814–9.
Peng GH, Ahmad O, Ahmad F, Liu J, Chen S. The photoreceptor-specific nuclear receptor Nr2e3 interacts with Crx and exerts opposing effects on the transcription of rod versus cone genes. Hum Mol Genet. 2005;14:747–64.
Haider NB, Demarco P, Nystuen AM, Huang X, Smith RS, McCall MA, et al. The transcription factor Nr2e3 functions in retinal progenitors to suppress cone cell generation. Vis Neurosci. 2006;23:917–29.
Jacobson SG, Sumaroka A, Aleman TS, Cideciyan AV, Schwartz SB, Roman AJ, et al. Nuclear receptor NR2E3 gene mutations distort human retinal laminar architecture and cause an unusual degeneration. Hum Mol Genet. 2004;13:1893–902.
Sharon D, Sandberg MA, Caruso RC, Berson EL, Dryja TP. Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch Ophthalmol. 2003;121:1316–23.
Gerber S, Rozet JM, Takezawa S, dos Santos LC, Lopes L, Gribouval O, et al. The photoreceptor cell-specific nuclear receptor gene (PNR) accounts for retinitis pigmentosa in the crypto-Jews from Portugal (Marranos), survivors from the Spanish Inquisition. Hum Genet. 2000;107:276–84.
Coppieters F, Leroy BP, Beysen D, Hellemans J, De Bosscher K, Haegeman G, et al. Recurrent mutation in the first zinc finger of the orphan nuclear receptor NR2E3 causes autosomal dominant retinitis pigmentosa. Am J Hum Genet. 2007;81:147–57.
Fishman GA, Jampol LM, Goldberg MF. Diagnostic features of the Favre-Goldmann syndrome. Br J Ophthalmol. 1976;60:345–53.
Jacobson SG, Román AJ, Román MI, Gass JDM, Parker JA. Relatively enhanced S cone function in the Goldmann-Favre syndrome. Am J Ophthalmol. 1991;111:446–53.
Bonilha VL, Fishman GA, Rayborn ME, Hollyfield JG. Retinal pathology of a patient with Goldmann-Favre syndrome. Ophthalmic Genet. 2009;30:172–80.
Kuniyoshi K, Uno N, Irifune M, Shimomura Y. Electroretinography of short-wavelength-sensitive cones with a LED built-in electrode and its normal values. Doc Ophthalmol. 2003;106:311–8.
Mizunoya S, Kuniyoshi K, Arai M, Tahara K, Hirose T. Electroretinogram contact lens electrode with tri-color light-emitting diode. Acta Ophthalmol Scand. 2001;79:497–500.
PolyPhen (polymorphism phenotyping). 2012. http://genetics.bwh.harvard.edu/pph/data/. Accessed 29 Apr 2016.
PolyPhen-2 (polymorphism phenotyping v2). 2016. http://genetics.bwh.harvard.edu/pph2/. Accessed 29 Apr 2016.
SIFT. 2011. http://sift.jcvi.org/. Accessed 29 Apr 2016.
Align GVGD. In: International Agency for Research on Cancer, World Health Organization. 2016. http://agvgd.iarc.fr/. Accessed 29 Apr 2016.
LOVD v3.0 (Leiden Open Variation Database). 2016. http://www.lovd.nl/3.0/home. Accessed 29 Apr 2016.
National Center for Biotechnology Information (NCBI). 2016. http://www.ncbi.nlm.nih.gov/. Accessed 29 Apr 2016.
Human Gene Mutation Database (HGMD). 2016. https://www.qiagenbioinformatics.com/products/human-gene-mutation-database/. Accessed 29 Apr 2016.
Human Genetic Variation Database (HGVD). 2016. http://www.genome.med.kyoto-u.ac.jp/SnpDB/. Accessed 29 Apr 2016.
Acknowledgments
We thank the patients and their families for their kind participation in this study; Professor Kunihiko Shiraki and Doctor Kumiko Hirayama of Department of Ophthalmology and Visual Science, Graduate School of Medicine, Osaka City University, for providing precise clinical data on the parents of patient 1; Professor Emeritus Duco I. Hamasaki of the Bascom Palmer Eye Institute of the University of Miami, for his critical discussion and final manuscript editing. This research was supported in part by research grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan [Grant-in-Aid for Scientific Research (C) 2546738] and the Japan Agency for Medical Research and Development (Practical Research Project for Rare/Intractable Diseases, 15ek0109072h0002 and 26310601).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
K. Kuniyoshi, None; T. Hayashi, None; H. Sakuramoto, None; H. Mishima, None; H. Tsuneoka, None; K. Tsunoda, None; T. Iwata, None; Y. Shimomura, None.
About this article
Cite this article
Kuniyoshi, K., Hayashi, T., Sakuramoto, H. et al. New truncation mutation of the NR2E3 gene in a Japanese patient with enhanced S-cone syndrome. Jpn J Ophthalmol 60, 476–485 (2016). https://doi.org/10.1007/s10384-016-0470-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-016-0470-0