Summary
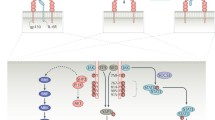
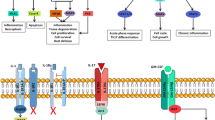
Proinflammatory cytokines are centrally involved in the pathogenesis of various rheumatic diseases. Interleukin (IL)-6 is a prototypic representative of this family. Basic research has uncovered a multitude of functions for this cytokine, such as immune regulation, hematopoiesis, inflammation, and oncogenesis (Fig. 1). In recent years, agents blocking the actions of IL-6 have been developed for the therapy of rheumatologic inflammatory diseases. While in some diseases, most notably rheumatoid arthritis, the clinical efficacy of these drugs was excellent, the results of clinical trials in other chronic inflammatory diseases were heterogeneous. In this review, we will summarize the data currently available on IL-6 blockade in chronic inflammatory diseases and will also discuss the safety issues of blocking this cytokine.
Zusammenfassung
Proinflammatorische Zytokine sind zentrale Faktoren in der Pathogenese von entzündlich rheumatischen Erkrankungen. Interleukin 6 ist ein prototypisches Beispiel dieser Zytokinfamilie. Interleukin 6 spielt eine wichtige Funktion in der Regulierung des Immunsystems und von Entzündungsreaktionen, der Blutbildung und der Onkogenese. Die Erkenntnisse aus der Grundlagenforschung legten den Grundstein für die Entwicklung von therapeutischen Ansätzen, die dieses Zytokin blockieren. Diese erwiesen sich in einigen Krankheiten, vor allem in der rheumatoiden Arthritis, als sehr effizient, in anderen nicht. In diesem Review werden die derzeit vorhandenen Daten aus diversen klinischen Prüfungen diskutiert und sowohl hinsichtlich Effektivität als auch hinsichtlich Sicherheit zusammengefasst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Tocilizumab in rheumatoid arthritis
The clinical efficacy of tocilizumab (TCZ) was evaluated in seven phase III randomized controlled trials (Table 1). Following the phase III clinical trials, several phase IIIb/IV studies were conducted.
Tocilizumab combination therapy
The OPTION (TOcilizumab Pivotal Trial in Methotrexate Inadequate RespONders) trial was the earliest global phase III study showing the efficacy of TCZ combined with methotrexate (MTX) in patients with rheumatoid arthritis (RA) who had an inadequate response to MTX. Other synthetic disease-modifying antirheumatic drugs (sDMARDs) were discontinued prior to the start of the study, and the patients were mostly naïve to biological DMARDs (bDMARDs). A total of 625 patients were randomized to intravenous administration of TCZ 8 mg/kg, 4 mg/kg, or placebo combined with stable MTX therapy (10–25 mg/week). At week 24, the primary end point, the ACR20 response was achieved in significantly more patients receiving TCZ, 8 mg/kg (59 %) and 4 mg/kg (48 %), compared with those receiving placebo (26 %). Secondary end points including ACR50, ACR70, and disease activity score (DAS)28 remission showed also superiority for TCZ over placebo control group [1].
The Tocilizumab in cOmbination With traditional DMARD (TOWARD) study, including more than 1200 patients, examined the combination of TCZ with any sDMARD, including MTX (75 %) and hydroxychloroquine (20 %), gold (0.5 %), salazopyrine (14 %), azathioprine (2 %), and leflunomide (14 %). This study included patients who had an inadequate response to these sDMARD but excluded patients who were previously treated with bDMARDS.
The patients were randomized in a 2:1 ratio to the TCZ (8 mg/kg every 4 weeks) or the placebo treatment groups in combination with sDMARDs at stable doses. ACR20 responses as well as secondary end points, including the ACR50, ACR70 improvements,DAS28, EULAR responses, and systemic markers of the acute-phase response (APR), were assessed at week 24. The ACR20 response rate was significantly higher at week 24 in patients receiving TCZ + sDMARD (61 versus 25 %; p < 0.0001) than placebo. Patients responded to TCZ regardless of the type and amounts of the accompanying sDMARDs with the exception of patients who had a background therapy consisting of at least three DMARDs, where the response to TCZ was less pronounced. In addition, all secondary end points were significantly different in favor of TCZ at week 24 [2].
To test the efficacy of TCZ in patients who had previously failed on tumor necrosis factor (TNF) inhibitors, the RADIATE (Research on Actemra Determining EffIcacy after Anti-TNF FailurEs) trial was performed. In this study, 499 patients with an inadequate response to one or more TNF inhibitors were randomized to receive a dose of TCZ 8 mg/kg or 4 mg/kg or a placebo plus MTX (10–25 mg weekly for 24 weeks). At week 24, the proportion of the ACR20 responders was significantly higher in both treatment groups (50.0 % in the 8 mg/kg, 30.4 % in the 4 mg/kg, compared with only 10.1 % in the control group (p < 0.001)). While the ACR20 response was not related to the type and number of prior TNF inhibitor therapies in the 8 mg/kg group, ACR50 and 70 response rates were much lower in patients who had previously received three TNF blockers. Interestingly, DAS28 remission was significantly higher only in the TCZ 8 mg/kg group than placebo, but not in the TCZ 4 mg/kg group [3]. The 8 mg/kg regimen was also found to normalize C-reactive protein (CRP) levels in patients, but not in the 4 mg/kg group, where CRP levels dropped on treatment, but remained above the upper limits of the normal (ULNs). In this context, it needs to be borne in mind that due to the dramatic effect of TCZ on acute-phase reactants, the use of the DAS28 score, which is heavily weighted on erythrocyte sedimentation rate (ESR) or CRP, does not deem ideal; especially, DAS28 remission rates may be exaggerated and, indeed, in the RADIATE trial, were even higher than 50 % improvement (ACR50) [4].
In the TociLIzumab Safety and THE Prevention of Structural Joint Damage (LITHE) study, 1196 patients with RA, who had an inadequate response to MTX, were randomized to the TCZ 8 mg/kg or 4 mg/kg treatment groups or a placebo control treatment group along with stable doses of MTX (ratio 1:1:1). In this study, the focus was on the effect of TCZ on radiographic progression. Primary end points were defined as the change from baseline in the total Genant-modified Sharp score and physical function (area under the curve of the Health Assessment Questionnaire Disability Index (HAQ-DI)), and secondary end points included the ACR response rates. The analysis of radiographic data demonstrated that TCZ plus MTX reduces radiographic progression by 74 and 70 % in the TCZ 8 mg/kg and TCZ 4 mg/kg groups, respectively, in comparison with the placebo group. The mean change in the total Genant-modified Sharp score was 0.29, 0.34, and 1.13 in the TCZ 8 mg/kg, TCZ 4 mg/kg, and the placebo group, respectively. In terms of physical function, the change in HAQ-DI score was significantly reduced in both treatment groups. In addition, the proportion of ACR20, 50, and 70 responders was also significantly higher in both TCZ groups at week 52 [5].
Tocilizumab monotherapy
The AMBITION (Actemra versus Methotrexate double-Blind Investigative Trial In mONotherapy) compared the efficacy of TCZ monotherapy with MTX monotherapy. A total of 673 patients, who were either MTX naive (66 %) or did not discontinue previous treatment with sDMARDs or bDMARDs because of lack of efficacy, were randomized to the TCZ 8 mg/kg treatment group or MTX treatment group. The study demonstrated that TCZ was more effective compared with MTX monotherapy. The proportion of ACR20, ACR50, and ACR70 responders were 70, 44, and 28 % in the TCZ treatment group versus 53, 34, 15 % in the MTX group, the difference being highly significant [6].
The Japanese SAMURAI trial (Study of Active controlled Monotherapy Used for Rheumatoid Arthritis, an IL-6 inhibitor), where the treatment was open label, but radiographic assessment was done blinded, compared TCZ 8 mg/kg with DMARDs (mostly MTX) and was ongoing for 52 weeks. This trial demonstrated significantly less radiographic progression in the TCZ group compared with the sDMARD group. Also, clinical response rates were significantly higher in the TCZ treatment group compared with sDMARD therapy (ACR20/50/70: 78, 64, and 44 % for TCZ-treated group versus 34, 13, and 6 % in the sDMARD-treated group) [7].
Moreover, in another Japanese trial, the SATORI (Study of active controlled Tocilizumab monotherapy for rheumatoid Arthritis) trial, the efficiency of TCZ monotherapy in patients with an inadequate response to MTX was shown. A total of 125 patients were randomized to either TCZ 8 mg/kg or low-dose MTX therapy. At 24 weeks, the primary end point, the ACR20 response was achieved in 80.3 % in the TCZ group compared with 25 % in the control group. However, it must be noted that in both Japanese studies, MTX was dosed in only 8 mg/week, which is due to regional differences in the MTX dose used, as MTX 8 mg/week is the maximum dosage approved in Japan [8].
In the ADACTA study (ADalimumab ACTemrA), a phase IV study comparing TCZ monotherapy with TNFi monotherapy, 326 patients were randomly assigned to adalimumab monotherapy or TCZ monotherapy. The study showed that TCZ monotherapy was superior to adalimumab monotherapy. This is in line with earlier studies on adalimumab monotherapy leading to the current practice of prescribing anti-TNF therapies only as combination therapies [9]. To address the real-life question whether it is better to either add TCZ to MTX or switch MTX to TCZ monotherapy in patients who had an inadequate response to MTX alone, the ACT-RAY Study was performed where 556 patients were randomized to either continue MTX with the addition of TCZ 8 mg/kg or switch to TCZ monotherapy. While the data suggested that there was no significant superiority of the add-on strategy over the switch strategy in both DAS28-ESR remission rates (40.4 versus 34.8 %) and ACR20/50/70 response rates (71.5/40.2/25.4 % versus 70.3/40.2/25.4 %), low disease activity (DAS28 < 3.2) was reached in significantly more patients of the add-on strategy group compared with the switch strategy, and radiographic changes were also significantly lower with combination than monotherapy of TCZ [10, 11].
Therefore, these studies suggest that TCZ is efficacious as monotherapy, that it is significantly more efficacious as MTX monotherapy (both with regard to clinical response and inhibition of structural damage) or TNFi monotherapy (clinical response) [12]. However, the clinical trial comparing TCZ monotherapy with TCZ and MTX has been currently analyzed. Therefore current recommendations suggest the combination therapy. However, for patients who are intolerant to or experienced adverse events (AEs) from sDMARDS, TCZ monotherapy can be considered [13].
As mentioned earlier in the text, as IL-6 inhibition has a considerable effect on the hepatic APR, it is very likely that the response rates of the previous described studies were overestimated due to the large influence of APR on many disease scores. Therefore, Smolen and Aletaha [14] used data from the three randomized clinical trials (LITHE, OPTION, and TOWARD) and investigated the efficiency of TCZ on RA disease activity, using measures that do (DAS28 and simplified disease activity index (SDAI)) or do not (clinical disease activity index (CDAI)) comprise acute-phase reactants. Their study showed that disease activity is significantly reduced by TCZ therapy independent of the type of composite measures used to assess disease activity. However, when using DAS28 compared with SDAI and CDAI, the remission rates in the TCZ groups were much higher (30 against 7.7 and 6.4 %), which can be explained by the high weight of the ESR (and also CRP) in the DAS28 formula. Of note, using the CDAI index, the remission rates in patients treated with TCZ were similar to those treated with TNF inhibitors [14].
Safety profile of tocilizumab
Schiff et al. [15] analyzed in their study the cumulative safety of TCZ using data from five phase III trials (OPTION, AMBITION, RADIATE, TOWARD, LITHE), two extension trials, and one clinical pharmacology study. The overall AEs were 339.0/100 patient years (PY) in the placebo group, 358.0/100 PY in the 4 mg/kg group, and 381.6/100 PY in the 8 mg/kg group.
Infections were reported to be the most frequent AEs and serious AEs (SAEs) in both groups. The rate of serious infections was highest for the TCZ 8 mg/kg group (4.9/100 PY) compared with 3.5/100 PY for the TCZ 4 mg/kg group and 3.5/100 PY for the control group. Rate of serious infections were greater in older patients, overweight individuals, patients with pre-existing pulmonary disease or diabetes, and patients who were previously treated with TNFi, regardless of the treatment group. The most common infections were pneumonia and skin infections. In terms of Mycobacterium tuberculosis infection, seven cases were reported, of whom all received TCZ 8 mg/kg. As with SAEs, the rate of infections did not increase over time [15]. Another meta-analysis using data from six randomized controls trials also including RADIATE, OPTION, TOWARD, and AMBITION showed a significant greater risk for infections for the treatment of TCZ 8 mg/kg in combination with MTX compared with a control group [16]. A meta-analysis by Singh et al. [17] reported similar rates for serious infections in comparison with other bDMARDS, including TNFi. Although these analyses have to be interpreted with some due caution, the safety of TCZ seems to be comparable to other bDMARDS.
Regarding malignancies, the overall rate was 0.7/100, 1.4/100, and 0.7/100 PY in the control, TCZ 4 mg/kg, and TCZ 8 mg/kg groups. Again, the overall rate of malignancy of 1.1/100 PY in patients who received TCZ treatment was similar to published data on a large cohort of RA patients who were mostly treated with TNFi (62 %) with a malignancy rate of 1.3/100 PY [15, 18].
Schiff et al. reported on 18 cases of lower gastrointestinal (GI) perforations (1.9/1000 PY) during TCZ treatment, whereof 16 were associated with underlying diverticulitis. The majority of these patients were taking oral glucocorticoids, which are associated with risk of lower GI tract (GIT) perforation (3.9/1000 PY) [15, 19]. Of note, the rate of GIT perforations for TNFi was 1.3/1000 PY as analyzed from the data of the United Health Care database [19]. Thus, patients with previously reported diverticulitis (not diverticulosis) seem to have a slightly higher risk for lower GI perforations compared with TNFi. Glucocorticoids and/or nonsteroidal anti-inflammatory drugs (NSAIDs) put those patients in an additional risk. Therefore, abdominal pain should be taken seriously in all patients during TCZ treatment.
Increased liver enzymes alanine transaminase (ALT) and aspartate transaminase (AST) are associated with TCZ treatment. This increase was generally mild and reversible and importantly not associated with drug-induced liver injury [15]. An increase in ALT and AST more than three times the ULN was less frequent with TCZ monotherapy. In the case of ongoing liver enzyme elevation in a range of one to three times the ULN, a dose reduction to 4 mg/kg or interruption is recommended until normalization; if liver enzymes increase to more than three times the ULN, therapy should be discontinued and can be started again at a lower dose once liver enzymes are less than three times the ULN and resumed at 8 mg after liver enzyme normalization. For sustained liver enzyme elevation more than three times the ULN or for any elevation more than five times the ULN, therapy should be stopped [20].
Neutropenia was reported frequently during TCZ treatment and generally did not appear associated with an increased risk of infections [15]. Nevertheless, TCZ treatment should be stopped if neutrophil counts are < 500/mm3. At counts of 500–1000/mm3, treatment should be discontinued and started again at a lower dose when neutrophil counts are > 1000/mm3 [20]. In addition, decreases in mean platelet counts were observed in the TCZ groups at week 2 compared with control group. Grade 3 (thrombocytes 25–50 x10 9/L) and grade 4 (thrombocytes < 25 g/l) thrombocytopenia occurred in 32 patients treated with TCZ, of whom just 1 suffered a serious bleeding event (hemorrhagic stomatitis). Consequently, the treatment was stopped, and the event resolved without further complications [15].
Finally, a significant increase in mean cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides were reported in phase III clinical trials. As generally serum cholesterol and LDL levels are low in RA patients, this elevation of cholesterol might either be a drug effect or because TCZ reduces disease activity, and thus, lipid levels return to normal [21].
Concerning cardiovascular events during the phase III trials, the myocardial infarction rate was reported to be 0.49/100 PY in the control group, 0.18/100 PY in the TCZ 4 mg/kg group, and 0.17/100 PY in the TCZ 8 mg/kg group. The rate of strokes was 0.24/100 PY in the control group, 0/100 PY in the TCZ 4 mg/kg group, and 0.33/100 PY in the TCZ 8 mg/kg group. Altogether, the rate of cardiovascular events in these trials was low. Importantly, also the 5-year follow-up study of the STREAM trial showed no increased risk of cardiovascular disease during TCZ long-term treatment [22]. Recently, the MEASURE trial was published, which was performed to investigate the risk of TCZ treatment for vascular disease. Consistent with prior data, LDL and triglyceride levels increased in the TCZ treatment group versus placebo. No changes in mean small LDL, oxidized LDL, or total HDL were detected. However, TCZ significantly decreased lipoprotein a, fibrinogen, and D-dimer, which are also vascular risk factors. Thus, the net effect of TCZ on vascular risk is yet not clear [23].
Novel IL-6-blocking agents
Olokizumab, a humanized anti-IL-6 monoclonal antibody (mAb), has been tested in a 12-week phase IIb study in RA patients with moderate-to-severe disease activity despite TNF inhibitors [24]. Patients were randomized to receive placebo or olokizumab in various dosing regimens (60, 120, or 240 mg either every 4 weeks or every 2 weeks) and compared with TCZ 8 mg/kg, providing the first head-to-head comparison of drugs targeting the same molecule. All patients received background MTX. All olokizumab doses tested were better than placebo in inducing an ACR20 response. Olokizumab showed comparable efficacy to TCZ across multiple end points, and also AEs were similar to TCZ [24].
Sarilumab is a human anti-IL-6Ra mAb and therefore similar to TCZ. Patients with active RA despite MTX were randomized to receive placebo or sarilumab in various dosing regimens (100, 150, or 200 mg every other week or 100 or 150 mg weekly) with concomitant MTX therapy. The proportion of patients achieving an ACR20 response (primary end point) at week 12, compared with placebo, was only significantly higher for sarilumab 150 mg weekly (72.0 versus 46.2 %, p = 0.0203), 150 mg every other week (67 %; p = 0.0363), and 200 mg every other week (65 %; p = 0.0426), probably due to the high placebo response. For ACR50 and ACR70, the difference between treated and placebo was considerably better. Infections were the most common treatment-emerged adverse event (TEAEs). Cholesterol elevation was also reported [25].
Sirukumab, a human anti-IL-6 mAb was evaluated in two parts of a phase II study to assess the safety and efficacy of subcutaneous sirukumab in patients with active RA despite MTX [26]. In part A, 36 patients were randomized to placebo or sirukumab 100 mg every other week through week 10, with crossover treatment during weeks 12–22. In the dose-finding part B, 151 patients were randomized to receive sirukumab (100 mg every other week or 25, 50, or 100 mg every 4 weeks) until week 24, or placebo until week 10, with patients then being crossed over to sirukumab 100 mg every other week. The primary end point was an ACR50 response at week 12, which was achieved only with sirukumab 100 mg biweekly (26.7 versus 3.3 % with placebo; p = 0.026). The incidence of TEAEs was similar among the sirukumab and placebo groups. AEs and changes in laboratory values were consistent with previous reports of drugs of this class [26].
A dose-finding phase IIb study using clazakizumab, a humanized anti-IL-6 mAb, for RA patients has also been reported [27]. At day 1 and week 8, patients on a stable dose of MTX received clazakizumab (80, 160, and 320 mg) or placebo. The proportion of patients reaching an ACR20 response was significantly higher in patients receiving clazakizumab than placebo (27 %): 80 mg: 81 %, p < 0.0001 versus placebo; 160 mg: 71 %, p < 0.001 versus placebo; 320 mg: 82 %, p < 0.0001 versus placebo. Treatment with clazakizumab was associated with elevated liver enzymes and serum cholesterol, and no serious infections were reported [27].
Tocilizumab in other chronic inflammatory diseases
Tocilizumab in ankylosing spondylitis
Ankylosing spondylitis (AS) is a chronic inflammatory disorder primarily affecting younger male individuals (< 45 years) and is characterized by inflammation in the sacroiliacal joints and the spine, and also affects peripheral joints and entheses. Extraskeletal manifestations include eyes and the GIT. Biological therapies (TNF inhibitors) have revolutionized the treatment of this disease. Similar to TNF, IL-6 has been demonstrated to be elevated in patients with AS, and the levels of IL-6 have been demonstrated to correlate with disease activity [28–30]. In addition, treatment of patients with AS with anti-TNF therapies reduces the levels of IL-6. However, in an experimental model of peripheral arthritis and sacroiliitis, the TNFtg mouse model, IL-6 deficiency did not prevent sacroiliac joint inflammation and destruction [31]. In line with these findings, Sieper et al. [32] reported the results of the Builder 1 and the Builder 2 trial, where the efficacy and safety of TCZ (8 mg/kg of body weight) in patients with AS were analyzed. Both studies were multicenter double-blind, randomized, and placebo-controlled and enrolled patients fulfilling the modified New York criteria for AS who had active disease as assessed by the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) > 4. In Builder 1, patients were recruited who were responding inadequately or were intolerant to ≥ 1 current or previous NSAIDs, but were TNFi naïve, whereas in Builder 2, patients had to have responded inadequately to ≥ 1 TNFi agent(s) administered for ≥ 3 months and had to have elevated CRP levels (> 0.3 mg/dl).
At week 12, there was no difference between placebo and TCZ, as similar proportions of patients reached an ASAS 20 response (37.3 and 27.5 %, respectively; p = 0.2823) or ASAS 40 response (11.8 and 19.6 %; p = 0.2694). The trial was stopped prematurely on account of these results, so no patients were recruited into the Builder 2 study. Therefore, no data are available for the role of TCZ in TNFi nonresponders. Also, the analysis of peripheral arthritis did not reveal differences between the treatment and the placebo group. AEs were similar between the two treatment arms, and there were no opportunistic infections, serious infections, AEs leading to study withdrawal or deaths reported. There was, however, a reduction in CRP levels in patients treated with TCZ, which was in accordance with the idea that IL-6 is important in the APR, indicating sufficient IL-6 receptor blockade in this trial. Therefore, TCZ is not an effective treatment for patients with AS.
Tocilizumab in psoriatic arthritis
In contrast to RA, where TCZ has been studied extensively, data concerning the use of this drug in psoriatic arthritis are limited to case reports, and the reported results were mixed [33, 34].
Tocilizumab in SLE
Systemic lupus erythematosus (SLE) is a prototypic autoimmune disease characterized by autoantibody production, complement activation, and leukocyte infiltration in target organs such as the skin, kidney, lung, joints, and others. B cells are considered one of the major cell types involved in the pathogenesis of this disease. Animal studies have suggested an important role of IL-6 in lupus-like diseases, and the important role of this cytokine in regulating B cells has long been recognized, highlighted by the fact that one of the older names for IL-6 was B-cell stimulating factor [35–39]. Lupus patients display elevated serum levels of IL-6, and some studies demonstrated a correlation of IL-6 serum levels with disease activity [38, 39]. Despite the clear rationale for a role of IL-6 in the pathogenesis of also human SLE, data of randomized controlled trials are missing. However, there has been a report on a phase I open-label study by Illei et al. [40] at the NIH. In this study, 16 patients fulfilling the American College of Rheumatology classification criteria for SLE were enrolled. Inclusion criteria were somewhat complicated, but overall included patients with moderately active SLE according to the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI). As one of the anticipated effects of IL-6 receptor blockade in SLE was an effect on either the acute-phase reaction or the production of autoantibodies, the presence of at least one of the following serological markers had to be present: serum anti-dsDNA antibody level > 30 IU, anticardiolipin antibody level > 20 IgG phospholipid units/ml, a CRP level of > 0.8 mg/dl, or an ESR > 25 mm/h in men and > 42 mm/h in women. There was a significant decrease in anti-dsDNA antibodies, whereas there was no change in anti-SSA, anti-SSB, or anticardiolipin antibodies. In addition, plasma cell counts were also reduced significantly, while overall T and B lymphocyte counts remained stable. As expected, ESR and fibrinogen levels dropped significantly after treatment with TCZ. The authors also noted a decrease in complement levels after treatment. As complement is an acute-phase reactant, and is also used to monitor serological activity in SLE (low complement indicating complement consumption, which indicates active disease), the authors measured complement activation products in those patients. They found decreased levels of complement activation products, which led to the conclusion that TCZ most likely decreased complement production due to its inhibitory effects on the acute-phase reaction.
Analyzing the efficacy of TCZ in this small open-label trial, the authors showed a reduction in various disease activity scores. Subanalysis revealed an effect on arthritis in SLE patients, with the mean number of swollen joints improving from 7.7 at baseline to 1.1 at the end of the treatment. In five patients with renal disease at baseline, there was no change in the mean level of proteinuria. With regard to side effects, infections were the most common, with approximately 60 % of the patients experiencing at least one infection during the study period, which was higher than expected. However, most of the infections were mild and required no treatment. To fully be able to estimate the risk of AEs of TCZ in patients with SLE, larger studies are necessary.
Tocilizumab in Castleman disease
Castleman disease (also known as giant or angiofollicular lymph node hyperplasia, lymphoid hamartoma, angiofollicular lymph node hyperplasia) is a very rare disorder with lymphoproliferative features. It occurs in a unicentric and a multicentric form. Whereas in the unicentric form only one location in the body is affected, and surgery is the best option for curative treatment, the multicentric form requires systemic therapy. The disease is characterized by systemic symptoms such as fever, fatigue, splenomegaly, hepatomegaly, skin rash, and severe growth retardation. Abnormal laboratory findings include anemia, hypergammaglobulinemia, and increased levels of acute-phase proteins. Increased production of IL-6 by the affected lymph nodes is thought to be responsible for the systemic manifestations of the disease. Due to the rarity of the condition, no controlled trials of IL-6 blockade in this disease are available, but there are a number of small series and case studies reporting successful treatment and induction of remission with TCZ [41, 42].
Tocilizumab in JIA
TCZ has also been successfully used in the treatment of systemic juvenile idiopathic arthritis (JIA), which is the most severe form of JIA. In this disease of children and adolescents, chronic arthritis is accompanied by systemic manifestations such as fever, hepatosplenomegaly, lymphadenopathy, serositis, and substantially elevated markers of systemic inflammation. The concentrations of IL-6 in serum have been shown to reflect the extent and severity of not only joint involvement but also fever patterns and platelet counts [43]. Children and adolescents 2–19 years of age with disease onset before their 16th birthday were included if they met the International League of Associations for Rheumatology classification criteria for systemic-onset JIA. They had to have active disease, defined by an increase in CRP concentrations (CRP ≥ 15 mg/l) and an inadequate response to corticosteroids (at ≥ 0.2 mg/kg prednisolone equivalent) for longer than 3 months. Conventional DMARDs had to be stopped 2 weeks and biological DMARDs 12 weeks before the first administration of TCZ. The trial consisted of two phases, the first being an open-label lead-in phase, where all the patients enrolled received TCZ (8 mg/kg). All patients responding favorably to therapy as measured by an ACR Pedi 30 went on to a second double-blinded phase. In this second phase, 20 patients continued treatment, and 23 were allocated to receive placebo. A total of 80 % of the patients receiving TCZ showed maintained or improved response, whereas only 17 % of the placebo-treated patients did. Laboratory indicators of acute-phase reactants changed rapidly after the first infusion of TCZ. White-blood-cell, neutrophil, and platelet counts also decreased in patients receiving treatment. In addition, body temperature returned to normal and the median hemoglobin concentrations increased. AEs consisted mainly of mild to moderate infections and infusion reactions.
Another study analyzing the efficacy of TCZ in systemic JIA used a randomized, double-blind, placebo-controlled design and included 112 patients aged 2–17 years with an inadequate response to NSAIDs and glucocorticoids. A significantly higher proportion of patients in the TCZ group than in the placebo group had a JIA ACR70 response (71 versus 8 %), or even a JIA ACR90 response (37 versus 5 %). In addition, also the systemic symptoms (fever and rash) and laboratory abnormalities (anemia, thrombocytosis) significantly improved after treatment with TCZ.
Tocilizumab in large-vessel vasculitis
IL-6 has been shown to be expressed in the inflamed arteries of patients with giant cell arteritis (GCA) and Takayasu arteritis (TA) [44], and serum IL-6 levels have been found to be raised and to correlate with disease activity in these patients [45]. Recently, TCZ has also been reported to improve large-vessel vasculitides such as giant cell arteritis and Takayasu arteritis [46–48]. Although there are only case reports yet, the clinical data look encouraging. Patients treated with TCZ had a multitude of prior treatments and required continuously high doses of glucocorticoids. After receiving TCZ, almost all patients achieved clinical remission, and steroids could be reduced significantly. That said, randomized controlled trials are needed to confirm the positive effect of anti-IL-6 therapy in these diseases.
Tocilizumab in adult-onset Still’s disease
Adult-onset Still’s disease is a systemic autoinflammatory disease characterized by fever, joint involvement, and rashes of a distinctive salmon-like color. In addition, various other systemic symptoms can occur, such as serositis, lymphadenitis, and fatigue, among others. The treatment of this rare disease is limited to steroids and IL-1-blocking agents. Successful open-label treatments with TCZ of patients suffering from this disease refractory to standard treatment have been reported; however, controlled trials have not been performed yet [49].
In summary, evidence from numerous clinical trials shows that IL-6 blocking plays an important role in the treatment of RA, with a safety profile similar to other biological DMARDs. Excellent clinical data also exist for JIA, leading to the approval of TCZ for this disease in Europe and the USA. In addition, Castleman disease can be treated successfully with this drug. No effect has been demonstrated for TCZ in AS. For some systemic rheumatologic diseases such as SLE, large-vessel vasculitides, adult-onset Still’s disease, or psoriatic arthritis, available data do not allow to draw conclusions yet. However, some encouraging open-label treatments might lead to the development of controlled clinical trials.
Conflict of interest
The authors declare that there are no actual or potential conflicts of interest in relation to this article.
References
Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, Woodworth T, Alten R, Investigators O. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–97.
Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, Woodworth T, Gomez-Reino JJ. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–80.
Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, Alecock E, Lee J, Kremer J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–23.
Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute-phase reactants. Arthritis Rheum. 2011;63:43–52.
Kremer JM, Blanco R, Brzosko M, Burgos-Vargas R, Halland AM, Vernon E, Ambs P, Fleischmann R. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2010;63:609–21.
Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, Siri DA, Tomsic M, Alecock E, Woodworth T, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2009;69:88–96.
Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Murata N, van der Heijde D, Kishimoto T. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–7.
Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, Kishimoto T. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19:12–9.
Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, Sharp J, Perez JL, Spencer-Green GT. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37.
Dougados M, Kissel K, Sheeran T, Tak PP, Conaghan PG, Mola EM, Schett G, Amital H, Navarro-Sarabia F, Hou A, et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis. 2013;72:43–50.
Dougados M, Kissel K, Conaghan PG, Mola EM, Schett G, Gerli R, Hansen MS, Amital H, Xavier RM, Troum O, et al. Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: the ACT-RAY study. Ann Rheum Dis. 2014;73:803–9.
Singh JA, Beg S, Lopez-Olivo MA. Tocilizumab for rheumatoid arthritis: a Cochrane systematic review. J Rheumatol. 2011;38:10–20.
Smolen JS, Schoels MM, Nishimoto N, Breedveld FC, Burmester GR, Dougados M, Emery P, Ferraccioli G, Gabay C, Gibofsky A, et al. Consensus statement on blocking the effects of interleukin-6 and in particular by interleukin-6 receptor inhibition in rheumatoid arthritis and other inflammatory conditions. Ann Rheum Dis. 2013;72:482–92.
Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute-phase reactants. Arthritis Rheum. 2010;63:43–52.
Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther. 2011;13:R141.
Campbell L, Chen C, Bhagat SS, Parker RA, Ostor AJ. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford). 2011;50:552–62.
Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, Filippini G, Skoetz N, Francis D, Lopes LC, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011:CD008794.
Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: analyses from a large US observational study. Arthritis Rheum. 2007;56:2886–95.
Gout T, Ostor AJ, Nisar MK. Lower gastrointestinal perforation in rheumatoid arthritis patients treated with conventional DMARDs or tocilizumab: a systematic literature review. Clin Rheumatol. 2011;30:1471–4.
Smolen JS, Schoels MM, Nishimoto N, Breedveld FC, Burmester GR, Dougados M, Emery P, Ferraccioli G, Gabay C, Gibofsky A, et al. Consensus statement on blocking the effects of interleukin-6 and in particular by interleukin-6 receptor inhibition in rheumatoid arthritis and other inflammatory conditions. Ann Rheum Dis. 2013;72:482–92.
Md Yusof MY, Emery P. Targeting interleukin-6 in rheumatoid arthritis. Drugs. 2013;73:341–56.
Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis. 2009;68:1580–4.
McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, Dahmen G, Wollenhaupt J, Schulze-Koops H, Kogan J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis. 2013;73:349–56.
Genovese MC, Fleischmann R, Furst D, Janssen N, Carter J, Dasgupta B, Bryson J, Duncan B, Zhu W, Pitzalis C, et al. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb study. Ann Rheum Dis. 2014;73:1607–15.
Huizinga TW, Fleischmann RM, Jasson M, Radin AR, van Adelsberg J, Fiore S, Huang X, Yancopoulos GD, Stahl N, Genovese MC. Sarilumab, a fully human monoclonal antibody against IL-6Ralpha in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis. 2014;73:1626–34.
Smolen JS, Weinblatt ME, Sheng S, Zhuang Y, Hsu B. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2014;73:1616–25.
Mease P, Strand V, Shalamberidze L, Dimic A, Raskina T, Xu LA, Liu Y, Smith J. A phase II, double-blind, randomised, placebo-controlled study of BMS945429 (ALD518) in patients with rheumatoid arthritis with an inadequate response to methotrexate. Ann Rheum Dis. 2012;71:1183–9.
Gratacos J, Collado A, Filella X, Sanmarti R, Canete J, Llena J, Molina R, Ballesta A, Munoz-Gomez J. Serum cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in ankylosing spondylitis: a close correlation between serum IL-6 and disease activity and severity. Br J Rheumatol. 1994;33:927–31.
Bal A, Unlu E, Bahar G, Aydog E, Eksioglu E, Yorgancioglu R. Comparison of serum IL-1 beta, sIL-2R, IL-6, and TNF-alpha levels with disease activity parameters in ankylosing spondylitis. Clin Rheumatol. 2007;26:211–5.
Falkenbach A, Herold M. In ankylosing spondylitis serum interleukin-6 correlates with the degree of mobility restriction, but not with short-term changes in the variables for mobility. Rheumatol Int. 1998;18:103–6.
Hayer S, Niederreiter B, Nagelreiter I, Smolen J, Redlich K. Interleukin-6 is not a crucial regulator in tumour necrosis factor-mediated bilateral sacroiliitis. Ann Rheum Dis. 2010;69:1403–6.
Sieper J, Porter-Brown B, Thompson L, Harari O, Dougados M. Assessment of short-term symptomatic efficacy of tocilizumab in ankylosing spondylitis: results of randomised, placebo-controlled trials. Ann Rheum Dis.2014;73:95–100.
Ogata A, Umegaki N, Katayama I, Kumanogoh A, Tanaka T. Psoriatic arthritis in two patients with an inadequate response to treatment with tocilizumab. Joint Bone Spine. 2012;79:85–7.
Atzeni F, Ventura D, Batticciotto A, Boccassini L, Sarzi-Puttini P. Interleukin 6 blockade: tocilizumab in psoriatic arthritis. J Rheumatol. 2012;39:97–9.
Suzuki H, Yasukawa K, Saito T, Narazaki M, Hasegawa A, Taga T, Kishimoto T. Serum soluble interleukin-6 receptor in MRL/lpr mice is elevated with age and mediates the interleukin-6 signal. Eur J Immunol. 1993;23:1078–82.
Tang B, Matsuda T, Akira S, Nagata N, Ikehara S, Hirano T, Kishimoto T. Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol. 1991;3:273–8.
Finck BK, Chan B, Wofsy D. Interleukin 6 promotes murine lupus in NZB/NZW F1 mice. J Clin Invest. 1994;94:585–91.
Chun HY, Chung JW, Kim HA, Yun JM, Jeon JY, Ye YM, Kim SH, Park HS, Suh CH. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol. 2007;27:461–6.
Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–23.
Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, Fleisher T, Balow JE, Lipsky PE. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62:542–52.
Kurzrock R, Voorhees PM, Casper C, Furman RR, Fayad L, Lonial S, Borghaei H, Jagannath S, Sokol L, Usmani SZ, et al. A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clin Cancer Res. 2013;19:3659–70.
Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–64.
de Benedetti F, Massa M, Robbioni P, Ravelli A, Burgio GR, Martini A. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 1991;34:1158–63.
Weyand CM, Hicok KC, Hunder GG, Goronzy JJ. Tissue cytokine patterns in patients with polymyalgia rheumatica and giant cell arteritis. Ann Intern Med. 1994;121:484–91.
Roche NE, Fulbright JW, Wagner AD, Hunder GG, Goronzy JJ, Weyand CM. Correlation of interleukin-6 production and disease activity in polymyalgia rheumatica and giant cell arteritis. Arthritis Rheum. 1993;36:1286–94.
Beyer C, Axmann R, Sahinbegovic E, Distler JH, Manger B, Schett G, Zwerina J. Anti-interleukin 6 receptor therapy as rescue treatment for giant cell arteritis. Ann Rheum Dis. 2011;70:1874–5.
Unizony S, Arias-Urdaneta L, Miloslavsky E, Arvikar S, Khosroshahi A, Keroack B, Stone JR, Stone JH. Tocilizumab for the treatment of large-vessel vasculitis (giant cell arteritis, Takayasu arteritis) and polymyalgia rheumatica. Arthritis Care Res (Hoboken). 2012;64:1720–9.
Nishimoto N, Nakahara H, Yoshio-Hoshino N, Mima T. Successful treatment of a patient with Takayasu arteritis using a humanized anti-interleukin-6 receptor antibody. Arthritis Rheum. 2008;58:1197–1200.
de Boysson H, Fevrier J, Nicolle A, Auzary C, Geffray L. Tocilizumab in the treatment of the adult-onset Still’s disease: current clinical evidence. Clin Rheumatol. 2013;32:141–7.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Puchner, A., Blüml, S. IL-6 blockade in chronic inflammatory diseases. Wien Med Wochenschr 165, 14–22 (2015). https://doi.org/10.1007/s10354-014-0321-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-014-0321-x