Abstract
Rich and diverse trocholinid assemblages have been recorded from the lower to middle Cenomanian Altamira and Bielba formations of the Bascocantabrian Basin, northern Spain. They occur in bioclastic for-algal grainstones and near-reefal rudstones referred to platform margin environments. This material, partially impregnated by iron-rich solutions, is unusually well-preserved and offers the unique opportunity to carry out a detailed study of the last diversified assemblages of the aragonitic foraminiferal order Involutinida. It notably allows (1) describing one new subfamily (Coscinoconinae n. subfam.), two new genera (Hensonipapillus n. gen. and Paracoscinoconus n. gen.), and five new species (Coscinoconus discoideus n. sp., Frentzenella pygmaea n. sp., Hensonipapillus cantabricus n. sp., H. altamirensis n. gen., n. sp., and Paracoscinoconus semiinvolutus n. sp.); (2) clarifying the relationship between the umbilical canal system and the laminar deposits in Late Jurassic–Cretaceous coscinoconins; (3) emending and providing new combinations for “Trocholina” lenticularis Henson, 1947 “T.” minima Henson, 1947 and “T.” burlini Gorbatchik, 1959 which have been repeatedly misidentified in the literature; (4) discussing the epibenthic mode of life of trocholinids; and (5) proposing an explanation for their extinction at the Cenomanian–Turonian boundary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trocholinid benthic foraminifers are typical constituents of Mesozoic carbonate platforms, in both muddy lagoonal and external to well-agitated near-reefal environments (e.g., Blau 1987a, b; Arnaud-Vanneau et al. 1988; Senowbari-Daryan et al. 2010; Rigaud et al. 2013). Owing to their original aragonitic nature (Reichel 1955), (micro)structural details of their tests are commonly destroyed by diagenetic alteration and thus only rarely discernible. This is of importance since certain skeletal and architectural features are either considered to have specific or generic significance. The taxonomy and phylogeny of trocholinids has therefore been highly disputed (e.g., Koehn-Zaninetti 1969; Piller 1978, 1983; Arnaud-Vanneau et al. 1988; di Bari and Laghi 1994; Neagu 1994, 1995; Rigaud et al. 2013). In the Cretaceous literature, the usual poor preservation of trocholinids and the redundant introduction of the same taxa as new have produced significant confusion. Common misidentification of types and inadequately selected type material have led to approximative determinations and misuse of the group.
A diverse and abundant association of trocholinid foraminifers is reported from lower to middle Cenomanian strata of the Altamira and Bielba formations (northern Cantabria, Spain). The material, particularly well preserved, permits the recognition of usually obliterated structural details, allowing a thorough description of new and hitherto poorly known Cenomanian representatives of the family Trocholinidae.
Geology
Geological setting
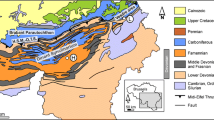
The study area is located in the northern part of the Spanish province Cantabria, along the coast of the Bay of Biscay, where Cretaceous strata are superbly exposed (Fig. 1a). Following two rifting phases between the European and Iberian plates in the Triassic and in the Late Jurassic/earliest Cretaceous, a zip-like sea-floor spreading proceeded eastward into the Gulf of Biscay from the early Aptian onwards (e.g., Olivet 1996; Fig. 1b). Due to related distensional movements, numerous sedimentary intra-shelf basins developed on the tectonically very active North Iberian passive margin, commonly as halfgrabens due to the tilting of fault blocks (Fig. 1c). The North Cantabrian Basin (NCB; Wiese and Wilmsen 1999) represents one of these extensional basins. During mid-Cretaceous times, the NCB was a gulf-like, E–W-elongated embayment opening to the strongly subsiding Basque Basin/Navarro-Cantabrian Ramp to the east while progressively shallowing to the west (Wilmsen 1997, 2000, 2005). To the south, it was delimited by the Cabuerniga Ridge (an east–west-trending horst-like structure formed of Palaeozoic and Triassic rocks) and to the north by a swell located in the Bay of Biscay (“Liencres High” of Wiese 1995; Fig. 1c). Deposition occurred under “wet (sub-)tropical” climatic conditions at a palaeo-latitude of about 30°N (Rat 1989; Philip and Floquet 2000). The mid-Cretaceous (Aptian–Turonian) depositional history of the basin has been treated in detail by Wilmsen et al. (1996), Wiese (1997), Wilmsen (1997, 2000, 2005), Wiese and Wilmsen (1999), and Najarro et al. (2011)
Regional topographical and geological framework. a The study area indicating the La Rabia, Cobreces–Toñanes and Liencres sections (modified from Wiese 1997). b Cenomanian palaeogeographical framework of the western Tethys (modified from Philip and Floquet 2000) indicating the North Cantabrian Basin (asterisk). c Structural setting of the North Cantabrian Basin (modified after Wilmsen 2000)
The thin-section material studied has been predominantly sampled from the Cenomanian Altamira Formation (Fig. 2). Previous studies of this unit focused on isolated sections (Reitner 1987) or on special features such as hardgrounds or patch reefs (Reitner 1989; Reitner et al. 1995; Wilmsen 1996). The first detailed approach based on an integrated study of numerous sections and a regional multistratigraphic framework of the complete Cenomanian succession was presented by Wilmsen (1997, 2000). The Cenomanian transgression, demonstrably pulsatory in character, progressively onlapped onto deltaic siliciclastics of latest Albian to earliest Cenomanian age (Bielba Formation, lower member; Figs. 2, 3). The pronounced Cenomanian 2nd-order sea-level rise was subdivided by Wilmsen (2000) into an earlier constructive phase and a later destructive phase, separated by a significant low sea-level stand at the early/middle Cenomanian boundary interval (global sequence boundary SB Ce 3). In the first phase (earliest Cenomanian), the flooding of the north Iberian continental margin caused a withdrawal of terrigenous sediment sources onto the Iberian Meseta, promoting the development of a carbonate ramp from which few of our specimens come from (Bielba Formation, upper member). This ramp evolved into a shelf-attached platform as soon as the early Cenomanian (Altamira Platform) (Fig. 3). The pronounced sea-level fall marking the early/middle Cenomanian boundary interval exposed the platform, increasing fluvial input and ending the constructive phase. During the middle to early late Cenomanian destructive phase, the Altamira Platform drowned stepwise, first on its east side, then on its west side (Wilmsen 2000). An associated Fe-rich, diachronous (middle–upper Cenomanian), condensed discontinuity surface known as “Nivel ferruginizado” (Carreras Suárez and Ramírez del Pozo 1971) or “Hardground 99” caps the Altamira Limestone (Wilmsen 2000; Fig. 3).
Lithostratigraphy of the Albian/Cenomanian in the study area (after Wilmsen 1997)
Localities and stratigraphy
Thin-sections from the following sections have been studied.
(a) The Liencres standard section ca. 10 km west of Santander (Figs. 1a, 3). Base of the section is at 43°28′11.58″N, 3°56′39.79″W, top at 43°28′10.68″N, 3°56′11.85″W
The Liencres section is the standard section for the Cenomanian of the North Cantabrian Basin (Wilmsen et al. 1996). The ca. 205-m-thick succession (Fig. 3) exposed in the eastern part of Playa de Somocuevas (coast of the Bay of Biscay, north of Liencres) comprises the uppermost Albian to lowermost Cenomanian Bielba Formation (ca. 130 m) and the ca. 75-m-thick Altamira Formation (lower to lower middle Cenomanian; see also Wilmsen 1997, pp. 37–51, figs. 18, 20). The trocholinid-bearing samples (samples S 86–S 180) are from the middle to upper part of the Altamira Formation (Fig. 3). Two additional samples (AM 1 and 2) come from the upper part of the Altamira Formation of a nearby section east of Playa de Las Dunas, ca. 1 km to the southwest of the Playa de Somocueveas (43°27′ 46.69″N, 3°57′0.07″W).
(b) The Cobreces–Toñanes section (Fig. 1a). Base of the section is at 43°23′46.59″N, 4°13′5.05″W, top at 43°23′54.69″N, 4°11′53.98″W
The 330-m-thick succession of Cobreces–Toñanes is exposed along the coast between the eponymous villages. There, the Bielba Formation is 195 m thick and the Altamira Formation, 135 m thick. The section has been described in detail by Wilmsen (1997, pp. 88–97, detailed log in fig. 46) and includes a patch reef complex (Wilmsen 1996). The sample To 30 is from the lowermost Cenomanian (Bielba Formation). Other thin-sections have been made from samples of the Altamira Formation: samples Co 27, 33, 35, 37, 39 and 41T from the upper lower Cenomanian (below the patch reefs) and samples CT 3–4 and BT 23 (found above that level), early middle Cenomanian in age.
(c) The La Rabia section, ca. 2 km west of Comillas (Fig. 1a). Base of the section is at 43°23′30.51″N, 4°18′41.15″W, top at 43°23′26.34″N, 4°19′03.46″W
The ca. 300-m-thick succession is exposed along the coast, east of the mouth of the Rio Turbio. The section starts above the upper Albian Caprina Limestones (Fig. 2) with the ca. 160-m-thick Bielba Formation followed by the Altamira Formation with a thickness of ca. 140 m. A comprehensive description including a detailed log of the section has been provided by Wilmsen (1997, pp. 97–105, fig. 49). The samples LR 110–LR-2 have been collected from the interval 185 m (LR 110, lowermost sample) to 297 m (sample LR-2), corresponding to the lower (LR 110, 149) and middle Cenomanian (LR-2).
Microfacies, micropalaeontology, and stratigraphy
The thin-sections studied comprise bioclastic grain- to pack-stones containing calcareous algae, benthic foraminifers, echinoderm fragments, and debris of corals and sponges (Fig. 4a, b), assigned to a shallow-water platform association (Wilmsen 2000, fig. 7/6). Some of the samples investigated represent rudstones comprising large metazoan bioclasts (corals and sponges, e.g., Acanthochaetetes) (Fig. 4c) surrounded by a matrix particularly rich in trocholinids and calcareous algae (Fig. 4d). Trocholinids show the best preservation in the middle Cenomanian “arenite-oncoid facies” of Reitner (1987, e.g., pl. 44, fig. 8). This widespread facies displays brownish iron-stained micritic coatings related to influx of weathering products from nearby emerged areas (Fig. 4e). In this microfacies type, trocholinids are typically associated with the dasycladale Trinocladus tripolitanus Raineri and debris of halimedaceans (Fig. 4d).
Microfacies aspect of the lower–middle Cenomanian trocholinid-bearing limestones of the Altamira Formation, North Cantabrian Basin, Spain. a, b Grain- to pack-stones with trocholinids, other benthic foraminifera (Lenticulina in b), and fragments of calcareous algae. c Rudstone with large bioclast of corals, sponges [here: Spirastrella (Acanthochaetetes) sp.] (S), calcareous algae, trocholinids (black circles) and other benthic foraminifera. d Packstone with sparitized tests of trocholinids. e Wackestone with trocholinids and calcareous algae, Trinocladus tripolitanus Raineri (T). f Iron-impregnated lump containing a trocholinid showing lamellar test preservation (arrow). Thin-sections: S 164 (a), S 180 (b), AM 2 (c), AM 1 (d), Co 33 (e), CT 4 (f). Scale bars 1.0 mm
Apart from trocholinids, benthic foraminifers are well diversified and notably include Altamirella biscayana (see Schlagintweit et al. 2015), Gavelinella sp. (Fig. 5g), Lenticulina sp. (Fig. 4b), epistominids (Fig. 5r, s), Dictyopsella cf. libanica Saint-Marc (Fig. 5h), nezzazatids (Fig. 5o), Merlingia aff. cretacea Hamaoui and Saint-Marc (Fig. 5k), Phenacophragma cf. assurgens Applin, Loeblich and Tappan (Fig. 5j), Troglotella incrustans Wernli and Fookes (Fig. 5q), miliolids such as Meandrospira washitensis Loeblich and Tappan (Fig. 5l), or extremely rare small alveolinids (Fig. 5i), and orbitolinids such as Orbitolina concava (Lamarck) (Fig. 5a), Conicorbitolina corbarica (Schroeder) (Fig. 5c), and Conicorbitolina conica (d’Archiac) (Fig. 5b). These orbitolinids indicate an early to middle Cenomanian age (e.g., Arnaud et al. 1981). The presence of abundant small rotaliids, i.e. Rotorbinella mesogeensis (Tronchetti), is worth to mention (Fig. 5e, f). This species, interpreted as the possible ancestor of all rotaliid foraminifers (Martinez 2007), was originally described by Tronchetti (1981) as “Rotalia mesogeensis” from the lower Cenomanian of Provence, SE-France. According to Caus et al. (2009, fig. 4), R. mesogeensis would be missing in the Iberian Ranges and would only appear in the late Cenomanian in both the Pyrenees and the Betic Cordillera. Its co-occurrence with early Cenomanian orbitolinids in Cantabria proves that this view is incorrect. Planktonic foraminifera are represented by the finely pustulose tests of the robertinid Favusella washitensis (Carsey) (sensu Rigaud et al. 2015c) (Fig. 5d) and more rarely by the rotaliid Rotalipora (Fig. 5n). In the near-reefal rudstones, we also observed the encrusting acervulinoid Menaella bustamantei (Fig. 5p) described by Cherchi and Schroeder (2005) from the uppermost Albian of the Caniego Limestone of northern Spain. It is here reported for the first time outside its type locality and in slightly younger strata. Associated with foraminifers are omnipresent calcareous green algae (Halimedaceae, Dasycladaceae), indicative of the upper photic zone. They are represented by Halimeda ssp., Boueina hochstetteri Toula, Boueina iberica Schlagintweit & Wilmsen, Terquemella sp., Trinocladus tripolitanus Raineri, Dissocladella bonardii Radoičić, Conrad and Carras, Dissocladella? n. sp. 1 sensu, Conrad and Peybernès (1972) and Neomeris cf. mokragorensis Radoičić and Schlagintweit. The characteristic association of T. tripolitanus and Dissocladella n. sp. was described by Conrad and Peybernès (1972) from the late Albian of the Pyrenean Mountains and the Basco-Cantabrian Chains. Rhodophycean algae only include some tiny fragments of solenoporaceans and some small thallus fragments of Marinella lugeoni Pfender. A similar microfauna and microflora was reported by Schroeder and Willems (1983, fig. 4.1) from the late Albian Caniego Limestone of northern Spain. Calcareous algae are treated in a separate paper (Schlagintweit and Wilmsen 2014).
Foraminifera from the upper lower–middle Cenomanian Altamira Formation of the North Cantabrian Basin, Spain. a Orbitolina concava (Lamarck). b Conicorbitolina conica (d´Archiac). c Conicorbitolina cf. corbarica (Schroeder). d Favusella washitensis (Carsey). e–f Rotorbinella mesogeensis (Tronchetti). g Gavelinella sp. h Dictyopsella cf. libanica Saint-Marc. i Small unknown alveolinid. j Phenacophragma cf. assurgens Applin, Loeblich and Tappan. k Merlingia aff. cretacea Hamaoui and Saint-Marc. l Meandrospira washitensis Loeblich and Tappan. m Conorboides? sp. n Rotalipora sp. o Nezzazata simplex Omara. p Menaella bustamantei Cherchi and Schroeder. q Troglotella incrustans Wernli and Fookes boring into a mollusc shell. r, s Epistominids with preserved lamellar microstructure. Thin-sections: S 171 (a, h), S 133 (b, e–g), Co 35 (c), S 176 (d), LR 110 (i), LR 149 (j), BT 23 (k, o), LR 198 (l), Co 33 (m), LR HG-5 (n), AM 2 (p), LR 182 (q), Co 41 (r), S 164 (s). Scale bars (a–c) 0.3 mm, (d–s) 0.2 mm
Material preservation
In our Cenomanian material, trocholinids present different states of preservation, even in the same thin-section and for the same morphotype (Figs. 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17). Four different types of test preservation have been observed: (1) completely recrystallized to sparry calcite (both test and lumina) (very poorly preserved, not illustrated), (2) recrystallized to sparry calcite or microsparite with lumina of the proloculi and tubular chambers filled with fine micritic sediments (poorly preserved, not illustrated), (3) superficially preserved specimens limited to the periphery of the test and the tubular chamber (moderately preserved, e.g., Fig. 16g–i), and (4) light-brown, iron impregnated tests nicely displaying the intimate lamellar structure (well to very well-preserved; e.g., Figs. 6, 14). The various states of preservation are caused by different diagenetic processes of test alteration and impregnation. In the Triassic, different preservational modes of involutinids were correlated with their early post-depositional diagenetic environments within the former carbonate platform systems (Schott 1983, “diagenetic belts of Involutinidae”). Mixing of the Cenomanian trocholinid assemblage studied can be accordingly inferred.
Coscinoninae microstructure (upper lower–middle Cenomanian Altamira Formation of Cantabria). b Hensonipapillus cantabricus n. gen., n. sp. i, l, m Paracoscinoconus semiinvolutus n. gen., n. sp. a, c–h, j, k, n, o indeterminate coscinoconins. c. s. canal system, f. l. folded lamina, l. lamina, p. perforation, p. n. polygonal nodes, s. e. sharp edge of the canal system, s. l shortened lamella (end if arrow), s. o. spiral opening (of the tubular chamber on the canal system), t. l. tapered lamina. Thin-sections: Co 41 (a, f, k, o), Co 37 (b), Co 35 (c, e, h, I, m), Co 27 (d, g, n), Co 31 (j), S 133 (l). Scale bars 0.3 mm, except (j, n, o) 0.2 mm
Trocholininae from the upper lower–middle Cenomanian of Cantabria a–b Frentzenella cf. involuta (Mantsurova in Mantsurova and Gorbatchik). c–g Frentzenella cf. odukpaniensis (Dessauvagie). h–p Frentzenella pygmaea n. sp. Holotpye specimen in (n). int interfingered lamellae, p. perforation, pap papillae, u. d. umbilical ditch. Thin-sections: S 176 (a, j–m, o), S 180 (b, d, n), S 164 (c, e), To 30 (h, p), Co 27 (i). a–g, i–o Altamira Formation; h, p Bielba Formation. Scale bars 0.2 mm
Coscinoconinae from the upper lower–middle Cenomanian Altamira Formation of Cantabria. a–g Coscinoconus discoideus n. sp. Holotype in d. c. s. canal system, d. s. depressed suture, s. o. spiral opening of the tubular chamber on the canal system. Thin-sections: S 133 (a), Co 39 (b), S 172 (c, d, f, g), S 176 (e). Scale bars 0.3 mm
Coscinoconinae from the upper lower–middle Cenomanian Altamira Formation of Cantabria. a–f Hensonipapillus altamirensis n. gen., n. sp. Holotype in e. c. s. canal system, pap papillae, p. n. polygonal nodes, Pr Proloculus, s. o. spiral opening of the tubular chamber on the canal system. Thin-sections: S 133 (a, c), Co 41 (b, d–f). Scale bars 0.3 mm
Coscinoconinae from the upper lower–middle Cenomanian Altamira Formation of Cantabria. a–i Hensonipapillus cantabricus n. gen., n. sp. Holotype in a. ap aperture, c. s. canal system, p. perforation, pap papillae, p. n. polygonal nodes, Pr Proloculus, s. o. spiral opening of the tubular chamber on the canal system, t. c. tubular chamber. Thin-sections: Co 35 (a, b, g), Co 41 (c, h), S 133 (d, f, i), CT 4 (e). Scale bars 0.3 mm
Coscinoconinae from the upper lower–middle Cenomanian Altamira Formation of Cantabria. a–f Hensonipapillus lenticularis (Henson) nov. comb.; g–l Hensonipapillus minimus (Henson) nov. comb. Juvenile specimens in (g) and (k). c. s. canal system, p. perforation, pap papillae, s. o. spiral opening (of the tubular chamber on the canal system). Thin-sections: LR 110 (a–f), S 133 (g, h), CT 3 (i, j, l), CT 4 (k). Scale bars 0.5 mm (a–f), 0.3 mm (g–l)
Coscinoconinae from the upper lower–middle Cenomanian Altamira Formation of Cantabria. a–f Paracoscinoconus burlini (Gorbatchik, 1959) n. gen., n. comb. g–o Paracoscinoconus semiinvolutus n. gen., n. sp. Holotype in (l). c. s. canal system, p. perforation, p. n. polygonal nodes, Pr Proloculus, s. e. sharp edge of the canal system, s. l. shortened lamella (end of arrow), s. o. spiral opening of the tubular chamber on the canal system. Thin-sections: S 133 (a, c, d), CT 3 (b), Co 27 (e), Co 41 (f, i, m, o), Co 33 (g, j), Co 37 (h, k, l), Co 35 (n), Scale bars 0.3 mm
Schematic preservational stages of Cenomanian trocholinids following Rigaud et al. (2013, fig. 7)
All tests show evidence of dissolution followed either by recrystallization or neomorphism, including inversion (see Hohenegger and Piller 1975 for details). These processes are destructive and tests owe their uncommon preservation to other, earlier diagenetic processes. As attested by the dark, micritic appearance of the test periphery of some trocholinids, part of the preservation is related to a superficial micritization (e.g., Fig. 10d–g). The well-preserved light-brown specimens displaying the laminae, in contrast, record an additional diagenetic step. In most samples, their good preservation is limited to the tubular chamber, pores and canal system (i.e., the most microporous parts of the test) and to the periphery of the test but in samples collected closer to the “Hardground 99”, which seals the Altamira Limestone, the whole test may display such remarkable preservation. In these tests, the preservation of microstructural details is related to a pervasive impregnation of test interstices by iron-rich fluids, prior to recrystallization. It is worth mentioning that other aragonitic constituents such as epistominids (Fig. 5r, s) and some aragonitic calcareous algae present in the same thin-sections also display this brownish type of preservation (see also Schlagintweit and Wilmsen 2014, fig. 4).
Systematic micropalaeontology
The following taxa were identified in thin-sections (Figs. 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16):
Family Trocholinidae Kristan-Tollmann, 1963, emend. Rigaud et al., 2013
Subfamily Lamelliconinae Zaninetti et al., 1987, emend. Rigaud et al., 2013
-
Licispirella? sp.
Subfamily Trocholininae Kristan-Tollmann, 1963, emend. Rigaud et al., 2013
-
Frentzenella cf. involuta (Mantsurova in Mantsurova and Gorbatchik, 1982)
-
Frentzenella cf. odukpaniensis (Desssauvagie, 1968)
-
Frentzenella pygmaea n. sp.
Subfamily nov. Coscinoconinae
-
Coscinoconus chouberti (Hottinger, 1976)
-
Coscinoconus discoideus n. sp.
-
Coscinoconus cf. perconigi (Neagu, 1994)
-
Coscinoconus cf. histeri (Neagu, 1994)
-
Hensonipapillus cantabricus n. gen., n. sp.
-
Hensonipapillus altamirensis n. gen., n. sp.
-
Hensonipapillus lenticularis (Henson, 1947) n. gen., n. comb.
-
Hensonipapillus minimus (Henson, 1947) n. gen., n. comb.
-
Paracoscinoconus burlini (Gorbatchik, 1959) n. gen., n. comb.
-
Paracoscinoconus semiinvolutus n. gen., n. sp.
The thin-sections are housed in the palaeozoological collections of the Senckenberg Naturhistorische Sammlungen Dresden. The samples from Liencres (Playa des Somocuevas), La Rabia, Cobreces, Cabo Toñanes and Las Dunas are abbreviated with S, LR, Co, CT, and AM, respectively. Cenomanian trocholinids are schematically represented according to their preservation type in Fig. 17. Dimensions are compiled in Table 1. For nomenclature glossary, refer to Rigaud et al. (2012, 2013).
-
Subphylum Foraminifera d’Orbigny, 1826
-
Class? Tubothalamea Pawlowski, Holzmann and Tyszka, 2013
-
Order Involutinida Hohenegger and Piller, 1977
-
Suborder Involutinina Hohenegger and Piller, 1977
-
Superfamily Involutinoidea Bütschli, 1880
-
Family Trocholinidae, Kristan-Tollmann, 1963 emend. Rigaud et al., 2013
-
Subfamily Lamelliconinae Zaninetti et al., 1987, emend. Rigaud et al., 2013
-
Genus Licispirella, Blau and Grün, 1997, emend. Rigaud et al., 2013
-
Type species: Semiinvoluta violae Blau, 1987
-
Licispirella? sp.
-
Fig. 7a, b
Remarks: The uncertainty about the generic status of this rare Cenomanian taxon is due to the obliquity of our sections that may have accentuated the apparent length of the laminar deposits, which appear shortened as in Licispirella. The genus Licispirella Blau and Grün comprises four species within the Late Triassic–Middle Jurassic interval (Rigaud et al. 2013). If its presence in the Cenomanian is confirmed, it would represent a good example of a lazarus taxon, reappearing after more than 75 million years of hidden evolution. Licispirella? sp. was observed in the Cobreces–Toñanes section (thin-sections Co 27 and Co 33) (Fig. 1).
Dimensions: Diameter 0.4–0.45 mm; height 0.18–0.22 mm; ratio d/h 1.9–2.4; number of whorls up to 7–8; apical angle 135°–150°.
-
Subfamily Trocholininae Kristan-Tollmann, 1963, emend. Rigaud et al., 2013
-
Genus Frentzenella Rigaud, Blau, Martini and Rettori, 2013
-
Type species: Frentzenella frentzeni Rigaud Blau, Martini and Rettori, 2013
*1982 Trocholina involuta n. sp.—Mantsurova in Mantsurova and Gorbatchik: 127, pl. 3, fig. 7, pl. 4, fig. 3
n.f. 2013 Frentzenella involuta (Mantsurova in Mantsurova and Gorbatchik) nov. comb.—Rigaud et al.: 332
Remarks: Only two isolated specimens of F. involuta have been illustrated by Mantsurova and Gorbatchik (1982): pl. 3, fig. 7, pl. 4, fig. 3). Our forms, which have only been observed in the Liencres section (thin-sections S 164 and S 180) (Figs. 1, 3), show more prominent umbilical masses and are smaller than the type specimens. However, our specimens possess, as the types, a finely perforated spiral side, a subrounded to subangular margin, and a very thick apical thickening.
Dimensions (data from Mantsurova, 1982 in parentheses): Diameter 0.40–0.60 mm (0.65–1.0 mm); height 0.2–0.4 mm (0.42–0.6 mm); ratio d/h: 1.4–1.8 (1.6–1.7); number of whorls up to 6–7 (up to 5); apical angle 85–110° (90°–100°).
*1968 Trocholina odukpaniensis n. sp.—Dessauvagie: 64–76, p. 1, figs. 1–4, p. 2, figs. 3–5, 7
n.f. 2013 Frentzenella odukpaniensis (Dessauvagie) nov. comb.—Rigaud et al.: 332
Remarks: Specimens of F. cf. odukpaniensis, very rare, have only been observed in the Liencres section (thin-sections S 164, S 176 and S 180) (Figs. 1, 2, 3). They show dimensions intermediate between the Callovian‒Tithonian species Frentzenella belorussica (Mityanina, 1963) and the Berriasian-Cenomanian species F. odukpaniensis (Desssauvagie, 1968), which are morphologically close. In our specimens, the umbilical ditch is not as much pronounced as in the specimens illustrated by Dessauvagie (1968: pl. 2, figs. 3, 4). However, the apparent depth of the umbilical ditch in Dessauvagie’s specimens is partly related to intense microboring activity. The deeper part of the umbilical ditch is indeed micritized and displays a sinuous aspect.
Dimensions (data from Desssauvagie 1968 in parentheses): Diameter 0.35 (juvenile)–0.6 mm (0.566–0.899 mm, mean 0.761 mm); height 0.15–0.45 mm (0.266–0.466 mm, mean 0.387 mm) ratio d/h 1.3–2.5 (1.57–2.16, mean 1.9); number of whorls up to 7 (5–6); apical angle 70°–110°.
-
Frentzenella pygmaea n. sp.
-
Fig. 8h–p
Derivatio nominis: pygmaea = Latin, referring to its small size compared to other Cretaceous trocholinids.
Holotype: Axial section illustrated in Fig. 8n, sample S 180.
Locus typicus: F. pygmaea has been observed in the Cobreces–Toñanes section (thin-section Co 27) and the Liencres section (thin-section S 176) (Fig. 1). The holotype comes from the Liencres section (Fig. 3).
Stratum typicum: uppermost lower to lower middle Cenomanian, Altamira Formation
Diagnosis: A low trochospiral Frentzenella with few whorls, thick spiral side laminar extensions, simple perforations on both sides of the test, and with a sudden enlargement of the tubular chamber in the last whorl.
Description: Test asymmetric, sub-lenticular with a rounded to subangular margin and a circular outline. It is formed by a globular proloculus (diameter: 0.025–0.04 mm) simply open on a low trochospirally coiled, undivided tubular chamber presenting, on its sides, well-developed lateral laminar extensions of the tube wall. In the first 5–6 whorls, the tubular chamber appears oval to kidney-shaped in section, depending on the level of development of a tube floor, which is optional. In the last whorl, the tubular chamber suddenly increases in size, becoming subtriangular in section. Laminar extensions of spiral side straight, interfingering, building a thick, smooth apical thickening. Laminar extensions of umbilical side interfingering, locally thickened, forming numerous rounded papillae at the test surface. Wall primarily hyaline and fibrous aragonitic but usually recrystallized into microsparite to sparite. Radially arranged perforations are developing from the tubular chamber periphery. Primary aperture not observed, most likely simple, terminal.
Dimensions: Diameter 0.20–0.40 mm; height 0.1–0.2 mm; ratio d/h 1.6–2.5; number of whorls: up to 6–7; apical angle 90°–140°.
Comparisons: The new species differs from F. odukpaniensis in the lack of a periumbilical ditch, in its smaller size, tubular chamber morphology (more rounded in section, at least in the first whorls), and lower number of whorls, and from F. involuta in its smaller size, lower trochospiral coiling, and greater number of papillae.
Remarks: In our material, one specimen of F. pygmaea has been found in probable attached position (Fig. 8o).
-
Coscinoconinae n. subfam
Type genus: Coscinoconus Leupold in Leupold and Bigler 1936.
Composition: The new subfamily Coscinoconinae includes the three genera Coscinoconus Leupold in Leupold and Bigler, 1936, Hensonipapillus n. gen., and Paracoscinoconus n. gen.
Diagnosis: Trocholinidae with an umbilical canal system developed from spiral openings of the tubular chamber and forming interconnected levels of polygonal networks.
Description: Test discoidal, lenticular or conical formed by a globular proloculus open on a trochospirally enrolled, undivided tubular chamber. An umbilical canal system, developed from spiral openings in the tubular chamber and interspersed with the laminar extensions of the tube wall, forms levels of polygonal networks, which are interconnected by pores or canals perpendicularly crossing the laminar deposits (Fig. 6). Wall hyaline, fibrous aragonitic, and perforate. Aperture, originally simple, terminal, may be functionally substituted by the canal system.
Remarks: The umbilical canal system and laminar arrangement is complex and alike in all representatives of the subfamily Coscinoconinae (see below for details). Differences, of specific value, chiefly occur in the size of the pores or canals forming the mesh. It is unlikely that such complex structuring evolved independently in the frame of a parallel evolution and we therefore propose a new subfamily to encompass the whole group. The Middle Jurassic, early evolution of the group, however, is poorly known and requires further investigation in order to clarify phylogenetic links between coscinoconins and their trocholinin ancestors.
In some specimens, “rudimentary septa” sensu Boudagher-Fadel (2008) are dimly recognizable. Depending on the section, they represent either edges of openings of the tubular chamber on the umbilical canal system (Fig. 6b, f, j) or internal thickenings related to a dorsal ornamentation (striae) (Fig. 6h).
Genus Coscinoconus Leupold in Leupold and Bigler, 1936, emend. Rigaud et al., 2013.
Synonyms: Hottingerella Piller, 1983; Andersenolina Neagu, 1994; Septatrocholina BouDagher-Fadel and Banner in Boudagher-Fadel, 2008 (no type fixed in the original publication, name of type species uncertain, possibly Septatrocholina banneri on p. 162, but not formally described).
Type species: Coscinoconus alpinus Leupold in Leupold and Bigler, 1936.
Remarks: The genus Coscinoconus was introduced by Leupold in Leupold and Bigler (1936) with the type species C. alpinus and another species, C. elongatus from Tithonian–Berriasian strata of Switzerland. Leupold noticed their great affinity with Trocholina Paalzow, 1922, but concluded (p. 618, translated) that “the structure of Coscinoconus is very strange and seems to be rather isolated within the foraminifera. The detection of two species with the same test architecture in the Jurassic-Cretaceous boundary interval suggests the expectation of a larger group that can be differentiated generically”. According to Leupold and Bigler (1936), the structured umbilical grid plate (“Gitterplatte”) represents the diagnostic generic criterion. It is worth mentioning that Coscinoconus has even been considered as a dasycladacean alga by some authors (Maslov 1958; Kerčmar 1961) and is still mentioned as such in online databases (e.g., Guiry and Guiry 2014). The synonymy of Coscinoconus and Trocholina has been postulated for more than half a century (Reichel 1955; Gorbatchik 1959; Guillaume 1963; Desssauvagie 1968; Koehn-Zaninetti 1969; Zaninetti 1984; Arnaud-Vanneau et al. 1988; Blau 1987; Loeblich and Tappan 1987; Boudagher-Fadel 2008).
In his description of Trocholina chouberti, Hottinger (1976) did not refer to Leupold’s “Gitterplatte” of Coscinoconus although the remarkable similarity is obvious (compare, for example, Leupold in Leupold and Bigler 1936, pl. 18, fig. 9, with Hottinger 1976 pl. 1, fig. 1). Piller (1983) recognized the differences between Hottinger’s species and Trocholina, and established the genus Hottingerella. Later, Neagu 1994 created the genus Andersenolina characterized above all by the presence of a perforated umbilical plate (or lamella), which would be added with each whorl. Its type species, A. bancilai, can hardly be distinguished from “Trocholina” campanella Arnaud-Vanneau, Boisseau and Darsac, 1988 (Neagu 1994 pl. 12, fig. 7 vs. fig. 8). Moreover, on account of the layered structure of the canal system and lamellae, isolated specimens will either display polygonal nodes (=level of polygonal network) or perforations (=pores or canals perpendicularly crossing the laminar deposits), depending on the level of abrasion of the test. In the historic review provided by Neagu (1994, 1995), incomprehensibly neither the genus Coscinoconus Leupold nor the genus Hottingerella Piller were discussed.
In the recent taxonomic revision of the family Trocholinidae by Rigaud et al. (2013), Coscinoconus was given priority over both Hottingerella and Andersenolina and was defined as follows: “A Trocholinidae showing reduced lamellae on the spiral side and, on the umbilical side, well-developed lamellae endowed with a relatively complex canal system”. In his revision, almost all Cretaceous “Trocholina” species were transferred to Coscinoconus Leupold in Leupold and Bigler, 1936. The two species T. involuta Mantsurova in Mantsurova and Gorbatchik., 1982 and T. odukpaniensis Desssauvagie, 1968 were assigned to Frentzenella Rigaud, Blau, Martini and Rettori, 2013. Thereby, the long-believed existence of the genus Trocholina (lacking canal system and apical thickening) in the Cretaceous was doubted (Rigaud et al. 2013, fig. 9).
The distinctive canal system of Coscinoconus had been already observed in the Valanginian Coscinoconus (see Hottinger 1976, 2006 for details). This observation was possible due to the infiltration of fine silty sediments into the porous network, prior to recrystallization of the test. In our Spanish material, the canal system, filled with light sparry calcite, can commonly be observed between the more or less impregnated laminae. We validate the description of the interconnected canal system and the tubular chamber as “spirally arranged radial passages” as assumed by Hottinger (1976, p. 815) and of the lamellae and the canal system as postulated by Rigaud et al. (2013, p. 329, 330). The canal system in Coscinoconus “is interspersed with the laminar deposits. Mainly developed from the sutural pores and limiting the L2 lamellae, it forms levels of polygonal networks. Each level is connected by large canals, generally straight, perpendicularly crossing the successive lamellae”.
A persistent uncertainty concerning the aperture of Coscinoconus can be found in the literature. Leupold and Bigler (1936, p. 614) remarked that the spirally coiled tubiform chamber lumen is completely masked by the “Gitterplatte” so that a true aperture is lacking. He assumed that “the mesh of the whole grid network obviously functioned as the aperture” (Leupold and Bigler 1936, p. 614, translated). This assumption was again picked up by Neagu (1994, p. 126) in his diagnosis of Andersenolina: “the aperture in the adult stage is absent being substituted by the umbilical pores”. In contrast, Henson (1947) and Emberger (1955) described the aperture as the open end of the tubular chamber. In the revision of Rigaud et al. (2013), this problem was not further discussed due to the lack of appropriate material.
*1976 Trocholina chouberti n. sp.—Hottinger: 819–820, fig. 1, pl. 1
? 1982 Trocholina gigantea n. sp.—Gorbatchik and Mantsurova in Mantsurova and Gorbatchik: 126, pl. 1, fig. 7, pl. 4, figs. 1a–c, 2a–c
1983 Hottingerella chouberti (Hottinger)—Piller: 197 (nov. comb.)
1988 Trocholina chouberti Hottinger—Arnaud-Vanneau et al.: 360–361, fig. 3, pl. 5, figs. 1, 2
? 1988 Trocholina lenticularis Henson—Arnaud-Vanneau et al.: 362–362, pl. 1, figs. 6–10, pl. 6, figs. 22–27
1994 Andersenolina chouberti (Hottinger)—Neagu: 130, pl. 1, figs. 1–25, pl. 8, fig. 11, pl. 9, figs. 1–19, pl. 10, figs. 34–40, pl. 12, figs. 1–12 (nov. comb.)
2013 Coscinoconus chouberti (Hottinger) nov. comb. – Rigaud et al.: 330, figs. 4/7–4/11
Remarks: C. chouberti represents a comparably low conical trocholinid with the greatest diameter/height (d/h) test ratio of all millimetre-sized Cretaceous trocholinids described so far (see also Arnaud-Vanneau et al. 1988, fig. 3). The number of whorls is often difficult to determine because of test erosion but, when discernible, it reaches up to nine. A similar, highly eroded taxon was described by Mantsurova and Gorbatchik (1982) as “Trocholina” gigantea from the Berriasian of Crimea, Ukraine. It was illustrated by one basal section (Mantsurova and Gorbatchik 1982, pl. 1, fig. 7) and two isolated specimens viewed from different sides (Mantsurova and Gorbatchik 1982, pl. 4, figs. 1a–c, 2a–c). Test abrasion hampers determining the shape of the tubular chamber but except for its smaller dimensions (d 0.77–1.4 mm; h 0.35–0.588 mm; d/h 2.0–2.75; number of whorls: 4; apical angle: 130°–145° according to Gorbatchik and Mantsurova 1982), this species shows similar features as Hottinger’s species.
C. chouberti was so far recorded from upper Berriasian–Valanginian strata (Arnaud-Vanneau et al. 1988; Neagu 1994; Mancinelli and Coccia 1999). Its stratigraphic range has to be significantly extended, up to the lower middle Cenomanian. It is present in the Cobreces–Toñanes section (thin-sections Co 39, CT 3, CT 4), the La Rabia section (thin-section LR 149) and the Liencres section (thin-section S 133).
Dimensions (data from Hottinger 1976 in partentheses): Diameter 0.95–1.4 mm (“largest diameter 1.4–2.2 mm”); height 0.2–0.4 mm; ratio d/h 2.5–4.0 (2.8–3.4); number of whorls up to 9 (5–6); apical angle 100°–150° (~90–~130°).
-
Coscinoconus discoideus n. sp.
-
Fig. 10a–g
Derivatio nominis: Referring to its flat test
Holotype: Axial section illustrated in Fig. 10d, sample S 172.
Paratypes: Specimens in Fig. 10c, f, g, thin-section S 172.
Locus typicus: C. discoideus was observed in the Cobreces–Toñanes (thin-section Co 39) and the Liencres sections (thin-sections S 133, S 172, S 176) (Fig. 1). The holotype comes from the Liencres section (Fig. 3).
Stratum typicum: uppermost lower to lower middle Cenomanian, Altamira Formation.
Diagnosis: A discoidal to very low conical Coscinoconus presenting a prominent apex, up to 9–10 whorls, a narrow canal system, and numerous small, subrounded umbilical polygonal nodes.
Description: Test discoid asymmetric to very low conical with a subrounded margin, a circular outline and a prominent apex, which is only observable in axial, subaxial, and oblique (sub-)centered sections. It is formed by a globular proloculus simply open on a low trochospirally enrolled undivided tubular chamber, forming up to 9–10 whorls. The lateral laminar extensions of the tube wall are reduced on the spiral side and well developed, mostly formed by the stacking of discontinuous to papillose laminae on the umbilical side, forming numerous small, subrounded polygonal nodes at the test surface. The trochospiral coiling slightly varies through ontogeny such as in axial sections of adult forms, successive lumina appear arranged in a curly bracket. Gradually enlarging during growth, the tubular chamber lumen initially appears oval in section, later becoming kidney- to chevron-shaped. The narrow canal system is arranged in levels of polygonal networks developed from the tubular chamber and connected by thin pores, perpendicularly crossing the laminar deposits. Wall calcareous, commonly recrystallized into microsparite to sparite, but originally hyaline, fibrous aragonitic, and radially perforate. Aperture not observed, most likely primarily simple, terminal.
Dimensions Diameter 0.4–1.1 mm; height 0.14–0.25 mm; ratio d/h 2.8–4.5; number of whorls up to 9–10; apical angle 150°–175°.
Remarks: The canal system is probably too narrow to functionally replace the aperture.
*1994 Andersenolina perconigi n. sp.—Neagu: 134, pl. 10, figs. 1–15, pl. 13, figs. 6–9, 13–17, text–fig. 3, figs. 5–6
1994 Andersenolina elongata (Leupold) – Neagu: 130, pl. 4, figs. 1–22, pl. 6, figs. 12–14, text–fig. 3, fig. 8
2001 Andersenolina perconigi Neagu—Pop and Bucur: pl. 7, fig. 9
n.f. 2013 Coscinoconus perconigi (Neagu) nov. comb.—Rigaud et al.: 330
Remarks: Cylindro-conical representative of Coscinoconus (diameter up to 0.45 mm, height up to 1.2 mm) with up to 14 discernible whorls in adult specimens and a convex base. Our specimens, rare, come from a single thin-section of the La Rabia section (thin-section LR 149) and show high variability in size (compare scales in Fig. 11a–d). They differ from the highly acute conical species Coscinoconus altispirus described by Henson (1947) from the early Cenomanian of Qatar, which shows an acute tubular chamber, as observable in axial sections. Our forms are higher than the type specimens of C. elongatus Leupold in Leupold and Bigler 1936 and possess a larger tubular chamber. However, type specimens of C. elongatus might represent juvenile specimens.
In his description of C. perconigi Neagu (1994), did not provide any reliable criterion for distinguishing his species from C. elongatus or C. limognensis (nom. nov. for Trocholina gigantea Pelissié and Peybernès, see Rigaud et al. 2013). His specimens seem to show higher tests than C. elongatus and more whorls for a same height than C. limognensis. In our material, the number of whorls for a same height seems to be an unsteady pattern. Therefore, Neagu’s species might be a synonym of C. limognensis but our material is too scarce to confirm it. So far, C. limognensis is only known from the Bathonian–Oxfordian (Pelissié and Peybernès 1982).
Dimensions: diameter 0.3–0.45 mm; height up to 1.1 mm; ratio d/h 0.3–0.6; number of whorls up to 14; apical angle 25°–35°.
*1994 Andersenolina histeri n. sp.—Neagu: 137, pl. 11, figs. 1–21, 23–25, 27–40, pl. 13, figs. 1–5, text–fig. 3, figs. 1–4
n.f. 2013 Coscinoconus histeri (Neagu) nov. comb.—Rigaud et al.: 330
Remarks: Coscinoconus cf. histeri has been found in the Cobreces–Toñanes section (thin-sections Co 37, Co 39 and Co 41) and the Liencres section (samples S 133, S 180). From the data we have and according to Neagu’s selection of type specimens, this species would show highly variable apical angles. A similar pattern has been observed in Coscinoconus palastiniensis (Henson 1947), a Jurassic species that possesses larger polygonal nodes. The two species, which show a well-marked marginal band (sensu) Henson 1947, are most likely phylogenetically related. C. intermedius (Henson 1947), common in coeval strata, is morphologically close to C. histeri but approximately twice the size.
Dimensions (data from Neagu 1994 in parentheses): diameter 0.5–0.85 mm (0.26–0.55 mm); height 0.4–0.5 mm (0.24–0.91 mm); ratio d/h 1.4–2.3; number of whorls: up to 9 (6–14); apical angle 90°–130°
-
Hensonipapillus n. gen.
Derivatio nominis: Named after F.R.S. Henson and its papillose spiral side.
Type species: Hensonipapillus cantabricus n. gen., n. sp.
Composition: The genus Hensonipapillus n. gen. comprises the species H. altamirensis n. gen., n. sp., H. cantabricus n. gen., n. sp., H. lenticularis (Henson, 1947) n. comb., and H. minimus (Henson, 1947) n. comb.
Diagnosis: Coscinoconinae with, at least in the juvenile stage, well-developed, papillose lamellae on the spiral side.
Description: Test sub-discoidal, -lenticular, -globular or -conical formed by a globular proloculus open on a primarily trochospirally enrolled undivided tubular chamber. The lateral laminar extensions of the tube wall are, at least in the juvenile stage, well-developed on both sides of the test, forming an apical thickening and an umbilical mass. Papillose on the spiral side, they form papillae at the test surface. Discontinuous to papillose on the umbilical side and interspersed with a canal system, they mostly form polygonal nodes at the test surface. The canal system, developed from the tubular chamber, forms levels of polygonal networks that are interconnected by pores or canals, perpendicularly crossing the laminar deposits, as observed in Coscinoconus. Wall hyaline, fibrous aragonitic, and perforate. Primary aperture simple, terminal, may be functionally replaced by the umbilical canal system.
Remarks: The new genus Hensonipapillus n. gen. includes the species “Trocholina lenticularis” of Henson (1947), which has been previously selected as the type species of the genus Hensonina Moullade and Peybernès, 1974. However, as already stated by Arnaud-Vanneau et al. (1988) and Rigaud et al. (2015a), among others, Hensonina has been interpreted in a sense other than that defined by its type species and represents a misidentified genus according to the International Code of Zoological Nomenclature. Introduced for planispiral Involutinidae presenting a bilateral reticulate ornamentation [illustrated in Moullade and Peybernès 1974: pl. 3, figs. 7–9 (the specimen illustrated in their pl. 3, fig. 6 belongs to Involutina hungarica)], Hensonina cannot be typified by an asymmetric, trochospiral species presenting papillae on the dorsal side and umbilical nodes on the umbilical side (see holotype of “Trocholina lenticularis” in Henson 1947, pl. 12, figs. 5, 6 for ornamental differences). This taxonomic mistake is most likely linked to a previous misidentification by Henson (1947) who proposed as a paratype of his new taxon “Trocholina lenticularis” a specimen similar to Moullade and Peybernès’ species (compare Henson 1947, pl. 12, fig. 2 with Moullade and Peybernès 1974, pl. 3, figs. 7–9). So far, the genus Hensonina has been regarded as a junior synonym of Involutina Terquem, as first proposed by Schlagintweit and Piller (1990).
-
H. altamirensis n. gen., n. sp.,
-
Fig. 13a–f
Derivatio nominis: Referring to the Altamira Formation
Holotype: Axial section illustrated in Fig. 13e, thin-section Co 41.
Paratypes: Specimens in Fig. 13b, d, f, thin-section Co 41.
Locus typicus: H. altamirensis was observed in the Cobreces–Toñanes section (thin-sections Co 35, Co 41, holotype) and the Liencres section (thin-section S 133) (Figs. 1, 3).
Diagnosis: Medium convexo-plane Hensonipapillus with a subangular margin, up to 7–8 trochospiral coils, a tubular chamber lumen becoming subtriangular, and shortened spiral side lamellae in the final whorls.
Description: Test medium convexo-plane, with a subangular margin and a circular outline, formed by a globular proloculus simply open on an enrolled undivided tubular chamber, forming 7–8 trochospiral whorls. The lateral laminar extensions of the tube wall are well-developed on both sides of the test in the juvenile stage, forming an apical thickening and an umbilical mass. The spiral side, riddled with large pores, is formed by interfingering papillose lamellae in the first whorls and shortened lamellae in the last whorls so that papillae are only observable around the apex. The umbilical side mostly displays discontinuous to papillose laminae that are interspersed with a canal system and form numerous polygonal nodes at the test surface. The canal system, developed from the tubular chamber, forms levels of polygonal networks that are connected by short canals, perpendicularly crossing the laminar deposits. Gradually enlarging during growth, the tubular chamber initially appears oval to kidney-shaped in section, later becoming subtriangular. Wall calcareous, commonly recrystallized into microsparite to sparite but originally hyaline, fibrous aragonitic, and radially perforate. Primary aperture not observed, most likely terminal, simple or functionally replaced by the canal system.
Remarks: H. altamirensis differs from H. cantabricus mostly in its convexo-plane shape, subangular margin, and less rounded and proportionally shorter tubular chamber. Moreover, adult/senile specimens of H. altamirensis never display a last streptospiral whorl.
Dimensions: Diameter 0.35–0.7 mm; height 0.15–0.3 mm; ratio d/h 2–4; number of whorls up to 7–8; apical angle 135°–160°.
-
Hensonipapillus cantabricus n. gen., n. sp.
-
Fig. 18 Way of life of Cenomanian trocholinids (upper lower–middle Cenomanian Altamira Formation, Cantabria). a, b Hensonipapillus cantabricus n. gen., n. sp. showing probable anchorage structure in (a) and attached to a textularid in (b). c, e, f Trocholinidae indet. displaying marginal appendices in (c) and an elevated attached position in (f). d Possibly attached Hensonipapillus sp. g Paracoscinoconus burlini (Gorbatchik) with marked polygonal nodes. A substrate is artificially added to highlight the significant space delimited by the polygonal nodes and papillae. Thin-sections: Co 35 (a), CT 4 (b), LR 110 (c, d), Co 27 (e), S 133 (f, g). Scale bars 0.2 mm, except (f) 0.5 mm
1947 Trocholina sp. 1—Henson: 456, pl. 8, figs. 1–4.
?1973 Trocholina gr. T. arabica Henson—Bilotte: pl. 3, fig. 2
Derivatio nominis: Referring to the autonomous province of Cantabria, northern Spain.
Holotype: Axial section illustrated in Fig. 14a, thin-section Co 35.
Paratypes: Specimens in Fig. 14b, c, g, h, thin-section Co 35.
Locus typicus: H. cantabricus was observed in the Cobreces–Toñanes section (thin-sections Co 35 holotype, Co 37, Co 41, CT 3) and the Liencres section (thin-section S 133) (Figs. 1, 3).
Stratum typicum: Upper lower Cenomanian, Altamira Formation.
Diagnosis: Sublenticular, asymmetric Hensonipapillus presenting a low to medium trochospiral coiling on up to 7–9 whorls, a high tubular chamber, and a final streptospiral coil, with a possible 90° change in the coiling axis.
Description: Test sublenticular, asymmetric, with a rounded margin and a circular to subcircular outline, formed by a globular proloculus simply open on an enrolled undivided tubular chamber, which forms 10–11 trochospiral whorls and a final streptospiral coil, with a possible 90° change in the coiling axis. In the first 7–9 whorls, the lateral laminar extensions of the tube wall are well-developed, interfingered on both sides of the test, forming an apical thickening and an umbilical mass. The spiral side, riddled by large pores, is formed by interfingered papillose lamellae in the first whorl, which form papillae at the test surface, and shortened lamellae in the few last whorls. The umbilical side mostly displays short discontinuous to papillose laminae that are interspersed with a canal system, forming numerous small polygonal nodes and few papillae at the test surface. The canal system, developing from the tubular chamber, forms levels of polygonal networks that are connected by short canals, perpendicularly crossing the laminar deposits. Gradually enlarging during growth, the high tubular chamber, initially appears oval in section and later becomes kidney-shaped. Wall calcareous, commonly recrystallized into microsparite to sparite but originally hyaline, fibrous aragonitic, and radially perforate. Primary aperture terminal, simple.
Remarks: Henson (1947, p. 456) recognized his “Trocholina sp. 1” from the Late Jurassic or Early Cretaceous of Iraq as a new species, but did not introduce a new taxon. Henson remarked that this taxon is “distinguished from other described species by its dorsal layer of perforate shell material”. H. cantabricus is the only known coscinoconin showing a last streptospiral coil.
Dimensions (data from Henson 1947 in parentheses): Diameter 0.35–0.9 mm (about 0.5 mm); heigh: 0.18–0.61 mm; ratio d/h 1.5–2.0; number of whorls up tp 10–11 (4); apical angle 90°–130°.
-
Hensonipapillus lenticularis n. comb., emend.
-
Fig. 15a–f
1947 Trocholina lenticularis n. sp.—Henson: 452–453, pl. 11, fig. 1, pl. 12, figs. 1, 3, 5–6, 7?, 8
non 1947 Trocholina lenticularis n. sp.—Henson: pl. 12, fig. 2
n.f. 2013 Coscinoconus lenticularis (Henson) nov. comb.—Rigaud et al.: 330
Emended diagnosis: Slightly asymmetric, fully ornamented, low- to medium-trochospiral, lenticular to globular Hensonipapillus presenting large perforations on the spiral side, a large umbilical canal system and, in the adult stage, shortened spiral side lamellae and kidney-shaped lumina.
Remarks: As mentioned by Brönnimann and, Koehn-Zaninetti (1969) Hensonipapillus lenticularis and H. minimus display marked differences in the morphology of their umbilical masses, resulting in a noticeable asymmetry. Actually, their spiral side, usually more prominent, presents papillae whereas their umbilical side mostly displays polygonal nodes. This difference is related to their intimate structure. As in other Hensonipapillus, H. minimus, and H. lenticularis possess papillose laminar extensions on the spiral side and lamellae interspersed with a canal system on the umbilical side. This marked difference is distinguishable in the holotypes illustrated by Henson (1947) but not in the paratypes illustrated in his pl. 12, figs. 2, 4, which represent sectioned forms of an undescribed involutinin.
Occurrences: H. lenticularis was observed in the La Rabia section (thin-section LR 110), the Cobreces–Toñanes section (CT 4) and the Liencres section (thin-section S 133) (Figs. 1, 3).
Dimensions (data from Henson 1947 in parentheses): Diameter: 0.50–1.55 mm (1.21–1.54 mm), height 0.25–0.75 mm (0.79–1.39 mm); ratio d/h 2.0–3.0; number of whorls up to 6–7 (8–9); apical angle 90°–130°.
*1947 Trocholina lenticularis var. minima sp. et var. nov.—Henson: 453–454, pl. 11, fig. 5
non 1947 Trocholina lenticularis var. minima sp. et var. nov.—Henson: pl. 12, fig. 4.
Emended diagnosis: Discoidal to sublenticular Hensonipapillus with 1–3 sub-planispiral coils in the juvenile stage, a narrow umbilical canal system, and, in the adult stage, a marked trochospiral arrangement, shortened lamellae on the spiral side and subtriangular to chevron-shaped tubular lumen.
Occurrence: H. minimus was observed in the Cobreces–Toñanes (thin-sections Co 27, Co 41, CT 3, CT 4) and Liencres sections (thin-section S 133).
Dimensions (data from Henson 1947 in parentheses): Diameter 0.25–1.1 mm (0.6–0.9 mm); height 0.13–0.55 mm (max. 0.4 mm); ratio d/h 2.3–3.8; number of whorls up to 10 (about 7); apical angle 135°–160°.
-
Paracoscinoconus n. gen.
Type species: Paracoscinoconus semiinvolutus n. gen., n. sp.
Derivatio nominis: para = from Greek beside, referring to the relationship to Coscinoconus.
Diagnosis: Coscinoconinae with, at least in the juvenile stage, shortened laminar deposits on the spiral side.
Description: Test discoidal, lenticular, or conical formed by a globular proloculus open on a trochospirally enrolled, undivided tubular chamber. The lateral laminar extensions of the tube wall (L2 lamellae sensu Piller 1978) are, at least in juvenile forms, shortened on the spiral side, entirely covering the spiral suture and partially covering previous whorl(s), and well-developed, interfingering on the umbilical side, forming an umbilical mass. On the umbilical side, laminae are mostly discontinuous to papillose, interspersed with a canal system, and typically form polygonal nodes at the test surface. The canal system, developed from the tubular chamber, forms levels of polygonal networks that are interconnected by pores or canals, perpendicularly crossing the laminar deposits, as observed in Coscinoconus. Wall hyaline, fibrous aragonitic, and perforate. Primary aperture simple, terminal.
Composition: The genus Paracoscinoconus n. gen. comprises the species Paracoscinoconus semiinvolutus n. sp. and P. burlini n. comb.
Comparisons: As can be deduced from the genus name, Paracoscinoconus is very close to Coscinoconus but its spiral side lamellae are shortened at least in the juvenile part, entirely covering the normally slightly depressed spiral suture.
*1959 Trocholina burlini n. sp.—Gorbatchik: 81–82, pl. 4, figs. 3–5
1982 Trocholina burlini Gorbatchik—Mantsurova in Mantsurova and Gorbatchik: 124, pl. 3, fig. 4
?1988 Trocholina lenticularis Henson—Arnaud-Vanneau: 362, pl. 6, figs. 23, 25, 27
Emended diagnosis: A low- to medium-trochospirally coiled, discoidal to conical Paracoscinoconus presenting a tubular chamber progressively becoming triangular in section and with shortened spiral side lamellae that entirely cover the spiral suture so that the test surface is smooth.
Remarks: According to Gorbatchik (1959), the species is very common in the Barremian and rarer in the Valanginian. Neagu (1995, p. 23) questionably assigned the species to the genus Ichnusella Dieni and Massari. This genus has been described as presenting a monocrystalline tubular chamber wall.
P. burlini was observed in the Cobreces–Toñanes section (thin-sections Co 27, Co 35, Co 37, Co 41, CT 3, BT 23), Liencres section (thin-sections S 133, S 164, S 176, S 178, S180), and La Rabia section (thin-section LR 110).
Dimensions (data from Gorbatchik 1959 in parentheses): Diameter 0.50–0.85 mm (0.36–0.64 mm); height 0.2–0.4 mm (0.1–0.15 mm); ratio d/h 1.8–3.4 (2.5–4.2); number of whorls up to 8–9 (4–6); apical angle 110°–160° (120°–130°).
Derivatio nominis: Referring to the semi-involute spiral side.
Holotype: Axial section illustrated in Fig. 16l, thin-section Co 37.
Locus typicus: P. semiinvolutus was observed in the Cobreces–Toñanes (thin-sections Co 27, 33, 37, 41, CT 3, 4) and the Liencres sections (thin-section S 133) (Figs. 1, 3).
Stratum typicum: Uppermost lower to lower middle Cenomanian, Altamira Formation.
Diagnosis: A medium-trochospirally coiled, sublenticular Paracoscinoconus presenting oval lumina rapidly becoming kidney-shaped and possessing thick but shortened spiral side lamellae.
Description: Test sublenticular asymmetric with a rounded margin and a circular outline. It is formed by a globular proloculus simply opening on a trochospirally enrolled, undivided tubular chamber, forming up to eight whorls. The lateral laminar extensions of the tube wall are shortened on the spiral side and well-developed, mostly formed by discontinuous to papillose laminae on the umbilical side, forming numerous medium-sized polygonal nodes at the test surface. Trochospiral coiling slightly accentuated with ontogeny. Rapidly enlarging during growth, the tubular chamber initially appears oval in section, then kidney-shaped. Canal system arranged in levels of polygonal networks developed from the tubular chamber and interconnected by large and spaced-out pores, perpendicularly crossing the laminar deposits. Wall calcareous, commonly recrystallized into microsparite to sparite but originally hyaline, fibrous aragonitic, and radially perforate. Aperture not observed, most likely primarily simple, terminal.
Dimensions: Diameter 0.6–0.75 mm; height 0.3–0.45 mm; ratio d/h 1.7–2.2; number of whorls up to 8–9; apical angle 130°–140°.
Relationship between the umbilical canal system and the laminar deposits in coscinoconins
So far, the lamellar structure in Coscinoconinae has only been assumed (Rigaud et al. 2013, fig. 7), although some coscinoconins showing a well-preserved lamellar structure had been illustrated in a French scholar book by Mathieu et al. (2011 “Trocholina”, photographs 10, 11, provided by J-P Masse). Loeblich and Tappan (1987, for Hottingerella) and, Neagu (1994, for Andersenolina) proposed that a single L2 lamella (sensu) Piller 1978 was formed with each whorl. According to our material, lamellae are clearly interfingering (Figs. 14b, e, 15a, c, d, g, h, 16o), as recently defined for all other Trocholinidae (Rigaud et al. 2013). It implies that at least two L2 lamellae are formed with each whorl, as in Piller’s Aulotortus model. The laminar structure of Coscinoconinae is the most complex of all Involutinida. The laminae (L1 lamellae sensu) Piller, 1978 forming the test are thin and numerous. Their apparent length and thickness varies considerably (e.g., Fig. 6n, o) and whorls may display very small, discontinuous (as in Triadodiscus) to almost continuous laminae, which can be either uniform (as in Lamelliconus), uneven (as in Coronipora), or locally thickened (as in Trocholina and Frentzenella) as defined by Rigaud et al. (2013). The end of some laminae appears tapering (e.g., Fig. 6n) or slightly folded (distorted in the nomenclature of Rigaud et al. 2013 = folded lamina in Fig. 6n), whereas other laminae appear sharply cut in place of the canal system (see sharp edge of the canal system in Fig. 6n, o). It indicates that, as proposed by Hottinger (1976) by comparison with the marginal canal system of nummulitids, the umbilical canal system of Coscinoconus was both generated by pre-existing structuring and resorption. In fact, the polygonal morphology of the canal system and associated polygonal nodes is related to resorption processes whereas papillae are formed by local thickening of the laminae. Both ornamental types are observable in coscinoconins.
Remarks on the phylogeny of trocholinids
Trocholinids have shown intense periods of diversification during Late Triassic, Early Jurassic, and end-Jurassic to mid-Cretaceous times. Their record between these radiation events, however, is very sporadic and our understanding of their stratigraphic ranges and phylogenetic links is strongly biased. Thanks to the study of Late Triassic–Early Jurassic forms, a complete revision of the trocholinid phylogeny and stratigraphic distribution has been started by Rigaud et al. (2013) but significant lacunas exist in the Middle Jurassic, hampering any reconstruction of the early evolution, origin, and diversification of coscinoconin genera. The existence of post-Cenomanian trocholinids is still a matter of controversy. According to illustrations provided by Tewari and Srivastava (1968: “Trocholina sahnii”), a possible lazarus Papillaconus (or Hensonipapillus if the mentioned “deposits of crystalline calcite” represent recrystallized polygonal nodes for the largest and pores for the smallest) survived until the Maastrichtian. In the Holocene, trochospirally coiled aragonitic tubular foraminifers re-appear with the genus Trocholinopsis Piller, 1983, but its structure requires clarification. According to Piller (1983), its test exhibits only one lamella per whorl on its umbilical side and would be devoid of spiral side lamellae. This feature, if confirmed, would exclude it from the family Trocholinidae.
Remarks on the way of life of Cretaceous trocholinids
For Cretaceous trocholinids, the inferred mode of life is “epi-faunal; semi-attached herbivorous (browsers) and deposit-feeders (grazing herbivores, detritivores)” (Koutsoukos and Hart 1990, p. 228). Concerning high-conical to cylindro-conical versus low-conical trocholinids, Arnaud-Vanneau and Darsac (1984) suggested different palaeoenvironments within Tithonian–Valanginian carbonate platforms that might also reflect different modes of life. Up to now, attached trocholinids have been only illustrated by Senowbari-Daryan et al. (2010) in the Late Triassic species Trocholina blaui. This taxon clearly shows marginal appendices or extensions: (Senowbari-Daryan et al. 2010, figs. 6b, d–g, 7a, b, d, g, i, p), allowing an attachment in elevated position. This structure can, to some extent, be compared with the so-called micro-aquarium (=“corridor” in Vachard and Krainer 2001) of Palaeozoic Tetrataxis (Vachard et al. 2010) and Late Jurassic Mohlerina (Schlagintweit 2012), both attached on their umbilical side. In our Cenomanian material, some specimens seem to be preserved attached to bioclastic hard substrates (Fig. 18) and similar appendices are observed (Fig. 18b–d). A distinct anchorage structure similar to that observed in “Globotetrataxis” may be discernible in Fig. 18a. In some cases, however, a simple passive cementation between trocholinids and other clasts cannot be excluded (Fig. 18d–f).
An unstable palaeoenvironment within external platform settings, influencing the trophic structure of foraminiferal palaeocommunities, can be deduced from the microfacies. The ability to occupy various modes of life would be an asset to survive in such conditions. In the best preserved specimens, polygonal nodes (columnar protusions) delimit free spaces (Fig. 18g). We have not observed such specimens in an attached life position but this umbilical structure may represent a specialized construction improving exchanges with the exterior whilst attached in an umbilical position. In attached specimens, free space between the test and the substrate is commonly distinguishable (e.g., Fig. 18a, c, f) evidencing the importance of an elevated position when attached on the apertural side and explaining the need, in various foraminiferal groups, to build marginal appendices.
Remarks on the Cenomanian extinction of trocholinids
The genus Coscinoconus has been reported from the Bathonian–Cenomanian interval, (Rigaud et al. 2013, fig. 9) with the youngest known representative being C. intermedius (Henson). There are several records in the literature from different and widely distributed areas where this species (reported as “Trocholina arabica”) exhibits a last occurrence (LO) at the Cenomanian–Turonian boundary (e.g., Saint-Marc 1978: Lebanon; Berthou 1984: Portugal; Ettachfini et al. 2005: Morocco). Others, such as Bilotte (1973), placed the LO at the middle/upper Cenomanian boundary. Nevertheless, there are no confirmed records of Coscinoconus from strata younger than the Cenomanian.
Caus et al. (2009) discussed the disappearance of the k-strategists among larger benthic foraminifera, which possess calcitic microgranular/agglutinated shells (sensu Rigaud et al. 2015b). According to them, around the Cenomanian–Turonian boundary, the eutrophication and expansion of the oxygen minimum zone across shelf areas, related to the background of the oceanic anoxic event (OAE2), affected their livelihood. This view, however, is not accepted by others (e.g., Gale et al. 2000). Concerning trocholinids, we may also be faced with other reasons because of their aragonitic test mineralogy. The general effect of OAEs on calcifying marine organisms (mainly microfossils) was reviewed by Hönisch et al. (2012, fig. 1) without providing evidence for an obvious event on calcareous benthic foraminifers. They did, however, not differentiate between calcitic and aragonitic benthic foraminifers. In contrast, Hönisch et al. (2012, p. 1061) mention indications of higher partial pressure of CO2 and lower pH values. It can be speculated that such a calcification crisis might have a stronger impact on the latter group, and on aragonitic forms in general. For example, an increase in susceptibility towards ocean acidification (lowering of pH) in aragonitic shelled thecosomatous pteropods (e.g., in-life shell dissolution) was documented by Wall-Palmer et al. (2012). To assess the impact of ocean acidification on the test surface ornamentation in the benthic foraminifer Haynesina germanica, culturing experiments under varying atmospheric CO2 partial pressures were conducted by Khanna et al. (2013). High CO2 levels resulted in partial shell dissolution and significant reduction and deformation of ornamentation. The authors concluded that “a reduction in functionally important ornamentation could lead to a reduction in feeding efficiency with consequent impacts on this organism’s survival and fitness”. We thus propose that the extinction of the aragonitic-walled, highly ornamented Trocholinidae during the middle–late Cenomanian was connected to ocean acidification during the middle Cenomanian MCE and the late Cenomanian OAE2, the former being a prelude to the OAE2 (e.g., Coccioni and Galeotti 2003) that also initiated the platform drowning in Cantabria (Wilmsen 2000).
Conclusions
The Altamira and Bielba formations hold the best preserved trocholinid assemblages ever described from Cretaceous rocks. Beyond allowing the description of several new taxa, this preservation has permitted identifying and emending Hensonipapillus lenticularis (Henson, 1947) and Paracoscinoconus burlini (Gorbatchik, 1959), two species that have been largely misidentified in the past. Usually poorly to moderately preserved, trocholinids are a tricky foraminiferal group, even for specialists. The intimate details of their structure and external shape provided in this paper and in Rigaud et al. (2013) are fundamental to allow their correct identification, and thus to revise their widely used but too often erroneous stratigraphic ranges.
In the Cenomanian, prior to their probable complete extinction, Trocholinidae were rich and highly diversified. Typically represented by advanced forms of the new subfamily Coscinoconinae, they predominantly lived in near-reefal and platform-margin environments. Possibly attached or anchored on their umbilical side thanks to marginal appendices, coscinoconins possessed a relatively complex umbilical canal system and a pronounced ornamentation, facilitating cytoplasm and water circulation. Their extinction might be correlated to the late Cenomanian Oceanic Anoxic Event 2, which most likely impeded a normal development of their aragonitic tests.
References
Arnaud A, Berthou PY, Brun L, Cherchi A, Chiocchini M, de Castro P, Fourcade E, García Ouintana A, Hamaoui M, Lamolda M, Luperto Sinni E, Neumann M, Prestat B, Schroeder R, Tronchetti G (1981) Tableau de répartition stratigraphique des grands foraminifères caractéristiques du Crétacé Moyen de la région Méditerranéenne. Cretac Res 2:383–393
Arnaud-Vanneau A, Darsac C (1984) Caractères et évolution des peuplements de Foraminifères benthiques dans les principaux biotopes des plates-formes carbonatées du Crétacé inférieur des Alpes du Nord (France). Géobios Mém Spéc 8:19–23
Arnaud-Vanneau A, Boisseau T, Darsac C (1988) Le genre Trocholina et ses principales espèces au Crétacé. Rev Paléobiol vol spéc 2:353–377
Berthou PY (1984) Répartition stratigraphique actualisée des principaux Foraminifères benthiques du Crétacé moyen et supérieur du Bassin Occidental Portugais. Benthos ´83, 2nd Int Symp Benthic Foraminifera (Pau, April 1983):45–54
Bilotte M (1973) Le Cénomanien des Corbières Méridionales (Pyrénées). Bull Soc Hist Nat Toulouse 109:7–22
Blau J (1987a) Neue Foraminiferen aus dem Lias der Lienzer Dolomiten. Teil I: Die Foraminiferenfauna einer roten Spaltenfüllung in Oberrhätkalken. Jb Geol BA 129:495–523
Blau J (1987b) Neue Foraminiferen aus dem Lias der Lienzer Dolomiten. Teil II (Schluss): foraminiferen (Involutinina, Spirillinina) aus der Lavanter Breccie (Lienzer Dolomiten) und den Nördlichen Kalkalpen. Jb Geol BA 130:5–23
Blau J, Grün B (1997) Neue Involutininen (Foraminifera) aus dem marmorea-Hartgrund (Hettangium/Sinemurium, Lias) von Adnet (Österreich). N Jb Geol Paläont Abh 204:247–262
Boudagher-Fadel M (2008) Evolution and geological significance of larger benthic Foraminifera. Dev Palaeontol Stratigr 21:1–571
Carreras Suárez F, Ramírez del Pozo J (1971) Estratigrafía del Cretácico superior del borde Nororiental del Macizo Asturiano (zona de Bielba-Labarces, prov. de Santander). I Congreso Hispano-Luso-Americano de Geología Económica, 1, Sección 1, Geología, pp 49–72
Caus E, Bernaus JM, Calonge E, Martín-Chivelet J (2009) Mid-Cenomanian separation of Atlantic and Tethyan domains in Ibera by a land-bridge: the origin of larger foraminifera bioprovinces? Palaeogeogr Palaeoclimatol Palaeoecol 283:172–181
Cherchi A, Schroeder R (2005) Menaella bustamantei n. gen., n. sp. (Acervulinacea, Foraminiferida) from the uppermost Albian of northern Spain. Boll Soc Paleontol Ital 44:1–10
Coccioni R, Galeotti S (2003) The mid-Cenomanian event: prelude to OAE 2. Palaeogeogr Palaeoclimatol Palaeoecol 190:427–440
Conrad MA, Peybernès B (1972) Une association de Dasycladales du passage Albien-Cénomanien dans les Pyrénées et les régions voisines (Chaînes Cantabriques, Provence). Géobios 15:775–781
d’Orbigny A (1826) Tableau méthodique de la classe des Céphalopodes. Ann Sci Nat 7(2):245–314
Desssauvagie TFJ (1968) Cenomanian trocholinas from Nigeria. Micropalaeontology 14:64–72
di Bari D, Laghi G (1994) Involutinidae Bütschli (Foraminiferida) in the Carnian of the northeastern Dolomites (Italy). Mem Sci Geol 46:93–118
Ettachfini EM, Souhel A, Andreu B, Caron M (2005) La limité Cénomanien-Turonien dans le Haut Atlas central, Maroc. Géobios 38:57–68
Gale AS, Smith AB, Monks AE, Young JA, Howard A, Wray DS, Huggett JM (2000) Marine biodiversity through the Late Cenomanian-Early Turonian: palaeoceanographic controls and sequence stratigraphic biases. J Geol Soc Lond 157:745–757
Gorbatchik TN (1959) New species of foraminifera from the Lower Cretaceous of Crimea and Northwest Caucasus. Paleont J 1:78–83 (in Russian)
Guillaume S (1963) Les Trocholines du Crétacé inférieur du Jura. Rev Micropaléont 5:257–276
Guiry MD, Guiry GM (2014) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org
Henson FRS (1947) Foraminifera of the genus Trocholina in the Middle East. Ann Mag Nat Hist Ser 11(14):445–459
Hohenegger J, Piller W (1975) Diagenetische Veränderungen bei obertriadischen Involutinidae (Foraminifera). N Jb Geol Paläont Mh 1975:26–39
Hohenegger J, Piller W (1977) Die Stellung der Involutinidae Bütschli und Spirillinidae Reuss im System der Foraminiferen. N Jb Geol Paläont Mh 1977:407–418
Hönisch B, Ridgwell A, Schmidt DN, et al. (2012) The geological record of ocean acidification. Science 335:1058–1063
Hottinger L (1976) An early umbilical canal system in Trocholina chouberti n. sp. from the Lower Cretaceous of north-eastern Morocco. Eclog Geol Helv 69:815–820
Hottinger L (2006) Illustrated glossary of terms used in foraminiferal research. Carnets Géol Noteb Geol Mem 2006/02 (CG2006_M02), 126 p
Kerćmar D (1961) The first findings of the Upper Jurassic algae in Slovenia. Geol Razpr Poroc 7:9–24 (English abstract)
Khanna N, Godbold JA, Austin WEN, Paterson DM (2013) The impact of ocean acidification on the functional morphology of Foraminifera. PLoS ONE 8(12):e83118. doi:10.1371/journal.pone.0083118
Koehn-Zaninetti L (1969) Les foraminifères du Trias de la région de l’Almtal (Haute-Autriche). Jb Geol BA Sbd 14:1–155
Koutsoukos EAM, Hart MB (1990) Cretaceous foraminiferal morphogroup distribution patterns, palaeocommunities and trophic structures: a case study from the Sergipe Basin, Brazil. Trans R Soc Edinb Earth Sci 81:221–246
Kristan-Tollmann E (1963) Entwicklungsreihen der Trias-Foraminiferen. Paläont Z 37:147–154
Leupold W, Bigler H (1936) Coscinoconus eine neue Foraminiferenform aus Tithon-Unterkreide-Gesteinen der Helvetischen Zone der Alpen. Eclog Geol Helv 28:606–624
Loeblich AR, Tappan H (1987) Foraminiferal genera and their classification, vol 2. Van Nostrand Reinhold, New York
Mancinelli A, Coccia B (1999) Le Trocholine dei sedimenti mesozoici di piattaforma carbonatica dell´ Appennino-centro-meridionale (Abruzzo e Lazio). Rev Paléobiol 18:147–171
Mantsurova VN, Gorbatchik TN (1982) New data on structure of Trocholina tests (foraminifers). Vopr Micropaleont 25:166–133 (in Russian)
Martinez CB (2007) Foraminiferos rotálidos del Cretácico superior de la Cuenca pirenaica. PhD thesis, University of Barcelona. http://www.tdx.cat/bitstream/handle/10803/3442/cbm1de1.pdf
Maslov VP (1958) Finds of algae of the genus Coscinoconus Leupold, in the Jurassic of Crimea, and its true nature. Dokl Akad Nauk SSSR 121:545–548 (in Russian)
Mathieu R, Bellier JP, Granier B (2011) Manuel de Micropaléontologie. Carnets Géol Noteb Geol CG 2011_B02, 123 p
Moullade M, Peybernès B (1974) Etude microbiostratigraphique del´Albien du Massif de Montgri (Prov. Gerona, Espagne). Description de Hensonina n. gen. (générotype Trocholina lenticularis Henson, 1947) (Foraminiferida, Involutinidae). Arch Sci Genève 26 (1973):173–182
Najarro M, Rosales I, Moreno-Bedmar JA, de Gea GA, Barrón E, Company M, Delanoy G (2011) High-resolution chemo- and biostratigraphic records of the Early Aptian oceanic anoxic event in Cantabria (N Spain): palaeoceanographic and palaeoclimatic implications. Palaeogeogr Palaeoclimatol Palaeoecol 299:137–158
Neagu T (1994) Early Cretaceous Trocholina group and some related genera from Romania Part I. Rev Esp Micropaleont 26:117–143
Neagu T (1995) The Cretaceous Trocholina group and some related genera from Romania Part II. Rev Esp Micropalent 27:5–40
Olivet JL (1996) La cinématique de la Plaque Ibérique (Kinematics of the Iberian Plate). Bull Centres Rech Explor Prod Elf Aquitaine 20:131–195
Paalzow R (1922) Die Foraminiferen der Parkinsoni-Mergel von Heidenheim am Hahnenkamm. Abh Naturhist Ges Nürnberg 22:1–35
Pawlowski J, Holzmann M, Tyszka J (2013) New supraordinal classification of Foraminifera: molecules meet morphology. Mar Micropaleontol 100:1–10
Pelissié T, Peybernés B (1982) Etude micropaléontologique du Jurassique moyen/supérieur du Causse de Limogne (Quercy), déscription des foraminiféres Trocholina gigantea n. sp., Parinvolutina aquitanica n. gen., n. sp. et Limognella dufaurei n. gen., n. sp. Rev Micropaléont 25:111–132
Philip J, Floquet M (2000) Late Cenomanian (94.7–93.5). In: Dercourt J, Gaetani M, Vrielynck B, Barrier E, Biju-Duval B, Brunet MF, Cadet JP, Crasquin S, Sandulescu (eds) Atlas Peri-Tethys palaeogeographical maps. CCGM/CGMW, Paris, pp 129–136
Piller WE (1978) Involutinacea (foraminifera) der Trias und des Lias. Beitr Paläont Österr 5:1–164
Piller WE (1983) Remarks on the suborder Involutinina Hohenegger and Piller, 1977. J Foram Res 13:191–201
Pop G, Bucur II (2001) Upper Jurassic and Lower Cretaceous sedimentary formations from the Vâlcan mountains (South Carpathians). Stud Univ Babeş Bolyai Geol 46(2):77–94
Rat P (1989) The Iberian Cretaceous: climatic implications. In: Wiedmann J (ed) Cretaceous of the Western Tethys. Proc 3rd Int Cret Symp Tübingen 1987, Schweizerbart, Stuttgart, pp 17–25
Reichel M (1955) Sur une trocholine du Valanginien d’Arzier. Eclog Geol Helv 48:396–408
Reitner J (1987) Mikrofazielle, palökologische und paläogeographische Analyse ausgewählter Vorkommen flachmariner Karbonate im Basko-Kantabrischen Strike Slip Fault-Becken-System (Nordspanien) an der Wende von der Unterkreide zur Oberkreide. Docum Nat 40:1–239
Reitner J (1989) Lower and Mid-Cretaceous Coralline Sponge communities of the Boreal and Tethyan Realms in comparison with the modern ones—palaeoecological and palaeogeographic implications. In: Wiedmann J (ed) Cretaceous of the Western Tethys. Proc 3rd Int Cret Symp Tübingen 1987. Schweizerbart, Stuttgart, pp 851–878
Reitner J, Wilmsen M, Neuweiler F (1995) Cenomanian/Turonian sponge/microbialite deep-water hardground community (Liencres, Northern Spain). Facies 3:203–212
Rigaud S, Martini R, Rettori R (2012) Parvalamellinae, a new subfamily for Triassic glomospiroid Involutinidae. J Foram Res 42:246–257
Rigaud S, Blau J, Martini R, Rettori R (2013) Taxonomy and phylogeny of the Trocholinidae (Involutinina). J Foram Res 43:317–339
Rigaud S, Blau J, Martini S, Rettori R (2015a) Taxonomy, phylogeny, and functional morphology of the foraminiferal genus Involutina. Acta Paleont Polon. doi:10.4202/app.2012.0056
Rigaud S, Vachard D, Martini R (2015b) Agglutinated versus microgranular foraminifers: end of a paradigm? J Syst Palaeontol. doi:10.1080/14772019.2013.863232
Rigaud S, Vachard D, Martini, R (2015c) Early evolution and new classification of the Robertinida (Foraminifera). J Foram Res 45(1):3–28
Saint-Marc P (1978) Biostratigraphie de l´Albien, du Cenomanien et du Turonien du Liban. Ann Mines Géol (Actes VIe Coll Africain Micropaléont—Tunis 1974) 28:111–118
Schlagintweit F (2012) Mohlerina basiliensis (Mohler, 1938): a Middle Jurassic-Early Cretaceous facultative (?) epilithic benthic foraminifer. Facies 58:637–647
Schlagintweit F, Piller W (1990) Involutina hungarica (Sido) from allochthonous Urgonian limestones of the Northern Calcareous Alps and remarks on the genus Hensonina Moullade and Peybernès, 1974. Beitr Paläont Österr 16:145–153
Schlagintweit F, Wilmsen M (2014) Calcareous algae (Dasycladales, Udoteaceae) from the Cenomanian Altamira Formation of Northern Cantabria, Spain. Acta Palaeont Roman 10(1–2):15–24
Schlagintweit F, Rigaud S, Wilmsen M (2015) Altamirella biscayana n. gen., n. sp. from the lower Cenomanian of Cantabria, N Spain: a post-Palaeozoic fusulinan? Cretac Res 52:1–8
Schott M (1983) Sedimentation und Diagenese einer absinkenden Karbonatplattform: Rhät und Lias des Brünnstein-Auerbach-Gebietes, Bayerische Kalkalpen. Facies 9:1–60
Schroeder R, Willems H (1983) Über einen submarinen Durchbruch des Diapirs von Villasana de Mena (Prov. Burgos, N- Spanien) an der Wende Unter-/Oberkreide. N Jb Geol Paläont Abh 166:65–85
Senowbari-Daryan B, Rashidi K, Torabi H (2010) Foraminifera and their associations of a possibly Rhaetian section of the Nayband Formation in central Iran, northeast of Esfahan. Facies 56:567–596
Tewari B, Srivastava R (1968) Foraminifera from the upper part of the Lower Ariyalur Stage of Trichinopoly Cretaceous. J Palaeont Soc India 10:31–48
Vachard D, Krainer K (2001) Smaller foraminifers of the Upper Carboniferous from the Auernig Group, Carnic Alps (Austria/Italy). Riv Ital Paleont Strat 107:147–168
Vachard D, Pille L, Gaillot J (2010) Palaeozoic Foraminifera: systematics, palaeoecology and responses to global changes. Rev Micropaléont 53:209–254
Wall-Palmer D, Hart MB, Smart CW, Sparks RSJ, Le Friant A, Boudon G, Deplus C, Komorowski JC (2012) Pteropods from the Caribbean Sea: variations in calcification as an indicator of past ocean carbonate saturation. Biogeoscience 9:309–315
Wiese F (1995) Das mittelturone Romaniceras kallesi-Event im Raum Santander (Nordspanien): Lithologie, Stratigraphie, laterale Veränderung der Ammonitenassoziationen und Paläobiogeographie. Berliner Geowiss Abh E 16:61–77
Wiese F (1997) Das Turon und Unter-Coniac im Nordkantabrischen Becken (Provinz Kantabrien, Nordspanien): Faziesentwicklung, Bio-, Event- und Sequenzstratigraphie. Berliner Geowiss Abh E 24:1–131
Wiese F, Wilmsen M (1999) Sequence stratigraphy in the Cenomanian to Campanian of the North Cantabrian Basin (northern Spain). N Jb Geol Paläont Abh 212:131–173
Wilmsen M (1996) Flecken-Riffe in den Kalken der Formación de Altamira (Cenoman, Cobreces/Tonanes-Gebiet, Prov. Kantabrien, Nord-Spanien): Stratigraphische Position, fazielle Rahmenbedingungen und Sequenzstratigraphie. Berliner Geowiss Abh E 18:353–373
Wilmsen M (1997) Das Oberalb und Cenoman im Nordkantabrischen Becken (Provinz Kantabrien, Nordspanien):Faziesentwicklung, Bio- und Sequenzstratigraphie. Berliner Geowiss Abh E 23:1–167
Wilmsen M (2000) Evolution and demise of a mid-Cretaceous carbonate shelf: the Altamira Limestones (Cenomanian) of northern Cantabria (Spain). Sediment Geol 133:195–226
Wilmsen M (2005) Stratigraphy and biofacies of the Lower Aptian of the Cuchia (Cantabria, northern Spain). J Iber Geol 31:253–275
Wilmsen M, Wiese F, Ernst G (1996) Facies development, events and sedimentary sequences in the Albian to Maastrichtian of the Santander depositional area, northern Spain. Mitt Geol Paläont Inst Univ Hamburg 77:337–367
Zaninetti L (1984) Les Involutinidae (foraminifères): proposition pour une subdivision. Rev Paléobiol 3:205–207
Acknowledgments
We thank two anonymous reviewers for their constructive comments and suggestions. S.R. gratefully thanks the Swiss Science National Foundation for the grant PBGEP2-145580.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schlagintweit, F., Rigaud, S. & Wilmsen, M. Insights from exceptionally preserved Cenomanian trocholinids (benthic foraminifera) of northern Cantabria, Spain. Facies 61, 416 (2015). https://doi.org/10.1007/s10347-014-0416-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10347-014-0416-2