Abstract
Escherichia coli is a commensal bacterium from the human and animal intestinal microbiota; however, some strains may be pathogenic and can cause a wide range of intestinal and extraintestinal diseases. The presence of pathogenic strains in wild animals, such as deer, may constitute a risk for humans and other animals. Thus, the aim of this study was to determine the antimicrobial resistance (AMR), serotype, phylogenetic groups and virulence genes (VGs) of 22 E. coli isolates obtained from red deer faeces (Cervus elaphus). Results showed that most isolates (17/22) were susceptible to the antimicrobials assessed, whereas only five of them were resistant to some of the tested antimicrobials (mainly to β-lactamics, and to trimethoprim/sulphamethoxazole). Regarding the phylogenetic groups, 19 isolates belonged to the B1 group and three to the B2 group. The identified VGs corresponded to stx1 (17/22), stx2 (12/22), estA (12/22), bfpA (6/22), and eae (4/22). Interestingly, hybrid strains containing VGs that belonged to STEC and EPEC (stx1, stx2, bfpA (n = 2) and stx1, bfpA (n = 1)) and to STEC and ETEC (stx1, estA (n = 1), stx2, estA (n = 1), stx1, stx2, bfpA (n = 6) and eae, stx1, stx2, bfpA (n = 1)) pathotypes were found. The identified serotypes were O:H21, O70:NM, O91:NM, O5:NM, O:NM, O23:H16, O23:H25, O38:H25, O6:H34, O6:NT and O75:H9. This work represents the first insight of potentially pathogenic E. coli strains in red deer from Mexico, and it establishes the importance of deer strain characterization. Moreover, the high frequency (12/22) of hybrid strains found in this study, along with a presumably pathogenic potential profile, could represent an additional risk for humans and animals, therefore further investigations on this issue would be needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Escherichia coli is a Gram-negative, facultative anaerobic bacterium, which belongs to the Enterobacteriaceae family. It colonizes the gastrointestinal tract within the first hours of life and is part of the normal intestinal microbiota in humans, mammals and birds. Some E. coli strains have medical significance, since they can become intestinal and extraintestinal pathogens (Gyles and Fairbrother 2004; Lillehaug et al. 2005). Pathogenic E. coli strains can be classified, according to their specific virulence factors (VFs) and disease mechanisms into seven different pathotypes: (1) enteropathogenic E. coli (EPEC), (2) Shiga toxin-producing E. coli (STEC) with its enterohemorrhagic E. coli subgroup (EHEC), (3) enterotoxigenic E. coli (ETEC), (4) enteroaggregative E. coli (EAEC), (5) enteroinvasive E. coli (EIEC), (6) diffusely adherent E. coli (DAEC) and (7) adherent-invasive E. coli (AIEC) (Croxen et al. 2013).
STEC strains have a particular medical interest, since their prevalence has risen in recent years and more than 450 serotypes belonging to this pathotype have been identified in humans (Blanco et al. 2004). Animal reservoirs (mainly dairy and beef cattle) have played a major role in the transmission of these strains (Hussein and Bollinger 2005). Different cervid species have also been included in the list of possible reservoirs. Obwegeser and coworkers (Obwegeser et al. 2012) described some STEC strains in red deer (Cervus elaphus), roe deer (Capreolus capreolus), chamois (Rupicapra rupicapra), and ibex (Capra ibex). These authors concluded that stx (Shiga toxins) and eae (intimin) markers have a significant prevalence in hunted wild ruminants. Furthermore, Sánchez and co-workers (Sánchez et al. 2009) reported STEC strains in hunted specimens of red deer, roe deer, fallow deer (Dama dama) and mouflon (Ovis musimon) in Spain, whereas Li et al. (2013) reported the presence of these strains in wild sika deer (Cervus nippon) in China. For O157:H7 in particular, most studies (Renter et al. 2001; Dunn et al. 2004) have reported low prevalences in deer (0.25 to 0.3 %). Also, these animals can harbor non-O157 STEC strains with prevalences of 16 to 20 % (Asakura et al. 1998; Leotta et al. 2006).
World game meat production is estimated in over 2 million tons annually, produced mostly in Africa and America (56.2 and 15.3 % of the total production, respectively) (FAO 2013). Therefore, the presence of STEC strains in game animals, such as deer, constitutes a STEC infection risk, since the carcasses and by-products (retail cuts, jerky, sausages, etc.) have been involved in foodborne diseases (Ahn et al. 2009; Keene et al. 1997; Martin and Beutin 2011; Nagano et al. 2004).
Other less common infection routes involving deer STEC strains have been described, such as the US outbreak with contaminated strawberries (Laidler et al. 2013) and an outbreak involving kids playing in a contaminated soccer field (Franklin et al. 2013). These studies have established a public health scenario where deer and human populations coexist. The aim of the present study was to determine the antimicrobial resistance (AMR), serotype, phylogenetic groups and virulence genes (VGs) of E. coli strains obtained from red deer faeces of a special wildlife management unit (UMA, for its initials in Spanish).
Material and methods
Samples and study area
The study was performed in a legal and sustained hunting UMA, located inside the Natural Protected Area of Sierra Fria (NPASF) in the state of Aguascalientes, Mexico. The NPASF is located approximately at 21° 52′ 50″ and 22° 19′ 46″ North and 102° 22′ 50″ and 102° 51′ 26″ West; it extends over 1120 km2, with an altitude ranging from 1800 to 3050 m above sea level. The entire NPASF deer population is composed by red deer (C. elaphus), white-tailed deer (Odocoileus virginianus couesi), and elk (Cervus canadensis). The deer density was considered to be as low as 4 deer/km2. Local wildlife authorities, in accordance with applicable laws, issued hunting permits to licensed hunters as part of the UMA operation process. To maintain confidentiality and objectivity in the use of obtained information, diagnostic procedures were implemented only when the hunters and landowners expressed their consent and willingness to provide access to carcasses of red deer (RD) during the harvesting process. All evaluated RD were males, >10 months of age, hunted in the UMA. Age in these animals was estimated via assessment of tooth eruption and tooth wear. Twenty-two red deer were hunted during the 2013 hunting season, from which rectal swabs were obtained and placed in an Amies transport medium with activated carbon and were sent to the Bacteriology Laboratory of the Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias (INIFAP) for their further analysis.
Identification and antimicrobial susceptibility
Samples were collected by direct rectum swabbing of hunted deer, before the hunters processed the carcasses. Each sample was cultured by conventional techniques in MacConkey agar without antibiotics. The plates were incubated under aerobic conditions for 24–48 h at 37 °C. After incubation, each colony was microscopically (Gram stain, form and bacterial grouping) and macroscopically (lactose fermentation) described. The identification and antimicrobial susceptibilities were determined by the MicroScan WalkAway 96 plus (Dade Behring, West Sacramento, CA), using standardized minimum concentration breakpoints for susceptibility (CLSI 2011). An E. coli ATCC 25922 was used as a control strain. Isolates were stored at −70 °C in a brain-heart infusion broth containing 50 % glycerol.
Serotyping and phylogenetic group determination
The strains were serotyped by an agglutination assay (Orskov and Orskov 1992) using 96-well microtiter plates and rabbit serum (SERUNAM), against the 181 somatic and 53 flagellar E. coli antigens, 45 Shigella somatic antigens and the E. coli 64474:H32 strain, a new enterovirulent strain with close relation to Shigella boydii 16 (Navarro et al. 2010).
The extraction and purification of the genomic DNA were performed through the guanidine thiocyanate previously described (Pitcher et al. 1989). The phylogenetic group of each isolate was determined by the PCR technique described by Clermont and co-workers (Clermont et al. 2013).
Identification of VGs
The eae, stx1, stx2, bfpA, elt, estA, ipaH VGs and the rfbEO157 and fliCH7 serotype-related genes were identified by PCR (DebRoy and Roberts 2006; Tornieporth et al. 1995). For the stx2, bfpA and ipaH genes, PCRs were performed individually. E. coli O78:H11:K80 (H10407/ETEC strain), E. coli O127:H6 (E2348/69/EPEC strain), E. coli O157:H7 (EDL933/EHEC strain) ATCC 70092, E. coli O157:H7 (Public Health Laboratory, School of Medicine, UNAM) and E. coli EIEC (Department of Enteric Pathogens, Central Public Health Laboratory, London, UK) were included as positive controls. The amplified products were visualized in agarose gels and stained with ethidium bromide.
Results
Identification and antimicrobial susceptibility
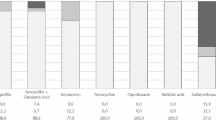
From the 22 isolates, 5 of them displayed AMR, mostly against β-lactam antibiotics (Table 1). Only one strain showed the presence of an extended-spectrum β-lactamases (ESBLs), whereas two were resistant to trimethoprim/sulphamethoxazole and ampicillin.
Serotyping
Fifteen isolates were typeable (68 %), whereas seven isolates were non-typeable (O?) (31.8 %); only one strain showed a rough O antigen (others were unreactive to the tested antisera). The most frequent isolated serogroup was O6 (5/22, 22.7 %), followed by O23 and O70 groups (3/22, 13.63 % each) and the O75, O91 and O38 groups, with just one strain each (4.5 % each). Subtyping of the 22 isolates using flagellar (H) antigens showed six strains with the H21 antigen (27.7 %), four with H34 (18.2 %), two with H16 (9 %), two with H25 (9 %) and one with H9 (4.5 %), and finally, seven isolates showed a lack of flagellum. The identified serotypes were O:H21 (5 strains), O70:non-motile (NM; 3 strains), O6:H34 (3 strains) and O23:H16 (2 strains), with one strain each for the O91:NM, O5:NM, O23:H25, O38:H25, O6:non-typable (NT), O75:H9, O?:NM and O rough (Rg):NM serotypes (Table 1).
Phylogenetic groups and virulence genes
Nineteen (86.4 %) and three (13.6 %) isolates belonged to the B1 and B2 phylogenetic groups respectively, according to the guidelines of Clermont et al. (2013).
Most of the strains carried VGs (21, 95.45 %), and several strains (15, 68 %) contained genes belonging to different pathotypes. Three strains harbored STEC (stx genes) and EPEC (bfpA gene), two had stx1, stx2 and estA genes and one had stx1 and bfpA genes. Furthermore, one strain had an EPEC-ETEC pattern, harboring estA and bfpA genes, and another strain contained STEC (stx1, stx2), EPEC (bfpA) and ETEC (estA) genes. Additionally, nine strains had STEC-ETEC hybrid patterns. From these, two had stx2 and estA, one had stx1 and estA, five harbored stx1, stx2 and estA and the last one—which was the most interesting—bore eae, stx1, stx2 and estA genes. Only one strain was a typical STEC strain, carrying stx1 and eae genes. None of the evaluated strains harbored the EIEC gene ipaH. Similarly, no O157:H7 was detected (all isolates were negative for rfbEO157 and fliCH7 genes) and only one isolate was negative for all the assessed genes (Table 1).
Discussion
The factors that determine the genetic structure of the E. coli population can be divided in host-related (diet, intestinal morphology and body mass) and environmental (taming and climate) factors; both of them seem to be predictors of the phylogenetic distribution (Gordon and Cowling 2003; DebRoy and Roberts 2006), aside from the genetic structure of the commensal E. coli. The interrelation between humans and animals may influence the phylogeny of a given strain from wild animals (Escobar-Paramo et al. 2006). The aim of this work was to characterize the E. coli strains present in a deer population from Mexico, in order to generate information about the circulating strains and to assess whether they constitute an animal and/or human health risk. Determining the phylogenetic groups showed that most of the studied isolates corresponded to the B1 group and only three were members of the B2 group. This finding was expected because the B1 phylogroup is associated with commensal strains, which are commonly reported in ruminants, humans and other animals; in addition, the B1 group has been reported in wildlife vertebrates and has been closely related to carnivorous mammals. In contrast, the B2 strains are not as common, since this group is thought to be more related to humans and pigs, but it also has been described in ruminant mammals (Carlos et al. 2010). The presence of these strains in red deer could be explained by the human presence in the UMA (veterinary personnel, park keepers, hunters), as well as livestock animals, and may be influenced by the animal diet.
In the present study, we observed that most of the isolated strains harbored VGs (21 of 22 carried at least one of the assessed genes). This fact must be taken into account for future risk assessment analyses, since most strains could be considered (based on their genetic carriage of virulence factors) as potentially pathogenic to humans. Strikingly, the presence of “hybrid” strains (carrying virulence factors corresponding to different pathotypes) was found: STEC-EPEC and STEC-ETEC, and (only one) STEC-EPEC-ETEC “hybrid” strain. This phenomenon has already been reported. Müller et al. (2007) reported strains with intermediate genetic profiles, carrying VGs from STEC, ETEC and EPEC in isolates from different countries (Mexico included). Another important example is the O104:H4 strain identified during the 2011 Germany’s E. coli outbreak, an agent carrying STEC (stx2) and EAEC (aatA, aggR, aap, aggA, aggC) virulence factors and antibiotic resistance (ECDC-EFSA 2011). This kind of hybrid strains may be more dangerous than a single pathotype alone, since it has more and diverse virulence factors, whose presence could interfere with a correct and quick diagnosis (Bielaszewska et al. 2014).
The occurrence for stx1, stx2 and eae matched the results reported by Baldy-Chudzik et al. (2008), in which 300 E. coli commensal isolates from healthy animals (herbivorous, carnivorous and omnivorous mammals) were screened in a Zoo in Poland; they found that the herbivorous isolates had the eae, stx1, stx2 VGs and belonged to phylogroup B1. This also coincides with the work performed by Girardeau et al. (2005), where the Shiga toxin-producing E. coli from cattle was more often classified as B1 than D groups. In the present study, we found 19 (54.54 %) non-O157 STEC isolates, which could represent a pathogenic risk for humans and/or animals, in particular due to the carriage of stx genes.
Concerning the MIC analysis, our results showed a low AMR frequency (5/22), mostly against old members of the β-lactam antibiotic family. The AMR of these five isolates may be associated to the human and livestock presence in the area and to the antimicrobial treatment that was likely applied to the animals. It is well known that the commensal and intestinal microbiota play a relevant role in antibiotic resistance. High bacterial density with a large genetic pool, combined with a constant antibiotic exposure due to human and veterinary medicine practices, yields the perfect combination for the selection of antibiotic resistance in the commensal microbiota (Griffin and Tauxe 1991). As expected, the B2 strains isolated in this study had no AMR, since the strains’ genetic background appears to affect the resistance patterns. Some strains from group A and those in group D (Deschamps et al. 2009) are particularly permissive to develop resistance to third-generation cephalosporins, in contrast to the B2 group strains, which are less resistant than others, regardless of the molecular mechanisms involved in the acquisition of resistance (e.g. mutation or gene acquisition) (Gordon and Cowling 2003; Skurnik et al. 2005; Tornieporth et al. 1995).
To our knowledge, there is a lack of information regarding the E. coli serotypes that may be present in red deer in Mexico. For instance, in a previous study in Spain, the most commonly reported serogroup in deer was O146, followed by O2, O8, O128, 0146, O174 and O166 (Sanchez et al. 2009). In the present work, all the identified serogroups have been previously reported as STEC. In particular, O5, O6, O75 and O91 have been found more than 30 times in human patients and healthy cattle. In addition, the O6 serotype has been identified as a cause of extraintestinal disease in dogs, whereas the O5 serogroup also has been linked to cats (Gyles and Fairbrother 2004). In contrast, O23, O38 and O70 have been reported less than 30 times according to Bettelheim (2007). Here, some isolates were non-typeable, and this fact could be explained because either the serogroup was not within those included in this research or, indeed, the serogroup was not identifiable, suggesting that they may be species specific. Additionally, the rough isolate described in this study may have been originated by mutations in the lipopolysaccharide O chain biosynthesis (Prehm et al. 1976).
The reported flagellar serogroups have not been previously reported in wild ruminants, but some of them have been described as STEC serogroups in sheep, goat, swine, beef cattle, beef products and human infections (Bettelheim 2007: Hussein 2007; Mora et al. 2011). However, seven strains were identified as non-motile because they do not react with the typing sera; this situation has been reported in several studies. The fliC gene PCR-restriction fragment length polymorphism is a perfect option to confirm the flagellar antigen (Fields et al. 1997). Furthermore, the H antigen (and fliC gene polymorphisms) characterization would give more detailed information; for example, the fliC characterization of the O91:NM strain could probably reveal if it was a “classical” O91 STEC or not. Also, 90.7 % of the reports for this serogroup comprise the following four serotypes: O91:H14, O91:H21, O91:H10 and finally, O91:H (usually harboring an H14 or H21 fliC pattern), with one third of the reports of this STEC serogroup being reported from human disease (Bettelheim 2007).
The combination of the study of different markers for each isolate (VG carriage, O and H antigens, adhesins, etc.) could draw a better image of the circulating E. coli strains within the deer population.
Conclusion
The present study represents the first characterization of E. coli strains in red deer performed in Mexico, demonstrating the presence of potentially pathogenic strains in this host, a fact that should be taken into consideration for epidemiological analyses. Also, the presence of hybrid-resistant STEC/EPEC, STEC/ETEC and STEC/EPEC/ETEC strains may alert us about the potential health risk that these deer strains may represent at the livestock-wildlife-human interface. Further studies would be required in order to expand our knowledge on this field.
References
Ahn CK, Russo AJ, Howell KR, Holt NJ, Sellenriek PL, Rothbaum RJ, Beck AM, Luebbering LJ, Tarr PI (2009) Deer sausage: a newly identified vehicle of transmission of Escherichia coli O157:H7. J Pediatr 155:587–589
Asakura H, Makino S, Shirahata T, Tsukamoto T, Kurazono H, Ikeda T, Takeshi K (1998) Detection and genetical characterization of shiga toxin-producing Escherichia coli from wild deer. Microbiol Immunol 42:815–822
Baldy-Chudzik K, Mackiewicz P, Stosik M (2008) Phylogenetic background, virulence gene profiles, and genomic diversity in commensal Escherichia coli isolated from ten mammal species living in one zoo. Vet Microbiol 131:173–184
Bettelheim KA (2007) The non-O157 shiga toxigenic (Verocytotoxigenic) Escherichia coli; under-rated pathogens. Crit Rev Microbiol 33:67–87
Bielaszewska M, Schiller R, Lammers L, Bauwens A, Fruth A, Middendorf B, Schmidt MA, Tarr PI, Dobrindt U, Karch H, Mellmann A (2014) Heteropathogenic virulence and phylogeny reveal phased pathogenic metamorphosis in Escherichia coli O2:H6. EMBO Mol Med 6:347–357
Blanco M, Blanco JE, Mora A, Dahbi G, Alonso MP, González EA, Bernárdez MI, Blanco J (2004) Serotypes, virulence genes, and intimin types of shiga toxin (verotoxin)-producing Escherichia coli isolates from cattle in Spain and identification of a new intimin variant gene (eae- ξ). J Clin Microbiol 42:645–651
Carlos C, Pires MM, Stoppe NC, Hachich EM, Sato MI, Gomes TA, Amaral LA, Ottoboni LM (2010) Escherichia coli phylogentic group determination and its application in the identification of the major animal source of fecal contamination. BMC Microbiol 10:161. doi:10.1186/1471-2180-10-161
Clermont O, Christenson JK, Denamur E, Gordon DM (2013) The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ Microbiol Rep 5:58–65
CLSI (2011) Performance standards for antimicrobial susceptibility testing. 21st Informational Supplement, M-100-S21. National Committee for Clinical Laboratory Standards, Wayne, Pennsylvania, USA
Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB (2013) Recent advances in understanding enteric pathogenic Escherichia coli. Clin Microbiol Rev 26:822–880
DebRoy C, Roberts E (2006) Screening petting zoo animals for the presence of potentially pathogenic Escherichia coli. J Vet Diagn Invest 18:597–600
Deschamps C, Clermont O, Hipeaux MC, Arlet G, Denamur E, Branger C (2009) Multiple acquisitions of CTX-M plasmids in the rare D2 genotype of Escherichia coli provide evidence for convergent evolution. Microbiology 155:1656–1668
Dunn JR, Keen JE, Moreland D, Thompson RA (2004) Prevalence of Escherichia coli O157:H7 in White-tailed deer from Louisiana. J Wildl Dis 40:361–365
EFSA/ECDC (2011) European Food Safety Authority/European Center for Disease Prevention and Control, 2011. Shiga toxin/verotoxin-producing Escherichia coli in humans, food and animals in the EU/EEA, with special reference to the German outbreak strain STEC O104, Stockholm:ECDC
Escobar-Paramo P, Le Menac’h A, Le Gall T, Amorin C, Gouriou S, Picard B, Skurnik D, Denamur E (2006) Identification of forces shaping the commensal Escherichia coli genetic structure by comparing animal and human isolates. Environ Microbiol 8:1975–1984
FAO, (2013) Food and Agriculture Organization of the United Nations, 2013. FAOSTAT (accesed 10.20.2015) faostat3.fao.org
Fields PI, Blom K, Hughes HJ, Helsel LO, Feng P, Swaminathan B (1997) Molecular characterization of the gene encoding H antigen in Escherichia coli and a development of a PCR-restriction fragment length polymorphism test for identification of E. coli O157:H7 and O157:NM. J Clin Microbiol 35:1066–1070
Franklin AB, VerCauteren KC, Maguire H, Cichon MK, Fischer JW, Lavelle MJ, Powell A, Root JJ, Scallan E (2013) Wild ungulates as disseminators of shiga toxin-producing Escherichia coli in urban areas. PLoS One 11;8(12):e81512. doi:10.1371/journal.pone.0081512
Girardeau JP, Dalmasso A, Bertin Y, Ducrot C, Bord S, Livrelli V, Vernozy-Rozand C, Martin C (2005) Association of virulence genotype with phylogenetic background in comparison to different seropathotypes of Shiga toxin-producing Escherichia coli isolates. J Clin Microbiol 43:6098–6107
Gordon DM, Cowling A (2003) The distribution and genetic structure of Escherichia coli in Australian vertebrates: host and geographic effects. Microbiology 149:3575–3586
Griffin PM, Tauxe RV (1991) The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev 13:60–98
Gyles CL, Fairbrother JM (2004) Escherichia coli. In: Gyles CL, Prescott JF, Songer JG, Thoen CO (eds) Pathogenesis of bacterial infections in animals. Blackwell Pub, Iowa, pp 193–214
Hussein HS (2007) Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products. J Anim Sci 85:63–72
Hussein HS, Bollinger LM (2005) Prevalence of Shiga toxin-producing Escherichia coli in beef cattle. J Food Prot 68:2224–2241
Keene WE, Sazie E, Kok J, Rice DH, Hancock DD, Balan VK, Zhao T, Doyle MP (1997) An outbreak of Escherichia coli O157:H7 infections traced to jerky made from red deer meat. JAMA 277:1229–1231
Laidler MR, Tourdjman M, Buser GL, Hostetler T, Repp KK, Leman R, Samadpour M, Keene WE (2013) Escherichia coli O157:H7 infections associated with consumption of locally grown strawberries contaminated by deer. Clin Infect Dis 57:1129–1134
Leotta GA, Deza N, Origlia J, Toma C, Chinen I, Miliwebsky E, Iyoda S, Sosa-Estani S, Rivas M (2006) Detection and characterization of Shiga toxin-producing Escherichia coli in captive non-domestic mammals. Vet Microbiol 118:151–157
Li R, He L, Hao L, Wang Q, Zhou Y, Jiang H (2013) Genotypic and phenotypic characterization of antimicrobial-resistant Escherichia coli from farm-raised diarrheic sika deer in Northeastern China. PLoS One 8(9), e73342. doi:10.1371/journal.pone.0073342
Lillehaug A, Bergsjo B, Schau J, Bruheim T, Vikoren T, Handeland K (2005) Campylobacter spp., Salmonella spp., verocytotoxic Escherichia coli, and antibiotic resistance in indicator organisms in wild cervids. Acta Vet Scand 46:23–32
Martin A, Beutin L (2011) Characteristics of shiga toxin-producing Escherichia coli from meat and milk products of different origins and association with food producing animals as main contamination sources. Int J Food Microbiol 146:99–104
Mora A, Herrera A, López C, Dahbi G, Mamani R, Pita JM, Alonso MP, Llovo J, Bernárdez MI, Blanco JE, Blanco M, Blanco J (2011) Characteristics of the Shiga-toxin-producing enteroaggregative Escherichia coli O104:H4 German outbreak strain and of STEC strains isolated in Spain. Int Microbiol 14:121–141
Müller D, Greune L, Heusipp G, Karch H, Fruth A, Tschäpe H, Schmidt MA (2007) Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR. Appl Environ Microbiol 73:3380–3390
Nagano H, Hirochi T, Fujita K, Wakamori Y, Takeshi K, Yano S (2004) Phenotypic and genotypic characterization of β-D-glucuronidase-positive shiga toxin-producing Escherichia coli O157:H7 isolates from deer. J Med Microbiol 53:1037–1043
Navarro A, Eslava C, Perea LM, Inzunza A, Delgado G, Morales-Espinosa R, Cheasty T, Cravioto A (2010) New enterovirulent Escherichia coli serogroup 64474 showing antigenic and genotypic relationships to Shigella boydii 16. J Med Microbiol 59:453–461
Obwegeser T, Stephan R, Hofer E, Zweifel C (2012) Shedding of foodborne pathogens and microbial carcass contamination of hunted wild ruminants. Vet Microbiol 159:149–154
Orskov F, Orskov I (1992) Escherichia coli serotyping and disease in man and animals. Can J Microbiol 38:699–704
Pitcher DG, Saunders NA, Owen RJ (1989) Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol 8:151–156
Prehm P, Schmidt G, Stirm S (1976) On the mutations responsible for the rough phenotype of Escherichia coli B. J Gen Microbiol 97:121–124
Renter DG, Sargeant JM, Hygnstorm SE, Hoffman JD, Gillespie JR (2001) Escherichia coli O157:H7 in free-ranging deer in Nebraska. J Wildl Dis 37:755–760
Sanchez S, Garcia-Sanchez A, Martinez R, Blanco J, Blanco JE, Blanco M, Dahbi G, Mora A, Hermoso de Mendoza J, Alonso JM, Rey J (2009) Detection and characterisation of Shiga toxin-producing Escherichia coli other than Escherichia coli O157:H7 in wild ruminants. Vet J 180:384–388
Skurnik D, Le Menac’h A, Zurakowski D, Mazel D, Courvalin P, Denamur E, Andremont A, Ruimy R (2005) Integron-associated antibiotic resistance and phylogenetic grouping of Escherichia coli isolates from healthy subjects free of recent antibiotic exposure. Antimicrob Agents Chemother 49:3062–3065
Tornieporth NG, John J, Salgado K, de Jesus P, Latham E, Melo MC, Gunzburg ST, Riley LW (1995) Differentiation of pathogenic Escherichia coli strains in Brazilian children by PCR. J Clin Microbiol 33:1371–1374
Acknowledgments
Mariana D. Carrillo-del Valle received scholarships from the Consejo Nacional de Ciencia y Tecnologia (CONACYT-24757). This work was supported by SEP-CONACYT, CB-2012-01, 179000, from Consejo Nacional de Ciencia y Tecnologia. The authors want to thank Luis Antonio León Alamilla (School of Medicine, Universidad Nacional Autonoma de Mexico) for his excellent laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Carrillo-Del Valle, M.D., De la Garza-García, J.A., Díaz-Aparicio, E. et al. Characterization of Escherichia coli strains from red deer (Cervus elaphus) faeces in a Mexican protected natural area. Eur J Wildl Res 62, 415–421 (2016). https://doi.org/10.1007/s10344-016-1015-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-016-1015-z