Abstract
Variation in the distribution and abundance of animals in space and time are key concepts of population ecology. We studied these variations in a population of red foxes (Vulpes vulpes) in the tundra and taiga of northern Sweden. We analysed 12 years (1974–1985) of snow tracking data from a large area of 65,375 km2. Specifically, we evaluated to what extent the distribution of red foxes was explained by the presence of prey and how this interacted with snow depth and altitude. We also tested for temporal linear trends in the distribution and abundance of red foxes during the study period. The distribution of red foxes was explained by the presence of rodents, hares, tetraonid species, and ungulates (i.e. carcasses). Snow depth had a negative effect on the impact of small prey on the distribution of the red foxes, whereas it had a positive effect on the impact of ungulates. The influence of hares increased with altitude. Neither distribution nor abundance of red foxes showed a positive or negative linear trend, suggesting a stable population in northern Sweden during our study. This study showed that the distribution of red foxes was not only influenced by the presence of their main prey (rodents), but also by interactions between alternative prey, altitude, and snow depth. This study also emphasizes the importance of ungulate carcasses for red foxes and for wildlife management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding how the distribution and abundance of animals vary in space and time is one of the main aims of ecology (Begon et al. 1996). The need for this knowledge is increasingly important today when human alteration of habitats and environmental changes are strongly affecting the majority of ecosystems worldwide (Walther et al. 2002). Food abundance and competition for resources are key determinants for the distribution of organisms (Osenberg and Mittelbach 1996; Persson et al. 1996) although several studies show that the mechanisms governing spatial and temporal variation in these distributions often are complex even in relatively simple food webs (e.g. Krebs et al. 1995; Post et al. 2000).
In recent years, much attention has been focused on top-down effects on the abundance and distribution of mesopredators due to the previous decline and recent return of apex predator populations, e.g. mesopredator release (Courchamp et al. 1999; Prugh et al. 2009; Ritchie and Johnson 2009). However, bottom-up effects mediated by changes in food availability may also affect mesopredator abundance and distribution (Ritchie and Johnson 2009). Increased human population densities and habitat fragmentation may provide new food resources and alter prey abundance, respectively (Kurki et al. 1998; Fahrig 2003).
The red fox (Vulpes vulpes Linnaeus, 1758) has one of the largest natural distributions of all mammals (Zimen 1980). It preys on a wide range of animal species and feed opportunistically on food resources such as berries and human garbage (Hartova-Nentvichova et al. 2010; Rosalino et al. 2010; Dell’Arte et al. 2007). The red fox occupies highly contrasting habitats (Lariviere and Pasitschniak-Arts 1996), and its distribution and abundance are determined to a large extent by food availability (Barton and Zalewski 2007; Rosalino et al. 2010; Gallant et al. 2012). In Scandinavia, the main prey of the red fox is microtine rodents (Lindström et al. 1987). Consequently, the production of young in Scandinavia is known to follow the 3–4 year cycle of rodents (Englund 1970; Lindström 1989; Lindström et al. 1994). During periods of low availability of main prey, the red fox includes a larger proportion of alternative food resources in its diet, including small game (Angelstam et al. 1984; Lindström et al. 1987) or ungulate carcasses (Jedrzejewski and Jedrzejewska 1992; Selås and Vik 2006; Killengreen et al. 2011). Recent studies have shown that moose carcasses are a main part of the winter diet of the red fox (Needham et al. 2014) and that red fox occupancy increases with higher densities of reindeer in the area (Henden et al. 2014).
The red fox population in Scandinavia increased rapidly from the 1940s to the 1970s (Selås and Vik 2006). This increase was probably facilitated by reduced harvest, absence of large carnivores, and increased food availability (Selås and Vik 2006; Elmhagen and Rushton 2007). The latter may have been caused by both direct and indirect human influences; an increase on human living standards providing more garbage (Killengreen et al. 2011), carcasses, and slaughter remains from increased ungulate populations including semi-domesticated reindeer (Ims et al. 2007; Angerbjörn et al. 2013) and increased abundance of rodents as a result of increased clear-cutting in forestry (Michal and Rafal 2014).
It has been suggested that the increase in the red fox abundance resulted in an expansion to higher altitudes and more northern latitudes since the early twentieth century (Hersteinsson and Macdonald 1992; Post et al. 2009; Killengreen et al. 2011). The overabundance of semi-domestic reindeer in northern Norway (Ims et al. 2007) has probably provided red foxes with carcasses as an important food resource during winter (Henden et al. 2010; Killengreen et al. 2011). Moreover, a warmer climate and an increase in primary production would ultimately lead to higher prey availability (Hersteinsson et al. 1989; Hersteinsson and Macdonald 1992). On the other hand, red foxes were already reported to breed in the open tundra in the first half of the twentieth century (Lönnberg 1926). It is thus unclear if the increased number of red foxes in the tundra is due to range expansion, or if it mirrored a general increase in abundance throughout Fennoscandia.
An increase in the red fox abundance may lead to elevated predation rates which may affect the structure and function of the ecosystem (Lindström et al. 1987, 1994; Danell and Hornfeldt 1987). For example, the densities of mountain hare (Lepus timidus), black grouse (Tetrao tetrix) and capercaillie (Tetrao urogallus) have increased in periods when the abundance of red foxes was reduced through control or disease (Lindström et al. 1987, 1994; Marcström et al. 1988; Smedshaug et al. 1999). Furthermore, Kjellander and Nordström (2003) found a negative correlation between the number of red fox litters and the ratio of roe deer fawns per doe.
Another example of the ecological impact of an increase in the red fox abundance is the case of the Scandinavian arctic fox (Vulpes lagopus). The arctic fox population in Scandinavia experienced a decline due to an intensive harvesting during the first part of the twentieth century (Lönnberg 1926) and has since not recovered. This has been partially attributed to competitive exclusion by red foxes (Hersteinsson and Macdonald 1992; Killengreen et al. 2007; Selås and Vik 2007; Henden et al. 2009) and therefore lethal control of red foxes has been used in experimental management in the tundra region (Angerbjörn et al. 2013). Despite the importance of red foxes and their anticipated positive response to climate change, information on the distribution and population trends of red foxes in the tundra and taiga region of northern Scandinavia is very limited.
The main objective of our study was to investigate how the distribution of red foxes varied in space in relation to the presence of main prey and alternative food resources and to depict the relationships between these bottom up effects, altitude and snow depth. We also investigated the relationship between the red fox distribution and prey species richness. In order to examine factors affecting the distribution of red foxes, we analysed snow tracking data on distribution and abundance of various wildlife species collected during 12 years in the tundra and taiga region of northern Sweden. Additionally, we also examined the relationship between linear temporal trends and altitude in the distribution and relative abundance of this red fox population.
Methods
Study area
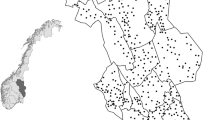
This study was part of a monitoring program for the tundra and taiga area in the northernmost county (Norrbotten) in Sweden from 1974 to 1985 (Fig. 1). The data collection was conducted by the Swedish Environmental Protection Agency (SEPA). The study area (65,375 km2) had a human population density of 0.33 people per km2 (Statistics Sweden (SCB) 2015) with ca. 5 % of the area covered by human settlements and consisted of four townships. There are several large national parks (5932 km2) situated in the study area, and most of the land (ca. 42,000 km2) is managed by the state. Traditional semi-domestic reindeer herding by the indigenous Sami people is extensive in the study area.
The landscape can be divided into three main categories: (1) treeless open tundra with limited vegetation, (2) mountain birch (Betulla pubescens tortuosa) forest, and (3) mixed boreal conifer forest dominated by Norway spruce (Picea abies) and Scots pine (Pinus sylvestris). The tree line in the study area is approximately 950 masl in the southern part and about 600 masl in the northern part. The highest peaks are about 2100 masl and more than 200 glaciers can be found in the area. Potential prey species for the red fox in the study area included mountain hare, red squirrel (Sciurus vulgaris), black grouse, capercaillie, hazel grouse (Bonasa bonasia), willow grouse (Lagopus lagopus), rock ptarmigan (Lagopus muta), and small rodents, which consisted of bank vole (Myodes glareolus), northern red-backed vole (Myodes rutilus), field vole (Microtus agrestis), tundra vole (Microtus oeconomus), grey-sided vole (Myodes rufocanus), water vole (Arvicola amphibius), and Norwegian lemming (Lemmus lemmus). Large carnivores present in the area included brown bears (Ursus arctos), wolverines (Gulo gulo), Eurasian lynx (Lynx lynx), wolves (Canis lupus), and golden eagles (Aquila chrysaetos).
Monitoring the abundance and distribution of mammals and birds
The study area consisted of 2080 squared sampling blocks of 25 km2 that followed the Swedish map grid (RT90) on 5 × 5 km intersections. The distribution and abundance of red foxes and potential prey species were monitored by recording the number of signs per species observed per sampling block. In each block, all signs of mammals and birds were recorded in areas that were accessible with snow mobiles. These signs included tracks in the snow, observations of animals, nesting sites, scats and signs of foraging. Observers attempted to scan and drive the whole study block and would scan the area with binoculars from natural observation points (e.g. knolls or ridges). The length driven within an area was dependent on the degree of tree cover and boulders obstructing the line of sight (5.56 ± 0.10 km per block in average). All tracks were registered as independent tracks unless further investigation showed otherwise. For example, observers followed the tracks in cases when they were uncertain if it was from one or two animals to check if tracks joined up or if two animals had walked in the same track. Snow depth, snow condition, cloud cover, wind and temperature data were also measured in the field. Sampling blocks were monitored once per year from January to May plus a second visit to sampling blocks where bears, wolverines, lynx and wolves had been detected. The same two observers would travel through the same areas, mostly following the same route.
Wind and precipitation can make it difficult to read tracks on the snow. Hence, we only included data collected during periods when favourable conditions of wind, precipitation and snow allowed for the detection of rodent tracks. We used box plots to identify outlayers and removed four observations with values higher than 100 times the average. The resulting data set consisted of 9984 surveys of the 2080 different sampling blocks. Among these blocks, 1195 were visited three or more years (lack of snow and severe weather prevented full coverage in all years).
Environmental data
We calculated the proportion of boreal forest and alpine tundra for each sampling block by using the Intersect function in ArcGis 9.3 (ESRI 2009) where we used a 1:250,000 map from the National Land Survey of Sweden as input data. We calculated mean elevation for each block by using the Zonal Statistics function in ArcGIS 9.3 where we used elevation data from the National Land Survey of Sweden as input data (the resolution of input data was 50 m).
Statistical analysis
Distribution of red foxes
We used model selection to assess how the distribution (presence/absence per sampling block) of red foxes varied in relation to the presence of potential prey species. For this, we fitted logistic mixed effect models by using the function ‘glmer’ in the R package lme4 (Bates et al. 2014; R Development Core Team 2012). We derived a set of 17 candidate models constituted by models with different combinations of the main prey and alternative prey species as explanatory variables, models with an index of prey species richness (number of prey species present in the sampling block), and models with snow depth and altitude interacting in different combinations with the variables listed above. All candidate models included sampling block ID as a random effect for the intercept to control for repeated measures. We were interested in explaining the variations in the data rather than obtaining a strong predicting power and therefore we included models that contained up to 14 explanatory variables. We used variation around the grand mean as a null model (i.e. model with no explanatory variables) of no effect of neither of the variables examined. We used Akaike’s information criterion (AIC) to rank models and considered models within two AIC units to be of similar quality (Anderson et al. 1998).
Prey species categories were mountain hares, tetraonid species (i.e. black grouse, capercaillie, hazel grouse, willow ptarmigan and rock ptarmigan), small rodents and ungulates (i.e. moose and reindeer as a source of carcasses). The index of species richness was defined as the number of different potential prey species present in the sampling block (i.e. small rodent, mountain hare, black grouse, capercaillie, hazel grouse, willow- and rock ptarmigans, moose and reindeer). We standardized altitude and snow depth variables by using the following equation: \( \left({x}_i-\overline{X}\right)/\left(2\times \mathrm{S}\mathrm{D}\right) \) (Gelman and Hill 2007). Altitude was highly correlated with both the proportion of boreal forest (Pearson’s r = −0.75, p < 0.001) and the proportion of tundra (r = 0.84, p < 0.001). Thus, the variable altitude represented both altitude and habitat composition and we excluded proportion of forest and tundra variables from the models. We checked for collinearity of the explanatory variables, and we found no correlation between any of them.
Temporal trends
When examining for temporal trends in the red fox distribution and relative abundance (average number of signs per sampling block), we restricted the analysis to only include sampling blocks that had been monitored six or more years (n = 756 sampling blocks). These blocks were surveyed a total of 6142 times.
We examined temporal trends in the red fox distribution and abundance by using mixed effect models (R Development Core Team 2012). In order to assess the relationship between the linear temporal trends and altitude, we defined three altitude categories by dividing the total altitude range, from 299 to 1419 masl, into three zones of 373.3 m altitude range each, i.e. “low altitude” (<672.5 masl, n = 3693 surveys of 452 sampling blocks), “medium altitude” (672.5–1016.1 masl, n = 1832 surveys of 224 sampling blocks) and “high altitude” (>1016.1 masl, n = 617 surveys of 80 sampling blocks). The altitude categories contained different proportions of boreal forest and tundra (low altitude: 61.51 % boreal forest, 13.10 % tundra; medium altitude: 16.35 % boreal forest, 74.80 % tundra; high altitude: 1.18 % boreal forest, 95.5 % tundra). Both models included sampling block ID as a random effect for the intercept to control for repeated measures.
Results
Distribution of red foxes
Red foxes were present in the majority of the study area in all years. On average, red fox signs were found in 84.29 % (±0.36 S.E.) of the sampling blocks in each year. Hare signs were present in 63.60 % (±0.50 S.E.) of the sampling blocks, rodents in 17.53 % (±0.38 S.E.), tetraonid species in 81.72 % (±0.39 S.E.) and ungulates in 54.18 % (±0.49 S.E.). The model in which the red fox distribution was explained by the species richness index was 170.23 AIC units away from the top model. Instead, the presence of rodents, hares, tetraonids and ungulates was important to explain the distribution of red foxes as indicated by these variables being included in the best models that accounted for 62 % of the overall model fit (Table 1). When excluding the main prey (i.e. rodents), the model showed a delta AIC of 69.57. The best model presented a marginal R 2 of 0.40 (see Nakagawa and Schielzeth 2013). The presence of hares, tetraonid species and ungulates as alternative prey had highly positive effects on the distribution of red foxes (Table 2). These variables interacted with snow depth and altitude in different ways. The interaction with altitude was positive for hares. The confidence intervals of the interaction with altitude for large ungulates and for tetraonids included zero and the effect was therefore inconclusive. The best model also showed a negative interaction with snow depth for hares and a less pronounced positive interaction between snow depth and ungulates. The interaction between tetraonids and snow depth included zero in the confidence intervals, and hence, we were uncertain of its effect (Table 2).
Temporal trends
The presence and number of signs of red foxes per sampling block varied among years (Fig. 2). However, there was no positive or negative linear temporal trend in these variables during the study period (Table 3). Similarly, there was no positive or negative linear temporal trend in any of the different altitude categories neither for the distribution nor the relative abundance of red foxes in the study area (Table 3).
Discussion
The close association between the distributions of red foxes and prey species observed in our study is in accordance with previous findings (Angelstam et al. 1984; Lindström et al. 1987; Hersteinsson and Macdonald 1992). Halpin and Bissonette (1988) found that snow depth affected habitat use of red foxes by shifting the availability of the prey species. However, we show that the magnitude of the effects of different prey categories on the distribution of red fox also depends on altitude and snow depth. This demonstrates that several interacting factors must be taken into account in order to predict the distribution of red foxes, and not only prey abundance or presence.
Ungulates as a source of carcasses have been shown to be a significant part of the diet of red foxes during the winter period or during low density phases in the rodent cycle (Jedrzejewski and Jedrzejewska 1992; Cagnacci et al. 2003; Killengreen et al. 2011; Needham et al. 2014). In our study, we show that the presence of ungulates also affects the spatial distribution of the red fox. Henden et al. (2014) recently showed that the occupancy of the community of scavengers, including the red fox, was higher where the density of reindeer was larger. Selås and Vik (2006) also found a positive correlation between the increase of red fox hunting bags and the increase of ungulate numbers in Norway. During the 12 years of our study, the population of moose experienced a dramatic increase in northern Sweden (Cederlund and Bergstrom 1996). Similarly, the reindeer population in our study area increased markedly and peaked in 1990 (Moen and Danell 2003). Accordingly, the availability of carcasses for the red fox probably increased. The origin of these carcasses possibly was death by disease, inanition under harsh winter conditions, human hunting and predation (Saether et al. 1996; Lavsund et al. 2003; Tveraa et al. 2003). Our results show that the importance of carcasses for the distribution of red foxes interacted with altitude and snow depth. This importance increased as snow depth increased. It is probably easier for the red fox to feed on carcasses rather than on live prey in areas where deep snow makes moving, and therefore hunting, difficult (see Lindström 1983). Also, our study shows that the interaction of altitude with the availability of carcasses was important for the distribution of red foxes. However, the confidence interval included zero, and hence, we are uncertain of the impact of this effect. Given the habitat use of moose and reindeer, the effect of this interaction could represent a bias towards the use of carcasses of one of these species. Nonetheless, the second ranked model in the model selection process differed from the best model in that it did not include the interaction of altitude and snow depth with ungulates. This model was 2.04 AIC points away from the best model. This tells us that the effect of these interactions might not be very strong, but it is important to take them into account, especially in areas with contrasting numbers of different ungulate species. In conclusion, the access to moose and reindeer carcasses may have sustained the population of red foxes in northern Sweden during harsh winters and years of low density of rodents (Killengreen et al. 2011; Needham et al. 2014). Nowadays, the recovery of large carnivores in Scandinavia (Chapron et al. 2014) may increase the availability of ungulate carcasses for red foxes. We believe that a higher availability of carcasses for the red fox could stabilize the temporal dynamics of the red fox populations in Scandinavia.
Tetraonids species such as black grouse and ptarmigan represent an important alternative prey for red foxes (Lindström et al. 1987, 1994; Smedshaug et al. 1999). Indeed, the presence of these species had a pronounced effect on the red fox distribution in our study. However, the confidence intervals of the interactions of tetraonids with altitude and snow depth contained zero. Although the interactions per se were important for the distribution of red fox, the direction of the effects was inconclusive. This uncertainty may be attributed to the fact that we did not differentiate different tetraonid species and pooled species with different ecologies like capercallies and rock ptarmigans. Future studies differentiating bird prey into species may reveal more detailed patterns.
The presence of hares was also an important factor explaining the distribution of the red fox in northern Sweden. Many studies have previously shown the role of this species as an alternative prey of the red fox (Danell and Hornfeldt 1987; Jedrzejewski and Jedrzejewska 1992; Lindström et al. 1994; Kauhala and Helle 2000). However, here, we show that the importance of this species decreased with snow depth. On the other hand, the interaction between hares and altitude was positive. In contrast to tetraonids, hares may be more exposed in the open landscape and thus easier to detect and catch by the red fox at higher altitudes.
According some authors (e.g. Hersteinsson and Macdonald 1992; Fuglei and Ims 2008), warmer temperatures during recent years may have facilitated an expansion of the red fox populations to higher altitudes. Even though a warming trend has been reported in our study area during the period of our study (Jonsell et al. 2013), neither the distribution nor the relative abundance of the red fox showed an increase in northern Sweden. Instead, our data showed a stable population that, despite annual fluctuations, did not manifest any positive or negative temporal linear trend during the study period (i.e. 1974–1985). The red fox was present in an average of 70 % (±2.27 S.E.) of the sampling blocks in the highest altitudes (>1016.1 masl) which were areas consisting almost solely of open tundra and glaciers. Hence, it seems that the red fox was well-established in high altitudes in northern Sweden during the study period. According to Lönnberg (1926), the red fox was breeding in the open tundra already in the early twentieth century.
The sarcoptic mange had a pronounced negative impact on the red fox population in Scandinavia on a large scale (Mörner 1992; Lindström et al. 1994). However, our data show no decrease in the distribution and relative abundance of the red fox despite sarcoptic mange appearing in our study area around 1977–1978 (Lindström and Mörner 1985). We therefore suggest that the sarcoptic mange had a low impact on the red fox population in this part of northern Sweden due to the low population densities and harsh winter conditions.
In our study, we have depicted factors explaining the distribution of the red fox in northern Sweden and their interactions with snow depth and altitude. Moreover, we show that there is a relationship between the distributions of red foxes and ungulates. Management of the Scandinavian red fox populations in high altitudes should take into account the increasing availability of carcasses given the increase in the abundance of several ungulate species (e.g. moose and semi-domestic reindeer; Ims et al. 2007). Furthermore, the relationship between small game, altitude and snow depth has not previously been described. Our study thus demonstrates that several interacting factors may be important for predicting the distribution and dynamics of the red fox in mountain ecosystems.
References
Anderson DR, Burnham KP, White GC (1998) Comparison of Akaike information criterion and consistent Akaike information criterion for model selection and statistical inference from capture-recapture studies. J Appl Stat 25:263–282
Angelstam P, Lindström E, Widen P (1984) Role of predation in short-term population fluctuations of some birds and mammals in fennoscandia. Oecologia 62(2):199–208. doi:10.1007/bf00379014
Angerbjörn A et al (2013) Carnivore conservation in practice: replicated management actions on a large spatial scale. J Appl Ecol 50:59–67. doi:10.1111/1365-2664.12033
Barton KA, Zalewski A (2007) Winter severity limits red fox populations in Eurasia. Glob Ecol Biogeogr 16:281–289. doi:10.1111/j.1466-8238.2007.00299.x
Bates D, Maechler M, Bolker B, Walker S (2014) lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7, http://CRAN.R-project.org/package=lme4
Begon M, Sait SM, Thompson DJ (1996) Predator–prey cycles with period shifts between two- and three-species systems. Nature 381:311–315. doi:10.1038/381311a0
Cagnacci F, Lovari S, Meriggi A (2003) Carrion dependence and food habits of the red fox in an Alpine area. Ital J Zool 70:31–38
Cederlund G, Bergstrom R (1996) Trends in the moose-forest system in Fennoscandia, with special reference to Sweden. In: DeGraaf RM, Miller RI (eds) Conservation of faunal diversity in forested landscapes. Chapman & Hall, London, pp 265–281
Chapron G et al (2014) Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346(6216):1517–1519. doi:10.1126/science.1257553
Courchamp F, Langlais M, Sugihara G (1999) Cats protecting birds: modelling the mesopredator release effect. J Anim Ecol 68(2):282–292. doi:10.1046/j.1365-2656.1999.00285.x
Danell K, Hornfeldt B (1987) Numerical responses by populations of red fox and mountain hare during an outbreak of sarcoptic mange. Oecologia 73(4):533–536. doi:10.1007/bf00379412
Dell’Arte GL, Laaksonen T, Norrdahl K, Korpimäki E (2007) Variation in the diet composition of a generalist predator, the red fox, in relation to season and density of main prey. Acta Oecol-Int J Ecol 31:276–281. doi:10.1016/j.actao.2006.12.007
Elmhagen B, Rushton SP (2007) Trophic control of mesopredators in terrestrial ecosystems: top-down or bottom-up? Ecol Lett 10:197–206. doi:10.1111/j.1461-0248.2006.01010.x
Englund J (1970) Some aspects of reproduction and mortality rates in Swedish foxes (Vulpes vulpes) 1961–63 and 1966–69. Viltrevy 8:1–82
ESRI (2009) ARCGIS desktop. Environmental Systems Research Institute, Redland
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515
Fuglei E, Ims RA (2008) Global warming and effects on the Arctic fox. Sci Prog 91(Pt 2):175–191. doi:10.3184/003685008x327468
Gallant D, Slough BG, Reid DG, Berteaux D (2012) Arctic fox versus red fox in the warming Arctic: four decades of den surveys in north Yukon. Polar Biol 35:1421–1431. doi:10.1007/s00300-012-1181-8
Gelman A, Hill J (2007) Data analysis using regression and multilevel/hierarchical models. Analytical methods for social research. Cambridge University Press, Cambridge
Halpin MA, Bissonette JA (1988) Influence of snow depth on prey availability and habitat use by red fox. Can J Zool 66:587–592. doi:10.1139/z88-086
Hartova-Nentvichova M, Salek M, Cerveny J, Koubek P (2010) Variation in the diet of the red fox (Vulpes vulpes) in mountain habitats: effects of altitude and season. Mamm Biol 75:334–340. doi:10.1016/j.mambio.2009.09.003
Henden JA, Ims RA, Yoccoz NG (2009) Nonstationary spatio-temporal small rodent dynamics: evidence from long-term Norwegian fox bounty data. J Anim Ecol 78:636–645. doi:10.1111/j.1365-2656.2008.01510.x
Henden JA, Ims RA, Yoccoz NG, Hellström P, Angerbjörn A (2010) Strength of asymmetric competition between predators in food webs ruled by fluctuating prey: the case of foxes in tundra. Oikos 119:27–34. doi:10.1111/j.1600-0706.2009.17604.x
Henden JA, Stien A, Bårdsen BJ, Yoccoz NG, Ims RA (2014) Community-wide mesocarnivore response to partial ungulate migration. J Appl Ecol 51(6):1525–1533. doi:10.1111/1365-2664.12328
Hersteinsson P, Macdonald DW (1992) Interspecific competition and the geographical distribution of red and arctic foxes vulpes-vulpes and alopex-lagopus. Oikos 64:505–515. doi:10.2307/3545168
Hersteinsson P, Angerbjörn A, Frafjord K, Kaikusalo A (1989) The arctic fox in fennoscandia and iceland: management problems. Biol Conserv 49:67–81. doi:10.1016/0006-3207(89)90113-4
Ims RA, Yoccoz NG, Bråthen KA, Fauchald P, Tveraa T, Hausner V (2007) Can reindeer overabundance cause a trophic cascade? Ecosystems 10:607–622. doi:10.1007/s10021-007-9060-9
Jedrzejewski W, Jedrzejewska B (1992) Foraging and diet of the red fox vulpes vulpes in relation to variable food resources in bialowieza-national-park, poland. Ecography 15:212–220. doi:10.1111/j.1600-0587.1992.tb00027.x
Jonsell U, Hock R, Duguay M (2013) Recent air and ground temperature increases at Tarfala Research Station, Sweden. Polar Res 32. doi:10.3402/polar.v32i0.19807
Kauhala K, Helle P (2000) The interactions of predator and hare populations in Finland - a study based on wildlife monitoring counts. Ann Zool Fenn 37(3):151–160
Killengreen ST, Ims RA, Yoccoz NG, Brathen KA, Henden J-A, Schott T (2007) Structural characteristics of a low Arctic tundra ecosystem and the retreat of the Arctic fox. Biol Conserv 135:459–472. doi:10.1016/j.biocon.2006.10.039
Killengreen ST, Lecomte N, Ehrich D, Schott T, Yoccoz NG, Ims RA (2011) The importance of marine vs. human-induced subsidies in the maintenance of an expanding mesocarnivore in the arctic tundra. J Anim Ecol 80:1049–1060. doi:10.1111/j.1365-2656.2011.01840.x
Kjellander P, Nordström J (2003) Cyclic voles, prey switching in red fox, and roe deer dynamics—a test of the alternative prey hypothesis. Oikos 101:338–344. doi:10.1034/j.1600-0706.2003.11986.x
Krebs CJ, Boutin S, Boonstra R, Sinclair A, Smith J, Dale M, Martin K, Turkington R (1995) Impact of food and predation on the snowshoe hare cycle. Science 269:1112–1115. doi:10.1126/science.269.5227.1112
Kurki S, Nikula A, Helle P, Lindén H (1998) Abundances of red fox and pine marten in relation to the composition of boreal forest landscapes. J Anim Ecol 67:874–886. doi:10.1046/j.1365-2656.1998.6760874.x
Lariviere S, Pasitschniak-Arts M (1996) Vulpes vulpes. Mamm Species 537:1–11
Lavsund S, Nygren T, Solberg EJ (2003) Status of moose populations and challenges to moose management in Fennoscandia. Alces 39:109–130
Lindström E (1983) Condition and growth of red foxes (vulpes-vulpes) in relation to food-supply. J Zool 199:117–122
Lindström E (1989) Food limitation and social regulation in a red fox population. Holarct Ecol 12:70–79
Lindström E, Mörner T (1985) The spreading of sarcoptic mange among swedish red foxes (vulpes-vulpes l) in relation to fox population-dynamics. Rev Ecol Terre La Vie 40:211–216
Lindström E, Angelstam P, Widen P, Andren H (1987) Do predators synchronize vole and grouse fluctuations?: an experiment. Oikos 48(2):121–124
Lindström E, Andrén H, Angelstam P, Cederlund G, Hörnfeldt B, Jäderberg L, Lemnell PA, Martinsson B, Sköld K, Swenson JE (1994) Disease reveals the predator: sarcoptic mange, red fox predation, and prey populations. Ecology 75:1042–1049. doi:10.2307/1939428
Lönnberg E (1926) Fjällrävstammen i Sverige 1926. Kungl. Svenska Vetenskapsakademiens skrifter i naturskyddsärenden, 0374–8820; 7. Almqvist & Wiksell
Marcström V, Kenward RE, Engren E (1988) The impact of predation on boreal tetraonids during vole cycles: an experimental study. J Anim Ecol 57:859–872. doi:10.2307/5097
Michal B, Rafal Z (2014) Responses of small mammals to clear-cutting in temperate and boreal forests of Europe: a meta-analysis and review. Eur J For Res 133:1–11. doi:10.1007/s10342-013-0726-x
Moen J, Danell O (2003) Reindeer in the Swedish mountains: an assessment of grazing impacts. Ambio 32:397–402. doi:10.1639/0044-7447(2003)032[0397:ritsma]2.0.co;2
Mörner T (1992) Sarcoptic mange in Swedish wildlife. Rev Sci Tech 11:1115–1121
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. doi:10.1111/j.2041-210x.2012.00261.x
Needham R, Odden M, Lundstadsveen SK, Wegge P (2014) Seasonal diets of red foxes in a boreal forest with a dense population of moose: the importance of winter scavenging. Acta Theriol 59:391–398. doi:10.1007/s13364-014-0188-7
Osenberg CW, Mittelbach GG (1996) The relative importance of resource limitation and predator limitation in food chains. In: Polis A, Winemiller KO (eds) Food webs: integration of patterns and dynamics. Chapman and Hall, pp 134–148
Persson L, Bengtsson J, Menge BA, Power ME (1996) Productivity and consumer regulation—concepts, patterns, and mechanisms. In: Polis A and Winemiller KO (ed) Food webs: integration of patterns and dynamics. Chapman and Hall, pp 396–434
Post DM, Conners ME, Goldberg DS (2000) Prey preference by a top predator and the stability of linked food chains. Ecology 81:8–14. doi:10.1890/0012-9658(2000)081[0008:ppbatp]2.0.co;2
Post E et al (2009) Ecological dynamics across the arctic associated with recent climate change. Science 325:1355–1358. doi:10.1126/science.1173113
Prugh LR, Stoner CJ, Epps CW, Bean WT, Ripple WJ, Laliberte AS, Brashares JS (2009) The rise of the mesopredator. Bioscience 59:779–791. doi:10.1525/bio.2009.59.9.9
R Development Core Team (2012) R: a language and environment for statistical computing, 2151st edn. R Foundation for Statistical Computing, Vienna
Ritchie EG, Johnson CN (2009) Predator interactions, mesopredator release and biodiversity conservation. Ecol Lett 12:982–998. doi:10.1111/j.1461-0248.2009.01347.x
Rosalino LM, Sousa M, Pedroso NM, Basto M, Rosario J, Santos MJ, Loureiro F (2010) The influence of food resources on red fox local distribution in a mountain area of the western mediterranean. Vie Et Milieu Life Environ 60:39–45
Saether BE, Andersen R, Hjeljord O, Heim M (1996) Ecological correlates of regional variation in life history of the moose Alces alces. Ecology 77(5):1493–1500. doi:10.2307/2265546
Selås V, Vik JO (2006) Possible impact of snow depth and ungulate carcasses on red fox (Vulpes vulpes) populations in Norway, 1897–1976. J Zool 269:299–308. doi:10.1111/j.1469-7998.2006.00048.x
Selås V, Vik JO (2007) The arctic fox Alopex lagopus in Fennoscandia: a victim of human-induced changes in interspecific competition and predation? Biodivers Conserv 16:3575–3583. doi:10.1007/s10531-006-9118-6
Smedshaug CA, Selås V, Lund SE, Sonerud GA (1999) The effect of a natural reduction of red fox Vulpes vulpes on small game hunting bags in Norway. Wildl Biol 5:157–166
Statistics Sweden (SCB) (2015). http://www.scb.se/en_/Finding-statistics/Statistics-by-subject-area/Population/Population-composition/Population-statistics/. Accessed 7 Dec 2015
Tveraa T, Fauchald P, Henaug C, Yoccoz NG (2003) An examination of a compensatory relationship between food limitation and predation in semi-domestic reindeer. Oecologia 137(3):370–376. doi:10.1007/s00442-003-1373-6
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin JM, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395. doi:10.1038/416389a
Zimen E (1980) The red fox (Biogeographica; v.18). Springer, Netherlands. doi:10.1007/978-94-017-5592-4
Acknowledgments
We would like to thank Robert Franzén in the Swedish Environmental Protection Agency for providing access to the entire wildlife census data used in this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carricondo-Sanchez, D., Samelius, G., Odden, M. et al. Spatial and temporal variation in the distribution and abundance of red foxes in the tundra and taiga of northern Sweden. Eur J Wildl Res 62, 211–218 (2016). https://doi.org/10.1007/s10344-016-0995-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-016-0995-z