Abstract
During the last century, the red fox (Vulpes vulpes) has expanded its distribution into the Arctic, where it competes with the arctic fox (Vulpes lagopus), an ecologically similar tundra predator. The red fox expansion correlates with climate warming, and the ultimate determinant of the outcome of the competition between the two species is hypothesized to be climate. We conducted aerial and ground fox den surveys in the northern Yukon (Herschel Island and the coastal mainland) to investigate the relative abundance of red and arctic foxes over the last four decades. This region has undergone the most intense warming observed in North America, and we hypothesized that this climate change led to increasing dominance of red fox over arctic fox. Results of recent surveys fall within the range of previous ones, indicating little change in the relative abundance of the two species. North Yukon fox dens are mostly occupied by arctic fox, with active red fox dens occurring sympatrically. While vegetation changes have been reported, there is no indication that secondary productivity and food abundance for foxes have increased. Our study shows that in the western Arctic of North America, where climate warming was intense, the competitive balance between red and arctic foxes changed little in 40 years. Our results challenge the hypotheses linking climate to red fox expansion, and we discuss how climate warming’s negative effects on predators may be overriding positive effects of milder temperatures and longer growing seasons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the competitive exclusion principle, species with similar habitat requirements (complete competitors) cannot coexist (Hardin 1960). One compelling case of competition is that of the red fox (V. vulpes) and the arctic fox (V. lagopus) in the Arctic, where the red fox occupies habitats of the arctic fox, an ecologically similar mesopredator indigenous to the tundra. Both species have largely overlapping ecological niches (Hersteinsson and Macdonald 1982; Frafjord 2000; Elmhagen et al. 2002) suggesting strong competitive interactions (Hersteinsson et al. 1989). They both largely rely on microtine rodents for food (Smits et al. 1989; Frafjord 2000; Elmhagen et al. 2002) and use similar strategies to subsist in the harsh arctic environment, like food caching (Sklepkovych and Montevecchi 1996; Careau et al. 2008) and foraging on the sea ice for alternative foods such as seal carcasses in winter (Andriashek et al. 1985; Fay and Stephenson 1989).
Red foxes use preexisting arctic fox dens (Dalerum et al. 2002; Frafjord 2003; Rodnikova et al. 2011). They can weigh twice as much as arctic foxes and can be up to 70 % longer (Hersteinsson and Macdonald 1982; Larivière and Pasitschniak-Arts 1996; Audet et al. 2002). They tend to be larger at higher latitudes (Davis 1977; Cavallini 1995). The red fox is thus physically dominant and can kill the smaller arctic fox (Frafjord et al. 1989; Tannerfeldt et al. 2002; Pamperin et al. 2006). The difference found in their overlapping realized food niches (Eberhardt 1977; Smits et al. 1989; Frafjord 1995) potentially results from interference competition. While they seem to coexist in some parts of the Arctic (Eberhardt 1977; Smits and Slough 1993), spatial segregation was observed in Fennoscandia, suggesting that the red fox excludes its smaller competitor (Tannerfeldt et al. 2002; Dalén et al. 2004). The segregation is altitudinal (Tannerfeldt et al. 2002; Dalén et al. 2004), with arctic foxes being relegated to presumably less productive inland areas at higher altitude.
The arctic fox is highly adapted to arctic conditions (Prestrud 1991). It has better insulation (Scholander et al. 1950a) and a lower critical temperature than the red fox (Scholander et al. 1950a, b; Irving et al. 1955). It is better adapted to travel in snow, with a lower footload than the red fox (Murray and Larivière 2002). The red fox has a higher resting metabolic rate than the arctic fox (Klir and Heath 1992), and the arctic fox can decrease its basal metabolic rate in winter (Fuglesteg et al. 2006). Thus, the larger red fox requires more food and a larger territory to sustain itself compared to its smaller competitor. Coexistence between the two species thus likely depends on a harsh environment or low ecosystem productivity to prevent the dominant red fox, which has a higher energetic burden, from occupying all of the landscape and completely excluding the arctic fox, through interference competition for food and dens.
The Earth’s climate is changing (Hansen et al. 2006), especially at high latitudes (IPCC 2007). During the twentieth century, red fox expanded its distribution into arctic tundra in Eurasia (Skrobov 1960) and North America (Marsh 1938), reaching Ellesmere Island in the Canadian Arctic during the 1960s (Macpherson 1964). The ratio of red fox to arctic fox pelts harvested in the Northwest Territories (Canada) during the twentieth century was positively correlated with summer temperatures (Hersteinsson and Macdonald 1992), leading to the hypothesis that the northern limit of the red fox’s geographic range is determined directly by resource availability and thus ultimately by climate. A warming Arctic could thus benefit red fox by reducing thermal stress and increasing prey availability through a bottom-up effect starting with increased primary production (Mack et al. 2004; Walker et al. 2006; Schuur et al. 2007).
Repeated field surveys of fox dens were performed in the Yukon coastal plain over four decades (Ruttan 1974; Ruttan and Wooley 1974; Smits et al. 1988; Smits and Slough 1993), providing a rare opportunity for a detailed study of trends in a region occupied by both species for centuries (Nagy 1988). Climate warming has been most intense in the western part of arctic North America, with annual mean surface air temperature anomalies of 1.6–2.1 °C in 2001–2005 compared with 1951–1980 (Fig. 1b in Hansen et al. 2006). We hypothesized that this climate warming led to increasing dominance of red fox over arctic fox in north Yukon. We conducted fox den surveys in the Yukon Coastal Plain Ecoregion (Smith et al. 2004) from 2008 to 2010, to test the prediction that the number of active red fox dens increased and the number of active arctic fox dens decreased during the preceding four decades.
Materials and methods
Study area
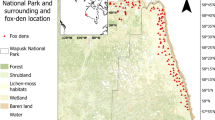
Our study area was on the Yukon Coastal Plain (Yukon, Canada) between the Yukon-Alaska border and a location (69°0′3.46′′N, 137°31′49.42′′W) between the Babbage and Blow rivers (Fig. 1), totaling approximately 2,550 km2 (Smits and Slough 1993) and mostly located within Ivvavik National Park. The southern inland limit was the point where terrain reached 100–150 m above sea level as in past surveys (Ruttan 1974; Ruttan and Wooley 1974; Smits et al. 1988; Smits et al. 1989; Smith et al. 1992; Smits and Slough 1993), and it included Herschel Island Territorial Park (110 km2, Fig. 1). The region has an arctic climate with annual mean temperatures of −10 to −12 °C and annual precipitation of 125–200 mm (Smith et al. 2004). Coldest and warmest months are February and July, with mean monthly low and high temperatures of −32 and −24 °C in February, and 8 and 10 °C in July, respectively (Smith et al. 2004). The region cooled from the mid-1940s to mid-1970s (Kittel et al. 2010) but has since undergone intense warming until the present day (Kittel et al. 2010, this study Fig. 2a–d), with a concurrent decline in the number of days with extreme cold in winter (Fig. 2e).
The study area (shaded) located in the Yukon Coastal Plain, modified from Smits et al. (1988). Circles represent dens used at least once by arctic foxes (V. lagopus), squares represent dens used at least once by red foxes (V. vulpes) and triangles represent dens used by both species over the surveyed years (1971–1972, 1984–1990, 2003, and 2008–2010). Small diamonds represent dens that were never occupied, while blackened shapes represent dens that have been used for reproduction at least once during the surveyed years. Years of red fox den usage are displayed
Meteorological data from Komakuk Beach Environment Canada weather station in the Yukon Coastal Plain, showing mean monthly air temperatures (1970–2010) for a spring (temperature = 0.080[year]−174.666, F 1,36 = 7.352, P = 0.010, R 2 = 0.170, n = 38), b summer (temperature = 0.019[year]−31.616, F 1,36 = 1.529, P = 0.224, R 2 = 0.041, n = 38), c autumn (temperature = 0.053[year]−113.812, F 1,36 = 3.486, P = 0.070, R 2 = 0.088, n = 38), d winter (temperature = 0.086[year]−195.855, F 1,38 = 7.525, P = 0.009, R 2 = 0.165, n = 40), and e percentages of days in February when average daily air temperature was −30 °C or less (1970–2006), indicating a statistically significant decline (percentage = 1668.003–0.821[year], F 1,32 = 6.303, P = 0.017, R 2 = 0.165, n = 34)
Soils in the area are mostly Cryosol (Smith et al. 2004). West of the Firth River, the plain was unglaciated during the Wisconsinan Glaciation (Mackay 1959; Dyke and Prest 1987) and has large alluvial deltas in sandy Regosol (Smith et al. 2004), while the East has undulating relief with hummocks created by ice movements (Rampton 1982). Thermokarst lakes, landslides, pingos, ice wedges, and polygons are common (Rampton 1982; Smith et al. 2004). Moraines are found east of Firth River (Rampton 1982). Herschel Island is hilly (mostly 60–185 m above sea level) and made of benthos sediments accumulated by the terminal moraine of a lobe of the continental ice sheet (Mackay 1959). Its soils are dominated by silt rich in ice and clay, with sand and gravel crests (Rampton 1982). Higher elevations of Herschel Island are mostly Cryosol Orthic Turbic (Smith et al. 1989). Elevated geological features such as moraines, pingos and crests attract denning foxes because of good drainage, protection from spring floods, higher irradiation by the sun which accelerates spring thaw, and good visibility for detecting predators.
The vegetation in the area is changing. For example, wideleaf polargrass (Arctagrostis latifolia) and arctic lupine (Lupinus arcticus) have increased in sloping highlands of Herschel Island in the last 20 years (Kennedy et al. 2001, Myers-Smith et al. 2011). Current photographs, compared to historical ones, through the twentieth century show increased willow (Salix spp.) cover on eastern Herschel Island (Myers-Smith et al. 2011), and on mainland fox dens south and southeast of the island (Ruttan and Wooley 1974; D. Gallant and B. G. Slough, unpublished data).
The porcupine caribou (Rangifer tarandus granti) herd migrates to the British Mountains and the Yukon Coastal Plain to breed (Fancy et al. 1994). Muskoxen (Ovibos moschatus) were reintroduced in Alaska in the late 1960s and have expanded through the northern Yukon (Smith et al. 2004). Small herds of caribou (200–250) and muskoxen (<45) resided year-round on Herschel Island during the study. There are no large seabird or goose breeding colonies. The main small mammal species are collared lemming (Dicrostonyx groenlandicus), brown lemming (Lemmus trimucronatus), and tundra vole (Microtus oeconomus) (Krebs et al. 2011). There are concentrations of arctic ground squirrel (Spermophilus parryii) colonies on the mainland, mostly south and southeast of Herschel Island (D. Gallant, unpublished data) but they are absent from the island.
Den surveys
Our study compares data from den surveys performed during 13 summers: 1971–1972 (Ruttan 1974; Ruttan and Wooley 1974), 1984–1990 (Smits and Slough 1993), 2003 (D. Cooley, unpublished data), and 2008–2010 (this study). We conducted an aerial den survey on the Yukon Coastal Plain between 1 and 6 July 2008. We used a Bell206B helicopter to conduct north–south parallel search transects at 500-m intervals, flying at a speed of 100 km/h at an altitude of 50–100 m. We used two observers, one on each side of the helicopter. Near den locations found during past surveys, we searched more intensively in order to relocate the dens. We landed at all observed den sites and documented signs of fox activity that could help determine den status (winter fur, scats, direct sightings, fox vocalizations, fresh diggings, prey remains). We considered dens with all burrows destroyed as “collapsed,” dens with functional burrows but without signs of recent fox activity as “inactive,” and dens with signs of activity dating from the current spring and summer as “active.” We identified reproductive dens by direct sighting of pups, characteristic juvenile barks, or small tracks. We determined species occupancy by identifying winter fur in burrows or by direct sightings. We surveyed Herschel Island in 2008 by foot, walking 500 m-spaced transects intermittently (2–21 June) over the whole island. We visited all island dens a second time in July. In 2009, we visited all Herschel Island dens monthly from 7 May to 4 August, and on the mainland, we visited as many known dens as possible by helicopter on 2 and 3 July. In 2010, volunteers visited some of the dens on Herschel Island while conducting other fieldwork.
We extracted historical data on fox den occupancy from the past den surveys. Den surveying in north Yukon was initiated by Ruttan and Wolley (1974) in 1971 and 1972. The August 1971 survey consisted of aerial searches using fixed-wing aircraft followed by helicopter access for intensive ground checks (Ruttan and Wooley 1974). From May to September 1972 (mostly in July), fixed-wing and helicopter surveys were coupled with extensive ground checks using dog teams, snowmobiles, or by foot, with multiple ground visits at most dens (Ruttan 1974). Another series of surveys was conducted from 1984 to 1990 (Smits and Slough 1993). In the 1980s, the methods and intensity of the surveys varied according to funding and logistical capabilities. In July 1984, a systematic aerial survey by helicopter used the same methods we used in 2008, with all detected dens being ground-checked (Smits and Slough 1993). In July of 1985, 1986, 1988, 1989, and 1990, opportunistic ground checks were undertaken at known dens during the course of other fieldwork (Smits and Slough 1993). Ground checks were limited to Herschel Island in 1986 and 1990. In July 1987, a helicopter survey was undertaken following a stratified random block design in search of new dens, along with ground checks at all known dens (Smits and Slough 1993). In July 2003, a multispecies helicopter survey was done on Herschel Island along its drainage basins at a speed of approximately 110 km/h and altitudes of 91–153 m, with sighted dens and foxes being documented without ground checks (D. Cooley, unpublished data).
During the 1984–1990 surveys, den locations were annotated on 1:250,000 scale maps for the mainland and a 1:50,000 scale map for Herschel Island (Smits and Slough 1993). We derived GPS coordinates from these maps and used both the coordinates and the maps during the 2008 survey to relocate previously known dens. We inspected maps and descriptions of den locations from Ruttan (1974) and Ruttan and Wooley (1974) to identify dens unique to the 1971–1972 surveys and those in common with other surveys. Most past surveys consist of single visits mostly in July, and it was thus not possible to distinguish between natal dens (where pups are born) and rearing dens (where broods can be relocated); our “reproductive den” category therefore included both natal and rearing dens. Relocation of broods are likely infrequent because we failed to observe any relocations during intensive fieldwork on Herschel Island in 2008 and 2009 (D. Gallant, unpubl. data).
Statistical analyses
Data from the 13 surveys constitute partially replicated samples with dens being surveyed an unequal number of years. The data thus constitute incomplete samples or partially paired data. We used all data, since restricting statistical testing to dens common to compared years produces less powerful statistical results (Tang and Tang 2004). We worked in two steps.
Firstly, most statistical procedures integrating incompletely paired datasets rely on the assumption that the so-called missing data are independent of treatment and outcome (Choi and Stablein 1982; Ekbohm 1982; Tang and Tang 2004). Our dataset violates this assumption; dens known to have been used by foxes in the past tended to be the focus of ground visits during years when it was impossible to visit all dens. Another common assumption of statistical testing integrating incompletely paired datasets is that both subgroups (paired and unpaired) in the dataset have the same proportion (Ekbohm 1982; Thomson 1995). Our dataset also violates this assumption, because the paired subgroup (dens often inspected) includes more active dens than the unpaired subgroup (dens less often inspected). We thus tested whether the proportion of surveyed dens used by red foxes and arctic foxes changed over time, using a procedure by Bland and Butland (unpublished, see description in “Appendix”), which incorporates both paired and unpaired survey data and does not rely on the two assumptions described above.
Secondly, we analyzed a specific subset of frequently surveyed dens to determine how species occupation of individual fox dens changed through time. We analyzed only dens visited on at least 4 years over a minimum span of 18 years. Based on available survey information, we classified each of these dens in one of the following categories: changed from red to arctic fox, changed from arctic to red fox, used by arctic fox once, used by red fox once, used by arctic fox several times, used by red fox several times, inactive, and used by unknown species. We made comparisons among combinations of these categories using the Scott and Seber (1983) test (p̂) for comparing two proportions within the same survey. The method applies the multivariate central-limit theorem to the multinomial distribution to approximate 95 % confidence intervals for the difference between two proportions (Scott and Seber 1983). Comparing dens changing from arctic fox to red fox to dens changing from red fox to arctic fox was particularly valuable to answer our overall objective, and we used a nonparametric sign test (P) (Zar 1999) to do so. We assumed sampling without replacement for both the Scott and Seber (1983) and Zar (1999) tests, thus relying on the binomial distribution as an approximation of the true distribution because we assumed that the number of dens included in a given test was a small subset of dens present in the northern Yukon.
Results
We visited 164 different den sites in 2008–2010. We were successful in finding 74 of the 136 dens known from past surveys of 1971–1972 and 1984–1990 while 90 new sites were found (Table 1). Limited time in 2009 precluded revisiting all dens visited in 2008 on the mainland (51 of 102 locations visited), so we prioritized dens based on past occupation by foxes because our main goal was documenting dens most likely to be used by foxes for making interspecific comparisons. We did not consider mainland dens as collapsed when not found, due to time constraints during the aerial survey. On the mainland, 26 of the 55 new sites visited were active or inactive ground squirrel colonies that had signs of past fox occupation, with some of the burrows apparently large enough for foxes. Such locations probably existed during past aerial surveys but may have been identified from a distance as ground squirrel colonies and thus skipped. On Herschel Island, our extensive ground searches led us to consider all unfound historical dens as collapsed by 2008. Den density is high on the island and both fox species reproduced over the last four decades (Fig. 1). On the island, where all or most dens important to reproduction are known, the shortest distances between natal red and arctic fox dens were 8.64, 4.81, 3.97, and 8.41 km in 1984, 1985, 1988, and 2009, respectively. In 2008 and 2009, red fox presence was only detected on Herschel Island and its vicinity (Fig. 1), suggesting a decrease in the spatial extent of red fox den usage.
Occupation and reproduction of the two fox species for monitored dens (Fig. 3) indicate that the relative abundance of red and arctic foxes changed little over four decades, with recent survey results falling within the range of past ones. There was a higher proportion of dens with signs of arctic fox usage in 1984–1990 compared to 2003–2010, and this difference was statistically significant with the range of the 95 % confidence interval falling outside the zero value (d 1984–1990 vs 2003–2010 = 0.178 ± 0.110, n = 171). However, there was no difference for the subset of paired data (i.e., dens visited in each of the two time periods), with 29 out of 60 dens (not the exact same ones) active in both periods. The subset of unpaired data was thus the sole contributor to the difference, with a proportion of 0.500 (n = 30) used by arctic fox for the period of 1984–1990 compared with a proportion of 0.086 (n = 81) for 2003–2010. The large number of previously unknown sites found on the mainland in 2008 is the cause of the lower proportion obtained for that period. Many of these newly found sites seemed to have been inactive for a long time and were occupied by ground squirrels. There was a slightly lower proportion of dens with signs of red fox usage in 1984–1990 compared with 2003–2010 but the difference was not statistically significant (d 1984–1990 vs 2003–2010 = –0.016 ± 0.037, n = 171).
Status of fox dens visited during ten surveys in north Yukon from 1971 to 2010. Numbers above bars indicate sample sizes. Survey years 1988 (85 dens), 1989 (19 dens), and 1990 (13 dens) are not shown because little information was collected on fox activity: numbers of reproductive dens were 7, 1, and 6 for arctic fox and 2, 1, and 0 for red fox in 1988, 1989, and 1990, respectively
The dynamics of den occupation by foxes also showed stability with regard to the presence of both species in the north Yukon (Fig. 4). A total of 62.3 % of dens for which we have ≥4 years of data spanning ≥18 years (n = 61) have been occupied by arctic foxes at least once without any detection of red fox usage (Fig. 4). This value is statistically different from that of all other categories combined (p arctic fox – p other = 0.492 ± 0.168, n = 61). The percentage of dens that changed from arctic fox to red fox occupation (8.2 %, 5 dens) was similar to that of dens that changed from red fox to arctic fox occupation (9.8 %, 6 dens) (P [x ≤ 5 or x ≥ 6] ≥ 0.999, n = 11). All dens used by red foxes in this subset were also used at least once by arctic foxes (Fig. 4).
Dynamics of den use by arctic fox and red fox in northern Yukon for 61 dens surveyed in ≥4 summers spread over a period of ≥18 years. Reproductive and nonreproductive dens are included in the analysis. Categories are mutually exclusive and include change from red fox to arctic fox use (RA), change from arctic fox to red fox use (AR), used by arctic fox once (A), used by red fox once (R), sporadically used by arctic fox (AA), sporadically used by red fox (RR), inactive (I), and used by unknown species (UU)
Discussion
We reject our hypothesis that climate warming has led to increasing dominance of red fox over arctic fox in tundra habitats of the north Yukon during the last four decades. Yet there has been a significant warming (Hansen et al. 2006; Wendler et al. 2010; Fig. 2 this study) and an increase in primary productivity (Kennedy et al. 2001; Sturm et al. 2001; Schuur et al. 2007) in this geographic area. Hersteinsson and Macdonald (1992) suggested that climate-driven primary productivity controls food abundance which in turn limits red fox distribution and thus abundance. Bartoń and Zalewski (2007) rather suggested that climate directly limits red foxes through winter thermal stress that increases energetic (i.e., food) requirements and through duration of snow cover that impedes access to prey (Fig. 5).
Our results spanning four decades of climate warming do not support Hersteinsson and Macdonald’s (1992) proposition that climate warming triggers a bottom-up chain of increased productivity leading to increased abundance of the larger red fox. Also, we observed unchanged red fox abundance as winters became warmer (Fig. 2 d and 2e), so neither the processes proposed by Hersteinsson and Macdonald (1992), nor those by Bartoń and Zalewski (2007), have resulted in expansion of red fox in north Yukon. We formulate the hypothesis that climate warming in the Arctic also had several negative effects on red foxes, and that these negative effects overrode the positive effects potentially arising from longer growing seasons and milder winters. We now explore this hypothesis in details and explore its consequences for our understanding of the anticipated effects of climate change on the tundra ecosystem.
In Norway, Killengreen et al. (2007) proposed that the dampening of small rodent cycles under climate warming was the cause of declines in arctic fox, while in Sweden Elmhagen and Rushton (2007) showed that ecosystem productivity was a limiting factor on increases in red fox abundance. Our survey data showed that where red foxes lived and reproduced on the Yukon Coastal Plain, they have not been able to do so consistently over time (Figs. 1 and 3) and arctic foxes could occupy locations vacated by red foxes (Fig. 4). This suggests that food availability is still a limiting factor in northern Yukon that is having more impact on the larger red fox and is maintaining the competitive balance between the two species.
Arctic foxes rely heavily on microtine rodents, especially lemmings, regardless of the availability of other food items (Angerbjörn et al. 1999; Frafjord 2000; Roth 2002). Their reproduction and population size fluctuate according to microtine abundance (Spaans et al. 1998; Tannerfeldt and Angerbjörn 1998; Angerbjörn et al. 1999). Microtines are also important to red foxes in the Arctic (Reid et al. 1997; Frafjord 2000; Elmhagen et al. 2002). In several parts of the North American Arctic, lemmings undergo cyclic population fluctuations with abundance peaks every three to 4 years (Spaans et al. 1998; Wilson et al. 1999; Gruyer et al. 2008). Brown and collared lemmings on the Yukon Coastal Plain appear not to be cyclic and persist at a relatively low density (1–4/ha) (Krebs et al. 2002, 2011) but undergo fairly low amplitude abundance fluctuations on Herschel Island (1–60/ha) (Krebs et al. 2011). The island has a high density of fox dens and had 2 years of high densities of reproductive dens in the 1980s (Smits and Slough 1993). Currently, both fox species are found on the island and red foxes possibly had an important presence there. Nolan et al. (1973) stated that it was dominated by red fox but did not provide data. The island’s den entrances are larger (Smits et al. 1988), suggesting more activity of the larger red fox compared with the mainland. Recent den surveys show concentration of red fox activity on Herschel Island and its vicinity (Fig. 1), indicating that it might provide more food.
Long-term data on small rodent abundance is lacking for the region, and the net effect of ongoing climate change on microtines requires investigation. Negative effects may be considerable and include reduced duration of snow cover affecting thermal and predation cover, and increased frequency of winter thaw events limiting access to food through ice cover. Even with potential positive effects of warming on microtines, food limitation for foxes may remain periodically significant. For example, any demographic response by microtines due to increased summer primary production would probably be consumed by the diverse predator assemblage (many raptors, least weasels and foxes) before the subsequent winter in this primarily top-down food web (Krebs et al. 2003).
Despite the periodic high densities of microtines on Herschel Island, red foxes have not excluded arctic foxes from the island, nor have they excluded arctic foxes from the coastal plain. On the mainland, the reason may be the limited distribution and abundance of alternative prey, such as arctic ground squirrels, which are much larger than microtines and can sustain red foxes during summers with low microtine density (Reid et al. 1997). Ground squirrels have not successfully colonized Herschel Island and are absent over large areas on the coastal plain, probably because of poor burrowing opportunities. Red fox dens on the mainland in 2008–2009 coincided with areas where we observed active ground squirrel colonies and landscapes with good burrowing substrates (e.g., alluvial deposits, eroded river banks), such as the alluvial fan at the mouth of the Firth River (Fig. 1). The current red fox distribution appears to be associated with two areas of higher food abundance: one linked to ground squirrels and the other to a fluctuating lemming population. When compared with the wider arctic fox distribution, this further indicates that food is a limiting factor that has a bigger impact on the larger red fox and is favouring coexistence between these competitors in northern Yukon.
Seal carcasses left by hunting Polar bears (Ursus maritimus) are an important seasonal food source for foxes (Roth 2002, 2003) and there are signs that seal accessibility is decreasing due to changing ice conditions. Duration of the ice-free season in the southern Beaufort Sea has increased during the current warming period (Wendler et al. 2010). Patterns of seasonal ice flow in the eastern Beaufort Sea have changed drastically (Macdonald et al. 1999; Stirling 2002), and the perennial ice is now considerably degraded (Barber et al. 2009). The polar bear population in the region is under stress (Regehr et al. 2006), suggesting fewer seal kills and thus fewer carcasses.
Anthropogenic food subsidies, which are capitalized upon by both fox species (Eberhardt 1977; Hersteinsson and Macdonald 1982; Eberhardt et al. 1983; Jędrzejewski and Jędrzejewska 1992), have diminished in north Yukon. The three Distant Early Warning stations (DEW line) in the Yukon were decommissioned and scaled down to automated North Warning System sites from 1963 to 1993. Inuvialuit activity has diminished, as family camps are now rare along the coast (L. J. Meyook, pers. commun.) and a permanent settlement on Herschel Island was dismantled in the early 1970s (Alunik et al. 2003). The north Yukon could be considered as a control site, because it has red fox presence but few locations constituting anthropogenic sources of food.
Continued food scarcity appears to be the most likely factor explaining the ongoing coexistence between red and arctic foxes for the last four decades despite the climate warming in the north Yukon. The situation in northern Yukon shows that climate change to date has not overcome the food limitation put on foxes and might have had a negative impact on food abundance for predators. We hypothesize that where red foxes have expanded in the arctic, this limitation has been lifted due to allochthonous food subsidies. For example, recent increase in red fox presence in Alaska was observed in Prudhoe Bay, a locality with considerable human activity (Sanzone et al. 2010). Red fox expansion in Scandinavia could have benefitted from an increase in ungulate abundance due to wolf (Canis lupus) extirpation (Selås and Vik 2006, but see Elmhagen and Rushton 2007). In northern Norway, Killengreen et al. (2011) determined that semi-domesticated reindeer (Rangifer tarandus L.) constituted the main food source helping to sustain red foxes during the critical winter season, especially during the low phase of the small rodent abundance cycle. The arctic fox could still face competitive exclusion by red foxes in parts of north Yukon if food abundance increases in the future. Models by Henden et al. (2010) showed the biggest negative impact on arctic foxes in situations when an area receives food subsidies and the dominant red fox monopolizes the resource. Oil and gas exploration is increasing in the region and bring risks of anthropogenic food supplementation.
Further investigation about the historical presence of red foxes in the Yukon Coastal Plain ecoregion is also needed because this species may have been present in this ecosystem for half a millennium (Nagy 1988). Currently, it is reasonable to consider red foxes as an integral part of the north Yukon tundra ecosystem, rather than as an invasive species whose presence has been enabled by climate change.
References
Alunik I, Kolausok ED, Morrison D (2003) Across time and tundra: the Inuvialuit of the Western Arctic. University of Washington Press, Seattle
Andriashek D, Kiliaan HPL, Taylor MK (1985) Observations on foxes, Alopex lagopus and V. vulpes, and wolves, C. lupus on the off-shore sea ice of northern Labrador. Can Field Nat 99:86–89
Angerbjörn A, Tannerfeldt M, Erlinge S (1999) Predator-prey relationships: arctic foxes and lemmings. J Anim Ecol 68:34–49
Audet AM, Robbins CB, Larivière S (2002) Alopex lagopus. Mamm Species No 713
Barber DG, Galley R, Asplin MG, De Abreu R, Warner K-A, Pućko M, Gupta M, Prinsenberg S, Julien S (2009) Perennial pack ice in the southern Beaufort Sea was not as it appeared in the summer of 2009. Geophys Res Lett 36:L24501
Bartoń KA, Zalewski A (2007) Winter severity limits red fox populations in Eurasia. Global Ecol Biogeogr 16:281–289
Careau V, Giroux J-F, Gauthier G, Berteaux D (2008) Surviving on cached food–the energetics of egg-caching by arctic foxes. Can J Zool 86:1217–1223
Cavallini P (1995) Variation in the body size of the red fox. Ann Zool Fenn 32:421–427
Choi SC, Stablein DM (1982) Practical tests for comparing two proportions in incomplete data. Appl Stat 31:256–262
Dalén L, Elmhagen B, Angerbjörn A (2004) DNA analysis on fox faeces and competition induced niche shifts. Mol Ecol 13:2389–2392
Dalerum F, Tannerfeldt M, Elmhagen B, Becker D, Angerbjörn A (2002) Distribution, morphology and use of arctic fox A. lagopus dens in Sweden. Wildl Biol 8:185–192
Davis S (1977) Size variation of the fox, V. vulpes, in the palaearctic region today, and in Israel during the late Quaternary. J Zool 182:343–351
Dyke AS, Prest VK (1987) Late Wisconsin and Holocene history of the Laurentide ice sheet (map 1703A, sheet 1). Géogr Phys Quatern 41:237–263
Eberhardt WL (1977) The biology of arctic and red foxes on the north slope. Dissertation, University of Alaska
Eberhardt LE, Garrott RA, Hanson WC (1983) Den use by arctic foxes in northern Alaska. J Mammal 64:97–102
Ekbohm G (1982) On testing the equality of proportions in the paired case with incomplete data. Psychometrika 47:115–118
Elmhagen B, Rushton SP (2007) Trophic control of mesopredators in terrestrial ecosystems: top-down or bottom-up? Ecol Lett 10:197–206
Elmhagen B, Tannerfeldt M, Angerbjörn A (2002) Food-niche overlap between arctic and red foxes. Can J Zool 80:1274–1285
Fancy SG, Whitten KR, Russell DE (1994) Demography of the Porcupine caribou herd, 1983–1992. Can J Zool 72:840–846
Fay FH, Stephenson RO (1989) Annual, seasonal, and habitat-related feeding habits of the arctic fox (A. lagopus) on St. Lawrence Island, Bering Sea. Can J Zool 67:1986–1994
Frafjord K (1995) Summer food habits of arctic foxes in the alpine region of southern Scandinavia, with a note on sympatric red foxes. Ann Zool Fenn 32:111–116
Frafjord K (2000) Do arctic and red foxes compete for food? Z Für Säugetierkd 65:350–359
Frafjord K (2003) Ecology and use of arctic fox A. lagopus dens in Norway: tradition overtaken by interspecific competition? Biol Conserv 111:445–453
Frafjord K, Becker D, Angerbjörn A (1989) Interactions between arctic and red foxes in Scandinavia–predation and aggression. Arctic 42:354–356
Fuglesteg BN, Haga ØE, Folkow LP, Fuglei E, Blix AS (2006) Seasonal variations in basal metabolic rate, lower critical temperature and responses to temporary starvation in the arctic fox (A. lagopus) from Svalbard. Polar Biol 29:308–319
Gruyer N, Gauthier G, Berteaux D (2008) Cyclic dynamics of sympatric lemming populations on Bylot Island, Nunavut, Canada. Can J Zool 86:910–917
Hansen J, Sato M, Ruedy R, Lo K, Lea DW, Medina-Elizade M (2006) Global temperature change. P Natl Acad Sci USA 103:14288–14293
Hardin G (1960) The competitive exclusion principle. Science 131:1292–1297
Henden J-A, Ims RA, Yoccoz NG, Hellström P, Angerbjörn A (2010) Strength of asymetric competition between predators in food webs ruled by fluctuating prey: the case of foxes in tundra. Oikos 119:27–34
Hersteinsson P, Macdonald DW (1982) Some comparisons between red and arctic foxes, V. vulpes and A. lagopus, as revealed by radio tracking. Symp Zool Soc Lond 49:259–289
Hersteinsson P, Macdonald DW (1992) Interspecific competition and the geographical distribution of red and arctic foxes V. vulpes and A. lagopus. Oikos 64:505–515
Hersteinsson P, Angerbjörn A, Frafjord K, Kaikusalo A (1989) The arctic fox in Fennoscandia and Iceland: management problems. Biol Conserv 49:67–81
IPCC (2007) Climate change 2007: synthesis report. In: Pachauri RK, Reisinger A (eds) Contribution of working groups I. II and III to the fourth assessment report of the intergovernmental panel on climate change IPCC, Geneva
Irving L, Krog H, Monson M (1955) The metabolism of some Alaskan animals in winter and summer. Physiol Zoöl 28:173–185
Jędrzejewski W, Jędrzejewska B (1992) Foraging and diet of the red fox V. vulpes in relation to variable food resources in Białowieża National Park, Poland. Ecography 15:212–220
Kennedy CE, Smith CAS, Cooley A (2001) Observations of change in the cover of polargrass, A. latifolia, and arctic lupine, L. arcticus, in upland tundra on Herschel Island, Yukon Territory. Can Field Nat 115:323–328
Killengreen ST, Ims RA, Yoccoz NG, Bråthen KA, Henden J-A, Schott T (2007) Structural characteristics of a low Arctic tundra ecosystem and the retreat of the Arctic fox. Biol Conserv 135:475–488
Killengreen ST, Lecomte N, Ehrich D, Schott T, Yoccoz NG, Ims RA (2011) The importance of marine vs. human-induced subsidies in the maintenance of an expanding mesocarnivore in the arctic tundra. J Anim Ecol 80:1049–1060
Kittel TGF, Baker BB, Higgins JV, Haney JC (2010) Climate vulnerability of ecosystems and landscapes on Alaska’s North Slope. Reg Environ Change 11:249–264
Klir JJ, Heath JE (1992) Metabolic rate and evaporative water loss at different ambient temperatures in two species of fox: the red fox (V. vulpes) and the Arctic fox (A. lagopus). Comp Biochem Phys A 101:705–707
Krebs CJ, Kenney AJ, Gilbert S et al (2002) Synchrony in lemming and vole populations in the Canadian Arctic. Can J Zool 80:1323–1333
Krebs CJ, Danell K, Angerbjörn A et al (2003) Terrestrial trophic dynamics in the Canadian Arctic. Can J Zool 81:827–843
Krebs CJ, Reid D, Kenney AJ, Gilbert S (2011) Fluctuations in lemming populations in north Yukon, Canada, 2007–2010. Can J Zool 89:297–306
Larivière S, Pasitschniak-Arts M (1996) V. vulpes. Mammal Species No 537
Macdonald RW, Carmack EC, McLaughlin FA, Falkner KK, Swift JH (1999) Connections among ice, runoff and atmospheric forcing in the Beaufort Gyre. Geophys Res Lett 26:2223–2226
Mack MC, Schuur EAG, Bret-Harte MS, Shaver GR, Chapin FS III (2004) Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature 431:440–443
Mackay JR (1959) Glacier ice-thrust features of the Yukon coast. Geogr Bull 13:5–21
Macpherson AH (1964) A northward range extension of the red fox in the eastern Canadian Arctic. J Mammal 45:138–140
Marsh DB (1938) The influx of the red fox and its colour phases into the barren lands. Can Field Nat 52:60–61
Murray DL, Larivière S (2002) The relationship between foot size of wild canids and regional snow conditions: evidence for selection against a high footload? J Zool 256:289–299
Myers-Smith IH, Hik DS, Kennedy C, Cooley D, Johnstone JF, Kenney AJ, Krebs CJ (2011) Expansion of canopy-forming willows over the twentieth century on Herschel Island, Yukon Territory, Canada. Ambio 40:610–623
Nagy MI (1988) Caribou exploitation at the Trail River site (northern Yukon). Dissertation, Simon Fraser University
Nolan JW, Goski BC, Wilde GW (1973) Atlas of wildlife habitat inventory maps for Environmental-Social Program, Northern Pipelines. Part of a wildlife habitat inventory of the Mackenzie Valley and the Northern Yukon. Canadian Wildlife Service, Ottawa
Pamperin NJ, Follmann EH, Petersen B (2006) Interspecific killing of an arctic fox by a red fox at Prudhoe Bay, Alaska. Arctic 59:361–364
Prestrud P (1991) Adaptations by the Arctic fox (A. lagopus) to the polar winter. Arctic 44:132–138
Rampton VN (1982) Quaternary geology of the Yukon Coastal Plain. Geol Surv Can Bull 317:1–49
Regehr EV, Amstrup SC, Stirling I (2006) Polar bear population status in the southern Beaufort Sea. US Geological Survey Open-File Report, Reston, No 2006–1337
Reid DG, Krebs CJ, Kenney AJ (1997) Patterns of predation on non-cyclic lemmings. Ecol Monogr 67:89–108
Rodnikova A, Ims RA, Sokolov A, Skogstad G, Sokolov V, Shtro V, Fuglei E (2011) Red fox takeover of arctic fox breeding den: an observation from Yamal Peninsula, Russia. Polar Biol 34:1609–1614
Roth JD (2002) Temporal variability in arctic fox diets as reflected in stable-carbon isotopes; the importance of sea ice. Oecologia 133:70–77
Roth JD (2003) Variability in marine resources affects arctic fox population dynamics. J Anim Ecol 72:668–676
Ruttan RA (1974) Arctic fox on the north slope of the Yukon Territory, 1972. In: Ruttant RA, Wooley DR (eds) Studies on furbearers associated with proposed pipeline routes in the Yukon and Northwest Territories. Biological Report Series, Canadian Arctic Gas Study Limited and Alaskan Arctic Gas Study Company Vol 9, pp 1–52
Ruttan RA, Wooley DR (1974) A study of furbearers associated with proposed pipeline routes in the Yukon Territory and Mackenzie River Valley, 1971. Biological Report Series, Canadian Arctic Gas Study Limited and Alaskan Arctic Gas Study Company, vol 8
Sanzone D, Streever B, Burgess B, Lukin J (eds) (2010) Long-term ecological monitoring in BP’s north slope oil fields: 2009 annual report. British Petroleum Exploration (Alaska) Inc, Anchorage
Scholander PF, Hock R, Walters V, Johnson F, Irving L (1950a) Heat regulation in some arctic and tropical mammals and birds. Biol Bull 99:237–258
Scholander PF, Walters V, Hock R, Irving L (1950b) Body insulation of some arctic and tropical mammals and birds. Biol Bull 99:225–236
Schuur EAG, Crummer KG, Vogel JG, Mack MC (2007) Plant species composition and productivity following permafrost thaw and thermokarst in Alaskan tundra. Ecosystems 10:280–292
Scott AJ, Seber GAF (1983) Difference of proportions from the same survey. Am Stat 37:319–320
Selås V, Vik JO (2006) Possible impact of snow depth and ungulate carcasses on red fox (V. vulpes) populations in Norway, 1897–1976. J Zool 269:299–308
Sklepkovych BO, Montevecchi WA (1996) Food availability and food hoarding behavior by red and arctic foxes. Arctic 49:228–234
Skrobov VD (1960) On the interrelations of the polar fox and the fox in the tundra of the Nenets national region. Zoologicheskii Zhurnal 39:469–471
Smith CAS, Kennedy CE, Hargrave AE, McKenna KM (1989) Soil and vegetation survey of Herschel Island, Yukon Territory. Yukon Soil Survey Report No 1, Agriculture Canada, Whitehorse
Smith CAS, Smits CMM, Slough BG (1992) Landform selection and soil modifications associated with arctic fox (A. lagopus) den sites in Yukon territory, Canada. Arctic Alpine Res 24:324–328
Smith CAS, Meikle JC, Roots CF (eds) (2004) Ecoregions of the Yukon Territory: biophysical properties of Yukon landscapes. PARC Technical Bulletin, Agriculture and Agri-Food Canada Bull 04–01, Summerland
Smits CMM, Slough BG (1993) Abundance and summer occupancy of arctic fox, A. lagopus, and red fox, V. vulpes, dens in the northern Yukon Territory, 1984–1990. Can Field Nat 107:13–18
Smits CMM, Smith CAS, Slough BG (1988) Physical characteristics of arctic fox (A. lagopus) dens in northern Yukon Territory, Canada. Arctic 41:12–16
Smits CMM, Slough BG, Yasui CA (1989) Summer food habits of sympatric arctic foxes, A. lagopus, and red foxes, V. vulpes, in the northern Yukon Territory. Can Field Nat 103:363–367
Spaans B, Blijleven HJ, Popov IU, Rykhlikova ME, Ebbinge BS (1998) Dark-bellied brent geese Branta bernicla bernicla forgo breeding when arctic foxes A. lagopus are present during nest initiation. Ardea 86:11–20
Stirling I (2002) Polar bear and seal in the eastern Beaufort Sea and Amundsen Gulf: a synthesis of population trends and ecological relationships over three decades. Arctic 55:59–76
Sturm M, Racine C, Tape K (2001) Increasing shrub abundance in the Arctic. Nature 411:546–547
Tang M-L, Tang N-S (2004) Exact test for comparing two paired proportions with incomplete data. Biometrical J 46:72–82
Tannerfeldt M, Angerbjörn A (1998) Fluctuating resources and the evolution of litter size in the arctic fox. Oikos 83:545–559
Tannerfeldt M, Elmhagen B, Angerbjörn A (2002) Exclusion by interference competition? The relationship between red and arctic foxes. Oecologia 132:213–220
Thomson PC (1995) A hybrid paired and unpaired analysis for the comparison of proportions. Stat Med 14:1463–1470
Walker MD, Wahren CH, Hollister RD et al (2006) Plant community responses to experimental warming across the tundra biome. P Natl Acad Sci USA 103:1342–1346
Wendler G, Shulski M, Moore B (2010) Changes in the climate of the Alaska North Slope and the ice concentration of the adjacent Beaufort Sea. Theor Appl Climatol 99:67–74
Wilson DJ, Krebs CJ, Sinclair T (1999) Limitation of collared lemming populations during a population cycle. Oikos 87:382–398
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Upper Saddle River
Acknowledgments
We thank Guillaume Szor, François Racine, Francis Taillefer, Andrew Fehr, Helen Slama, Alice Kenney, Elizabeth Hofer, Charles J. Krebs, and Scott Gilbert for field assistance. We thank Richard Gordon, Edward McLeod, Lee John Meyook, Jordan McLeod, Deon Arey, Sam McLeod, and Pierre Foisy for helping with field logistics. We thank helicopter pilots Florian Koch and Robert Ungar. We are grateful to Parks Canada for permission to work in Ivvavik National Park. We thank Bob Sagar for gathering climate data from the Komakuk Beach Environment Canada weather station database. Three reviewers made comments that improved this paper. Funding and support for this research come from the Natural Sciences and Engineering Research Council of Canada (grants to Dominique Berteaux, Alexander Graham Bell CGS-D graduate scholarship to Daniel Gallant), the International Polar Year program of Indian and Northern Affairs Canada, the ArcticNet Network of Centers of Excellence of Canada, the Wildlife Conservation Society Canada (Don Reid), the Polar Continental Shelf Program (PCSP), Natural Resources Canada, the Canada Research Chairs Program, the Aurora Research Institute, the Canadian Foundation for Innovation, the Centre d’Études Nordiques (CEN), the Northern Scientific Training Program (Indian and Northern Affairs Canada), and the Université du Québec à Rimouski. We thank J. Martin Bland at the University of York (York, United Kingdom) and Barbara K. Butland of St George’s Hospital Medical School (London, United Kingdom) for sharing their unpublished statistical procedure.
Author information
Authors and Affiliations
Corresponding authors
Appendix
Appendix
Procedure comparing proportions in overlapping samples (from Bland and Butland, unpublished)The procedure by J. M. Bland and B. K. Butland comparing proportions in overlapping samples (http://www.users.york.ac.uk/~mb55/overlap.pdf, accessed on March 4, 2011) calculates two differences of proportions: one for paired data (in our case, dens surveyed in each of the compared periods) and another for unpaired data (in our case, dens only surveyed in one of the periods). It then combines the calculated differences in a weighted average to produce a single difference of proportions. We used the procedure to obtain 95 % confidence intervals to decide whether differences between proportions were statistically significant. We pooled the survey data within the periods 1984–1990 and 2003–2010 and classified each den as having been used or not by foxes within each of the two survey periods. We describe below the calculations supporting Bland and Butland’s procedure, using symbols described in Table 2.
The difference (d) in the paired (p) dataset between the number of dens used in the first sample but unused in the second sample (n 10), and the number of dens unused in the first sample but used in the second sample (n 01) is:
with variance (Var):
while the difference in the number of dens used in the first (n x ) and second (n y ) samples in the unpaired (u) dataset is:
where k and m are the respective sample sizes of the two samples, with variance:
Combining these two difference estimates using a weighted (w) average, we get:
where:
and:
with variance:
The 95 % confidence interval is thus:
Rights and permissions
About this article
Cite this article
Gallant, D., Slough, B.G., Reid, D.G. et al. Arctic fox versus red fox in the warming Arctic: four decades of den surveys in north Yukon. Polar Biol 35, 1421–1431 (2012). https://doi.org/10.1007/s00300-012-1181-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-012-1181-8