Abstract
Drought seriously affects the agro-physiological and biochemical functioning of plants by influencing the interactions between plants and symbiotic microorganisms. Therefore, the objective of the present study was to implement a management approach to improve tomato growth, and drought tolerance using arbuscular mycorrhizal fungi (AMF) (pure strain (M) and consortium (M′)), and/or plant growth-promoting-rhizobacteria (Actinomycetes (A) and consortium with two bacteria Z2 and Z4 (B), and/or Olive-Mill-Wastewater-compost (OMWW-compost (C)). The potential for changes in physiological (stomatal conductance, chlorophyll fluorescence, photosynthetic pigments) and biochemical (sugar, protein, hydrogen peroxide (H2O2), malondialdehyde (MDA), phenols, and antioxidant enzymes) functioning in response to water stress was analyzed. Therefore, under 35% field capacity (FC), the application of AMF (M or M′)/PGPR (A and B) amended with compost stimulated biomass and improved stomatal conductance, chlorophyll and carotenoid contents and photosynthetic efficiency to a greater extent than in uninoculated and/or unamended plants. The compost application with double inoculation including M′A (CM′A) significantly improved sugar concentrations in leaves and roots by 34% and 30% as well as enhanced antioxidant activities notably catalase (CAT), polyphenoloxidase (PPO) peroxidase (POX) and superoxide dismutase activities of about 92%, 177%, 84% and 79%, respectively. The dual inoculation together with compost (CM′A) and (CM′B) resulted in a significant reduction of H2O2 contents by 14% and 13% and MDA by 93% and 92%, respectively. The application of locally produced compost with dual combinations of bacteria can overcome the challenges of water stress by improving the physiological, biochemical and tolerance of tomato.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water scarcity limits crops growth and productivity, leading to the most economic losses in agriculture (Meddich et al. 2018; Musolino et al. 2018). Currently, the scarcity of water resources represents the most critical threat to world food security (He et al. 2019; Kijne et al. 2003) and the future food demand for the population will aggravate by the drought effects (Mishra et al. 2021; Somerville and Briscoe 2001). More than 50% of the arable land will be affected by drought by 2050, resulting in damage to the growth and development of crops (Jaggard et al. 2010; Kasim et al. 2013). Drought influences water uptake and soil nutrient transport and availability while affecting plant morphology, physiology, and biochemistry particularly water content, leaf water potential, stomatal conductance, quantum yield and photosynthetic pigments, and phosphorus (P) and nitrogen (N) uptake (Baslam et al. 2014; Silva et al. 2017; Kour et al. 2020). Beside, it affects the defense system through the accumulation of reactive oxygen species (ROS) (Benaffari et al. 2022; Boutasknit et al. 2021b). In addition, exposure of plants to severe drought alters their photosynthetic mechanism, this may alter photosynthetic relationships, cell membrane, symbiotic interactions between plant and associated soil-borne microorganisms (Anli et al. 2020; Toubali et al. 2022; Yooyongwech et al. 2016). Abd El-Mageed et al. (2018) showed that the degradation of chlorophyll pigments and cell plasma membrane through ROS accumulation leads to decreased photosynthetic activity. In this regard, there is an urgent need to develop sustainable management strategies that allow plants to resist water stress conditions for further improvement of agricultural production.
The application of biostimulants such as beneficial symbiotic microorganisms and organic fertilizers has emerged as an innovative solution to promote increased yield and tolerance of crops under water stress conditions (Ben-Laouane et al. 2019). Proper management of plant water and mineral nutrition, growth and tolerance to water stress becomes a key element to increase crop yield (Baslam and Goicoechea 2012). In addition to regulating water uptake and nutrient acquisition, inoculation of plant growth promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) is presently an effective means to ensure stable, safe and sustainable crop production. Indeed, inoculation with rhizosphere microorganisms has beneficial effects related to solubilization of nutrients such as phosphorus (P), potassium (K) and iron (Fe), biodegradation of soil organic matter, production of phytohormones, improvement of soil structure through the formation of aggregates, and enhancement of plant resilience to water stress conditions (Chen et al. 2020). In addition, the use of beneficial soil microorganisms in combination with stable and mature organic amendments is an essential component to enhance plant resistance under stressful circumstances (Anli et al. 2021; Boutasknit et al. 2020, 2021c). The application of organic amendments is an environment friendly sources of plant nutrients (Ahmad et al. 2021; Boutasknit et al. 2021a). Likewise, the use of organic matter, especially compost, increases the water holding capacity of the soil in case of water deficit (Hirich et al. 2014; Paradelo et al. 2019). The incorporation of compost improves soil fertility and retains moisture at appropriate levels up to 30% (Bassouny and Abbas 2019). Furthermore, inoculation of AMF and/or PGPR in combination with compost could mitigate the adverse effects of water stress through enhancement of photosynthetic efficiency, production of antioxidant enzymes, and activation of induced resistance mechanism by bypassing plant defense (Anli et al. 2020; Duo et al. 2018).
Tomato (Solanum lycopersicum) one of the most important greenhouse vegetables in the Mediterranean area (Paucek et al. 2020). In Morocco, this crop (Lycopersicon esculentum Mill.) is currently one of the most important vegetable crops for export worldwide as well as one of the most consumed foods after potato (Fondio et al. 2013). Tomatoes are known for their wide range of minerals, vitamins, sugars and antioxidant compounds (Zare et al. 2011). With this in mind, tomatoes are becoming an increasingly important part of the human diet, hence the need to increase the production of this crop internationally (Berni et al. 2019). Tomatoes consume large amounts of water to grow. This need for water was increased during the growth phases and more particularly during the fruiting phase. Thus, in arid and semi-arid areas, of which Morocco is part, the limitation of water resources leads to an early ripening of tomatoes with reduced yields combined with poor quality.
Until date, the effect of combined application on tomato plant growth was rarely studied. Of our knowledge, data on the effect of combined application of OMWW compost, AMF and PGPR on tomato tolerance to drought stress and the underlying mechanisms are not available. In this context, and based on these findings, our study focused on the effect of the application of AMF and PGPR symbiotic inocula, in combination with compost on root colonization, photosynthetic and enzymatic activity of tomato plants submitted to water stress.
Therefore, the objective of the present study was to evaluate the effect of severe water stress on tomato with the application of microorganisms and a biological amendment at the physiological, biochemical, and cellular levels by characterizing the components of oxidative stress. Thus, the aim of this study was to improve the mechanisms of biofertilizers mediated plant protection responses and metabolic processes under tough water conditions in the context of sustainable global agricultural food production.
Material and Methods
Biological Materials
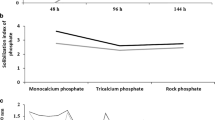
Two AMF species were used: a pure strain, exogenous Rhizoglomus irregulare, DAOM 197198 (M) provided by the Plant Biology Research Institute, University of Quebec, Montreal, Canada, and a consortium (M′) isolated from the Tafilalet palm grove, Morocco that composed of 15 species: Acaulospora delicata, Acaulospora leavis, Acaulospora sp., Claroideoglomus claroideum, Glomus aggregatum, G. clarum, G. claroides, G. deserticola, G. heterosporum, G. macrocarpum, G. microcarpum, G. versiforme, Glomus sp, Rhizophagus intraradices, and Pacispora boliviana. Maize (Zea mays L.) was used as a host plant to propagate the two AMF inocula, for three months under controlled greenhouse. AMF inoculation consisted of adding 25 g of soil consisting of mycorrhizal root fragments, spores and vesicles and hyphae. The number of AMF spores detected in this consortium was 47 spores/100 g of the soil sample. The bacterial inoculants used were a bacterial consortium named B composed of two bacterial strains (Z2 and Z4) and a pure bacterial strain Actinomycete (A)., isolated from date palm rhizospheric soil collected in Tafilalet palm grove, Morocco (31° 47′ 20.8″ N 04° 14′ 59.3″ W). Z2 was identified as Bacillus sp., while Z4 is reported to be Bacillus subtilis following molecular characterization of the 16S rDNA gene (sequencing and DNA-DNA hybridization (threshold 70%) and Delta melting T° below 5 °C). Analyses were done in the laboratory to test phosphate solubilization, indeed, a NBRIY CS Nautiyal (1999) was prepared and the phosphate solubilization index was calculated using the Eq. (1) for the towstrains after 10 days of culture at 28 °C (Fig. 1), Three sources of phosphate were used, namely: tricalcium phosphate (Ca3HPO4), monocalcium phosphate (CaHPO4) and rock phosphate with 5 g/L for each source. The resistance to polyethylene glycol PEG 6000 was also measured by following the optical density at 600 nm. The strains were grown in Tryptic Soy Broth (TSB) liquid culture with different concentrations of PEG (0, 10, 20, 30, 40 and 60%) at 28 °C (Fig. 1), then a salinity resistance test was used to detect the strains that resisted different concentration of NaCl (0.5M, 1M and 2M) (Table 1).
The OMWW-compost (C) used in this experiment is based on olive mill wastewater sludge mixed with green waste (50%, 50%) with the following characteristics, pH: 7.3; decomposition rate: 73.6% ± 0.5; polyphenols abatement rate: 78%; lipids abatement rate: 73.7%; moisture content: 55.1%; electrical conductivity: 8.2 mS cm−1; available phosphorus: 0.05 mg g−1 and C/N ratio: 15.6.
Tomato seeds, Solanum lycopersicum. Campbell 33 cultivar, of homogeneous size were selected and disinfected with sodium hypochlorite (bleach 5% v/v) for 5 min. They were rinsed three times with distilled water and germination was carried out at 28 °C. The seeds were performed in plastic petri dishes containing sterile filter paper discs. The dishes were incubated for 3 days at 28 °C in the dark. The germinated seeds were then transplanted into plastic trays containing previously sterilized peat. After 3 days, germinated seeds were transferred in plastic trays filled with commercial potting soil in the greenhouse. Then, the tomato plants were transplanted into perforated plastic pots (one for each pot).
Tomato plants inoculations were performed after one week of transplanting by adding 5 mL of the bacterial suspension (5 mL for B and 5 mL for A) near the roots. After one week, a second inoculation was performed to increase the level of bacteria in the soil and to ensure infection of the roots formed after the first inoculation. The inoculum were prepared by growing the strains in tryptic soy broth (TSB) liquid culture at 28 °C.
The experimental trial consisted of two irrigation regimes, 75% of field capacity (FC) and 35% FC, testing two strains of bacteria: Actinomycete A and bacteria strains consortium: Z2 and Z4 (B), two strains of AMF (Exogenous R. irregulare (M)), indigenous consortium (M′), and one compost type C among on tomato (Table 2).
The Greenhouse Equipment and Experiment Design
The experiment was carried out in the semi-controlled greenhouse with natural light (photon flux density between 500 and 750 μmol m−2 s−1), an average temperature of 23 °C (day/night air temperatures of 28/18 ± 4 °C), and an average relative humidity of 70% at the Faculty of Science, Marrakech-Morocco. The experimental design adopted was completely randomized design that contains 36 treatments with 5 biological replicates (Table 1). The total number of pots used in the experiment was 180 pots. Each pot was filled by 5 Kg of agricultural soil. Preliminarily sterilized for 4 h at 180 °C with the following characteristics: pH: 8.1; electrical conductivity: 0.2 mS.cm−1; organic matter: 1%; total organic carbon: 0.6%; available phosphorus: 7.3 mg Kg−1; total nitrogen: 0.9 mg g−1; calcium: 2.2 g Kg−1 and potassium: 0.6 mg g−1. The pots were wetted for 3 days in order to keep uniform soil water content distribution before transplantation.
Biological and Physiological Plants Trend
Several parameters such as growth and physiological, biochemical parameters were measured after the harvest.
Growth Parameters and AMF Colonization
At harvest, the plants were rinsed generously with tap water. The growth parameters such as shoot height, root length, total fresh and dry weight of the shoot and root parts, as well as the number of leaves, number of flowers were measured.
The washed, cleaned roots were placed in 10% KOH for 30 min at 90 °C, and then washed in tap water, acidified in 2% HCl for 10 min and stained with Trypan blue at 90 °C for 20 min according to Higo et al. (2015). Roots were cut into 1 cm pieces, mounted on slides in glycerol and analyzed to assess the following mycorrhizal parameters: mycorrhization frequency (F%), colonization intensity (I%) in individual mycorrhized roots according to equation (Eq. 1) given below (Trouvelot et al. 1986). At least 5 samples for each treatment, each composed of 12 pieces of roots, were studied using an optical microscope.
Where n5, n4, n3, n2, n1 number of fragments noted 5, 4, 3, 2 and 1, respectively, Class 5: more than 90%, Class 4: between 50% and 90%, Class 3: between 10% and 50%, Class 2: less than 10%, Class 1: trace and Class 0: no mycorrhization.
Stomatal Conductance, Chlorophyll Fluorescence and Leaf Water Potential
Stomatal conductance was measured using a portable porometer (Leaf Porometer, Decagon Device, INC), this was done on the 5th leaf of the 5 plants of each treatment. The values of this parameter are expressed in mmol H2O m−2 s−1.
The ratio of variable to maximum fluorescence (Fv/Fm) was measured using a fluorometer (OPTI-SCIENCE, OS30p) with tweezers to dark-adapt the leaves. After 30 min of dark adaptation, measurements were made on the 3rd leaf for each tomato plant per treatment.
Chlorophyll Content and Pigment Quantification
Arnon (1949) method has allowed determining the chlorophyll content leaves. This involves grinding 100 mg of fresh material (FM) in the presence of acetone 80%. After filtration, the optical density was read at 663 and 645 nm. Concentrations in chlorophyll were obtained from following formulas (Eq. 2 and 3 and 4):
Where, A = absorbance; V = final volume of the extract and FW = fresh weight.
Total Soluble Sugar Content
The total soluble sugar (TSS) content was measured according to the method developed by Dubois et al. (1956). The fresh material (0.1 g) homogenized with 4 mLof ethanol (80%) and then centrifuged at 5000 rpm. The supernatant was mixed with 0.25 mL of phenol (5%) and 1.25 mL of concentrated sulfuric acid (36N). After refortification, the TSS content was determined by measuring the absorbance at 485 nm using a spectrophotometer (UV-3).
Protein Content and Antioxidant Enzymes Activity
The harvested and frozen leaves were ground to a fine powder in liquid nitrogen with a pestle and mortar. The fine leaf powder (0.1 g) was homogenized with 4 mL of 1 M phosphate buffer (pH 7) containing 5% of polyvinylpolypyrrolidone. The homogenate was centrifuged at 18,000 × g for 15 min at 4 °C and the supernatant was used to determine the total soluble protein and antioxidant enzymes activity. Total soluble protein was measured following the method described by (Bonjoch and Tamayo 2001). The absorbance was measured at 595 nm.
Catalase activity (CAT) was determined according to Aebi (1984), in which a decrease in hydrogen peroxide (H2O2) level was monitored spectrophotometrically at 240 nm. The reaction mixture contained 50 mM K2HPO4, (pH = 7.0), 10 mM (H2O2), and 0.1 mL of enzyme extract. Results were expressed as units of H2O2/min/mg protein.
The method followed to determine peroxidase (POX) activity was that described by Tejera García et al. (2004). 0.1 mL of previously prepared enzyme extract was added 100 mM K2HPO4/KH2PO4 buffer (pH 6.5), 40 mM guaiacol, 10 mM H2O2. The absorbance was recorded at 436 nm and the results were expressed as Unit/mg protein.
Polyphenoloxidase (PPO) activity was estimated according to the method described by (Hori et al. 1997), which involves monitoring the oxidation of catechol at 410 nm. The reaction mixture contained 100 mM K2HPO4/KH2PO4 buffer (pH 6), 50 mM catechol, and enzyme extract. PPO activity was expressed as U/mg protein.
Superoxide dismutase (SOD) activity was assessed by the method described by (Beyer and Fridovich 1987). By monitoring the reduction of nitroblue tetrazolium (NBT) on formazan blue by spectrophotometry at 560 nm after 30 min under blue light. The reaction mixture contained 50 mM phosphate buffer (pH 7.8), 63 mM NBT, 13 mM methionine, and 60 μM of riboflavin solution were added to 100 μL of extract. The test tubes were exposed to light for 15 min before reading the optical density. SOD activity was expressed as U/mg protein.
Lipid Peroxidation Assessment and H2O2 Content
Lipid peroxidation was assessed by measuring the malondialdehyde (MDA) content of leaf samples according to the method described by Wu et al. (2016). The frozen leaf powder (100 mg) was mixed with 10% trichloroacetic acid (TCA) and 1 mL of cold acetone. The homogenate was centrifuged at 8000 × g at 4 °C for 15 min and the supernatant was added to 0.1% H3PO4 and 0.6% thiobarbituric acid. After incubation at 100 °C for 30 min, the mixture was cooled with an ice bath to stop the reaction. The absorbance was read at 532 nm. Nonspecific turbidity was corrected by subtracting A600 from A532, and the MDA content was calculated as follows in Eq. 5:
The determination of H2O2 content was done according to the method of Velikova et al. (2000). 100 mg of plant material was ground in 3 mL of 0.1% TCA, the mixture was centrifuged at 12,000 × g for 10 min, 0.5 mL of the supernatant (the enzyme extract) was added to 0.5 mL of potassium phosphate buffer (10 mM, pH = 7.0) and 1 mL of potassium iodine (1M) after incubation for 3 min at room temperature and 1 h in the dark, the absorbance was read at 390 nm.
Total Phenol Content
Total phenol content (TPC) was measured by grinding 1 g of fresh material in 8 mL of 80% methanol, the mixture was centrifuged at 1000 rpm for 5 min, 50 µl of the supernatant (phenolic extract) was diluted to 2 mL with distilled water, then 250 µL of 1/3 Folin-Ciocalteu reagent was added 3 min later, then 500 µL of a saturated sodium carbonate solution was added. After incubation at room temperature for one hour the optical density was read at 725 nm.
Data Analyses
Data were submitted to a two-way analysis of variance (ANOVA) by taking into account the effects of the biofertilizers, water regimes and their interactions using minitab 16 software. Significant differences between treatments were assessed by 1% level (P ≤ 0.01) followed by Tukey’s honest significant difference test. Normality of residuals was tested using the Andersan-darling test.
Results
Growth and Mycorrhization Parameters
The water stress (35% FC) has caused a significant reduction of growth parameters of the tomato plant (Table 3). Moreover, the application of bi- and tripartite combinations of bio-stimulants/biofertilizers (AMF (M and M′) and/or compost and/or bacteria) showed significant positive effects on the promotion of tomato shoot height (SH) and root length (RL) compared to the uninoculated and unamended plants under 75% and 35% FC. Application of M′, B and A in combination with compost improved SH compared to their separate application under drought stress conditions. In contrast, the application of M in combination with compost reduced SH compared to their separate application particularly under water stress conditions. Under 35% FC, the combined application of M and A improved RL by 68% compared to the control plants. Under 35% FC, the addition of compost to the M′B and MB combination improved RL by 175% and 67% respectively, compared to the control plants. In contrast, the application of A, B, M and M′ alone and in combination with compost inhibited RL compared to the controls under well watered conditions (75% FC). In addition, the combination of compost with B (CB) and with the double combination M′B (CM′B) increases the number of leaves by 32% and 45% respectively compared to the control plants under water stress conditions.
Tomato plants inoculated with AMF and compost showed greater root infection compared to the inoculated and unamended plants. Also, the application of water stress inferred significantly AMF infection and colonization parameters (Fig. 2). Application of M and M′ in combination with CB (CMB and CM′B) and with CA (CMA and CM′A) enhanced root colonization of tomato plants compared to their separate application. Application of M or M′ in combination with CB (CMB and CM′B) and with CA (CMA and CM′A) increased the root frequency of tomato plants by 62%, 66%, 61% and 67% respectively, compared to their separate application under water stress. Similarly, the intensity of root mycorrhization was improved by the application of M or M′ in combination with C and A (CMA and CM′A) or B (CMB and CM′B) by 75%, 66%, 80% and 64% respectively, compared to their separate application under drought stress conditions.
Physiological Parameters
Water stress negatively affected physiological parameters (Fig. 3). Under stress conditions 35% FC, tomato plants amended with compost and inoculated with M′ and A significantly improved Fv/Fm by 35% compared to the unamended and uninoculated plants (Fig. 3a). Similarly, the application of compost in combination with B (CB) or M′ (CM′) enhanced stomatal conductance by 76% and 50% respectively, compared to the control plants under water stress conditions 35% FC (Fig. 3b). Analysis of variance showed a highly significant difference between treatments and water regimes while there was no significance between interactions.
Sugar and Proteins Content
According to Fig. 4, a decrease in the amount of sugars was observed in the leaves and roots of tomato plants according to the different water regimes. Analysis of leaf and root sugars in tomato seedlings under water stress conditions revealed that tomato seedlings inoculated with B and M and/or amended with C increased leaf and root protein content compared to the control (Fig. 4a,b). This accumulation of leaf and root protein contents reached higher values in B‑inoculated and C‑amended (CB) plants under 35% FC by 6% and 4%, respectively, than in uninoculated and unamended plants. Furthermore, the sugar content of tomato leaves and roots was significantly reduced during the water stress conditions (Fig. 4c,d). However, the combination of compost with M′ and A (CM′A) significantly increased leaf and root sugar content by 34% and 30% compared to the control plants under water stress conditions. These results were confirmed by the analysis of variance, which detected highly significant differences between the two water regimes.
Chlorophyll a, b and Carotenoids
Chlorophyll a and b and carotenoid levels decreased significantly after the application of 35% FC of water stress, regardless of the presence/absence of bio-stimulants. Under 35% FC, the added bio-stimulants increased the concentrations of chlorophyll a and b and carotenoids in leaves of tomato plants compared to the controls (Fig. 5). The application of actinomycete A alone and/or in the presence of compost C and consortium M′ increased Chl “a”, “b” and carotenoids compared to the uninoculated and unamended plants under the 35% FC. Under 35% FC, the highest level of pigments was obtained in tomato plants treated with CM′A, where the concentrations of Chl “b” and carotenoids showed an improvement of 14% and 86% respectively, compared to the control plants. Chl “a” recorded an improvement of 6% and 2% respectively, with the application of A and CM′A in comparison with the control plants.
Antioxidant Enzyme Activities
Water stress induced a substantial increase in the activities of catalase (CAT), peroxidase (POX) and polyphenoloxidase (PPO) and superoxide dismutase (SOD) in tomato leaves compared to the well-watered 75% FC conditions (Fig. 6). The activities of these antioxidant enzymes were much higher in plants amended with C and inoculated with the M′ and A consortium (CM′A). Under water stress conditions, the activities of CAT, PPO, POX and SOD were improved by 92%, 33%, 177%, 84% and 79% respectively, for the CM′A treatment (Fig. 6) compared to the control.
Lipid Peroxidation Assessment, H2O2 and Total Phenol Content
Under 35% FC, H2O2 and MDA content increased strongly especially in the control plants compared to plants under 75% FC (Fig. 7). However, plants inoculated with AMF (M or M′) and/or bacteria (A or B) and/or amended with compost showed low levels of MDA in tomato leaves compared to the control plants regardless of the imposed water regime. Indeed, tomato control plants showed higher H2O2 levels of 14%, 13%, 11%, and 8% compared to CM′A, CM′B, CMA, and CMB treatments, respectively, under the water stress conditions (Fig. 7a). Similarly, we observed an increase in MDA content in unamended and uninoculated plants of 93%, followed by CM′B, CM′A, and CMB and CMA by 92%, 81%, and 78% respectively, compared to the control plants under 75% FC (Fig. 7b).
Analysis of leaf phenols during water stress revealed that both inoculated and amended plants significantly increased phenol content compared to the control (Fig. 7c). Application of compost in combination with M (CM) or B (CB) significantly boosted phenol levels by 98% and 76%, respectively, compared to the control plants under water stress conditions.
Principal Component Analysis
Principal component analysis revealed that AMF (M or M′) alone or in combination with compost and/or PGPR were the most effective treatments for improving growth, physiological traits, osmolytes and enzymatic and non-enzymatic antioxidant activity under favorable 75% and unfavorable 35% water conditions (Fig. 8). PC1 accounted for 41.05% and PC2 for 24.35% of the total variance. Figure 8 shows that all treatments inoculated with AMF and/or PGPR and/or amended with compost, were distinct from the control which segregated to the left of the PC1 component and was correlated with the traits H2O2, MDA and phenol content.
Principal component analysis (PCA) of all investigated traits. (SH shoots and RH roots height, LN leaf numbers, SDM shoots and RD roots dry matter, PL leaf and, PR root protein, TSSL leaf and TSSR root total soluble sugar, Chl a chlorophyll a, Chl b chlorophyll b, Car carotenoids, Fv/Fm chlorophyll fluorescence, gs stomatal conductance, MDA malonyldialdehyde, PT total phenol content, H2O2 hydrogen peroxide content, CAT Catalase, PPO polyphenol oxidase, POX peroxidase, SOD superoxide dismutase, F% AMF infection frequency and I% AMF infection intensity)
Discussion
Under water stress, tomato plants growth was significantly limited by decreasing root length, shoot height and number of leaves as well as shoot and root dry matters. This could be due to the rapid response of tomato to drought stress, which could be related to the closure of stomata as well as the decrease in transpiration and photosynthetic activity. However, the application of AMF (M′ or M), bacteria (A or B) and OMWW-compost, alone or in combination, resulted a significant increase in the different growth parameters. Previous studies showed a better growth of tomato and date palm inoculated with native and exogenous AMF and/or amended with organic fertilizer under drought stress conditions (Baslam et al. 2014; Tahiri et al. 2022a). Moreover, another study on chili and wheat, showed a significant improvement of growth plants inoculated with Bacillus sp. and Pseudomonas sp., respectively (Gou et al. 2020; Yaseen et al. 2019). This is in line with the obtained results in this study, as treatments with AMF+Compost+Bacteria showed a beneficial effect on tomato biomass under drought stress. The interactions between AMF, bacteria and compost can be rather specific, and have the potential to improve plant growth (Ben-Laouane et al. 2020; Ojuederie et al. 2019). This could be because compost, AMF, and bacteria change the soil structure and increase organic matter while simultaneously increasing the amount of plant-available nutrients, boosting plant growth (Armada et al. 2014; Tahiri et al. 2022b).
The intensity and frequency of mycorrhization recorded the highest values with the application of compost combined with bacteria in the presence of AMF under well-watered and drought stress conditions. Studies of other researchers support our observations (Bernardo et al. 2017; Mathur et al. 2019). The same results were obtained by Anli et al. (2020) and Boutasknit et al. (2021b), who showed significant improvement of date palm and carob tree roots colonization when AMF were combined with organic fertilizers and/or PGPR under drought stress. Other studies revealed an increase in root colonization when plants were treated by AMF combined with compost and bacteria (Aalipour et al. 2020; Anli et al. 2020; Azizi et al. 2021). These investigations clearly showed that a combination of AMF and PGPR might have a positive effect on root colonization.
In this study, water stress significantly decreased physiological parameters. This could be due to the restricted diffusion of CO2 into the leaf caused by the closure of stomata and the photosynthetic activity (Molero et al. 2019; Nadal and Flexas 2019; Yang et al. 2021). However, AMF/bacteria and/or compost improved tomato physiology by increasing chlorophyll fluorescence (Fv/Fm) as well carotenoids content. Previous studies reported that showed significant improvement of plant physiology when biofertilizers were applied under drought stress conditions (Boutasknit et al. 2021b; Meddich et al. 2021; Zoppellari et al. 2014). The improvement of physiological attributes in tomato leaves in the presence of compost and/or AMF and/or bacteria could also be explained by the availability of mineral elements with the addition of compost and better uptake by the AMF mycelium (Zhang et al. 2019). Mineral nutrients are known to play an important role in the improvement of photosynthesis (Dhalaria et al. 2020). In addition, bacteria and AMF could improve plant physiological traits by increasing soil aggregation and maintaining a higher water potential around the roots, which can increase pore size and the passage of solutes (Lemichez 2020; Vurukonda et al. 2016). It is known that AMF and/bacteria and/or compost improve the plant’s gas exchange by enhancing stomatal conductance and photosynthetic activity leading to a better acquisition and assimilation of CO2 by the treated-plants (Cheng et al. 2021; Sadeghi et al. 2020; Xu et al. 2016).
Considering protein content, our results showed no significant difference for applied biofertilizer treatments regardless the water regime applied compared to the control. This could explain by the fact that water deficit stimulates the protein synthesis of many enzymes including those involved in the detoxification of reactive oxygen species (ROS). This is in agreement with the findings of Choudhury et al. (2017). Furthermore, it is known that protein concentration is not already improved under water stress conditions (Dineshkumar et al. 2019). The improvement of protein is related to the induction shock specific proteins to protect plant tissues under abiotic stress in plant tissues (Avin-Wittenberg 2019). In addition, organic osmolytes, such as carbohydrates (sugars), are known to be key molecules for carbon budget. In this study, the concentration of sugars was significantly reduced under drought stress. Sugars’ increased concentration in plants might be explained as a response to soil drought, as they were able to lower osmotic potential in plants and act as water precursors (Brunner et al. 2015; Granot and Kelly 2019). However, under severe water stress conditions, compost combined with AMF and bacteria showed significant increment of sugars compared to the stressed control. The same findings were reported in sugarcane and date palm under water drought stress conditions (Anli et al. 2020; Ferreira et al. 2017). The application of AMF and/or compost and/or bacteria can cause an increase in growth parameters and physiological traits, which may influence the metabolism of sugars in cells (Secchi et al. 2017). Indeed, numerous studies revealed an increment of sugars concentration coupled with photosynthetic activity when plants were treated by biofertilizers in single or in combination (Ma et al. 2018; Zhang et al. 2019).
In this study, it was clear that the inoculation of tomato with AMF and/or PGPR and compost application were effective to increase the antioxidant enzymes activity and reduce ROS production. Peroxidase is hemoprotein with a hem prosthetic group: the Ferro proto porphyrin IX, 3 (Atamna et al. 2015). They are glycoprotein oxid-reductases that catalyze the oxidation of many organic and inorganic compounds by hydrogen peroxide (H2O2) (Huang et al. 2021). In this study, the recorded CAT, PPO, POX and SOD activities were significantly elevated in plants exposed to water stress and inoculated with AMF and/or bacteria and/or amended with compost. These results are in corroboration with those of Tahiri et al. (2022a), who showed that the induction of CAT, SOD, PPO, and POX constitute biochemical response of plant to oxidative stress caused by drought stress. They are enzymatic biomarkers, which are very important in the defense system and the detoxification of ROS production (Pirzadah et al. 2019) and protect cells against oxidative damage by toxic H2O2 (Xie et al. 2019). The increase of enzymatic activity under oxidative stress generated by water deficit revealed its important role in the elimination of H2O2 formed. POX reduces H2O2 to water molecule using ascorbate as electron donor resulting from dehydroascorbate (Pisoschi et al. 2021). The stimulation of these enzymes reflects the establishment of a state of tolerance in the cells of tomato plants inoculated by AMF/bacteria and/or amended by compost (Ait Rahou et al. 2021; Tahiri et al. 2022a).
H2O2 and MDA are considered a marker for the evaluation of ROS production and membrane of plasmalemma lipid peroxidation, respectively under environmental stress. Our findings showed in increment of H2O2 and MDA content in tomato plant under water stress conditions. However, they were significantly decreased when biofertilizers were applied alone or in combination, which linked with high antioxidant activity. This could be related to a decrease of ROS, membrane damage and oxidative stress and an improvement tolerance to oxidative stress increased (Adwas et al. 2019; Saleem et al. 2020). In addition, antioxidant enzymes such as CAT is very important for trapping H2O2 in the peroxisome by converting it to water (Corpas et al. 2019; Kapoor et al. 2019). In this organelle, H2O2 is produced from β‑oxidation of fatty acids and photorespiration (Yu et al. 2019). Moreover, high POX activity increases membrane stability and CO2 fixation in the Calvin cycle within chloroplasts, which are very sensitive to environmental stress (Hameed et al. 2021; Sharma et al. 2020). This could be related to the capacity of AMF/bacteria and/compost to reduce MDA and H2O2 by stimulating the activities of various biomolecules like osmoprotectants and antioxidant enzymes as well as secondary metabolites (Ghanbarzadeh et al. 2019; Meena et al. 2020; Ojuederie et al. 2019). AMF and PGPR inoculation and/or compost application could be able to reduce MDA content by decreasing membrane peroxidation and maintaining its structure and stability, which could improve plant stress tolerance (Ben-Laouane et al. 2019; Shafiq et al. 2021). According to an increase in antioxidant enzyme activities and osmolytes synthesis, plants are required to cope with drought stress by eliminating ROS (Ghanbarzadeh et al. 2019; Lahbouki et al. 2022). Under drought stress conditions, inoculated tomato plants produce less ROS and accumulate more antioxidant enzymes and osmolytes, which could be considered as a drought avoidance mechanism. This increment of enzymes activity would minimize the impacts of drought stress, particularly oxidative stress, by maintaining a higher water status, largely through increased water intake and/or reduced water loss, as well as mineral nutrient uptake (Halder et al. 2022; Inculet et al. 2019).
Overall, the findings revealed that soil amendment with compost combined with native AMF/PGPR/actinomycete led to higher growth, and stress-related photosynthetic features like stomatal conductance and compound buildup. It really is worth noting that application of autochthonous bio-products might be a novel way to promote tomato growth and tolerance, and it could be an ecological combination for tomato to sustain dry soils.
Conclusion
Altogether, the findings demonstrated that adding local compost to the soil along with native AMF/bacteria resulted in increased growth, and stress-related photosynthetic aspects such gas exchange and compound accumulation. It’s worth noting that employing autochthonous biofertilizers to promote tomato growth and tolerance could be a novel approach, and it could be a suitable combination for tomato plants to thrive in dry environments.
References
Aalipour H, Nikbakht A, Etemadi N, Rejali F, Soleimani M (2020) Biochemical response and interactions between arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria during establishment and stimulating growth of Arizona cypress (Cupressus arizonica G.) under drought stress. Sci Hortic 261:108923. https://doi.org/10.1016/j.scienta.2019.108923
Abd El-Mageed TA, El-Samnoudi IM, Ibrahim AEAM, El Abd Tawwab AR (2018) Compost and mulching modulates morphological, physiological responses and water use efficiency in sorghum (bicolor L. Moench) under low moisture regime. Agric Water Manag 208:431–439. https://doi.org/10.1016/j.agwat.2018.06.042
Adwas AA, Elsayed A, Azab AE, Quwaydir FA (2019) Oxidative stress and antioxidant mechanisms in human body. J Appl Biotechnol Bioeng 6:43–47
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ahmad A, Aslam Z, Bellitürk K, Iqbal N, Naeem S, Idrees M, Kaleem Z, Nawaz MY, Nawaz M, Sajjad M, Rehman WU, Ramzan HN, Waqas M, Akram Y, Jamal MA, Ibrahim MU, Baig HAT, Kamal A (2021) Vermicomposting methods from different wastes: An environment friendly, economically viable and socially acceptable approach for crop nutrition: a review. Int J Food Sci Agric 5:58–68. https://doi.org/10.26855/ijfsa.2021.03.009
Ait Rahou Y, Ait-El-Mokhtar M, Anli M, Boutasknit A, Ben-Laouane R, Douira A, Benkirane R, El Modafar C, Meddich A (2021) Use of mycorrhizal fungi and compost for improving the growth and yield of tomato and its resistance to Verticillium dahliae. Arch Phytopathol Plant Prot 54:665–690
Anli M, Baslam M, Tahiri A, Raklami A, Symanczik S, Boutasknit A, Ait-El-Mokhtar M, Ben-Laouane R, Toubali S, Ait Rahou Y, Ait-Chitt M, Oufdou K, Mitsui T, Hafidi M, Meddich A (2020) Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front Plant Sci 11:1560. https://doi.org/10.3389/fpls.2020.516818
Anli M, Symanczik S, El Abbassi A, Ait-El-Mokhtar M, Boutasknit A, Ben-Laouane R, Toubali S, Baslam M, Mäder P, Hafidi M, Meddich A (2021) Use of arbuscular mycorrhizal fungus Rhizoglomus irregulare and compost to improve growth and physiological responses of Phoenix dactylifera ‘Boufgouss’. Plant Biosyst 155:763–771. https://doi.org/10.1080/11263504.2020.1779848
Armada E, Portela G, Roldán A, Azcón R (2014) Combined use of beneficial soil microorganism and agrowaste residue to cope with plant water limitation under semiarid conditions. Geoderma 232–234:640–648. https://doi.org/10.1016/j.geoderma.2014.06.025
Arnon DI (1949) Copper enzymes in isolated chloroplasts. polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.900074
Atamna H, Brahmbhatt M, Atamna W, Shanower GA, Dhahbi JM (2015) ApoHRP-based assay to measure intracellular regulatory heme. Metallomics 7:309–321
Avin-Wittenberg T (2019) Autophagy and its role in plant abiotic stress management. Plant Cell Environ 42:1045–1053
Azizi S, Tabari Kouchaksaraei M, Hadian J, Fallah Nosrat Abad AR, Modarres Sanavi SAM, Ammer C, Bader MKF (2021) Dual inoculations of arbuscular mycorrhizal fungi and plant growth-promoting rhizobacteria boost drought resistance and essential oil yield of common myrtle. For Ecol Manag 497:119478. https://doi.org/10.1016/j.foreco.2021.119478
Baslam M, Goicoechea N (2012) Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza 22:347–359
Baslam M, Qaddoury A, Goicoechea N (2014) Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological and biochemical responses coping with water drought of date palm, Phoenix dactylifera. Trees 28:161–172. https://doi.org/10.1007/s00468-013-0939-0
Bassouny M, Abbas MHH (2019) Role of biochar in managing the irrigation water requirements of maize plants: the pyramid model signifying the soil hydro-physical and environmental markers. Egypt J Soil Sci 59:99–115
Ben-Laouane R, Meddich A, Bechtaoui N, Oufdou K, Wahbi S (2019) Effects of arbuscular mycorrhizal fungi and rhizobia symbiosis on the tolerance of medicago sativa to salt stress. Gesunde Pflanz 71:135–146. https://doi.org/10.1007/s10343-019-00461-x
Ben-Laouane R, Ait-El-Mokhtar M, Anli M, Boutasknit A, Ait Rahou Y, Raklami A, Oufdou K, Wahbi S, Meddich A (2020) Green compost combined with mycorrhizae and rhizobia: A strategy for improving alfalfa growth and yield under field conditions. Gesunde Pflanz. https://doi.org/10.1007/s10343-020-00537-z
Benaffari W, Boutasknit A, Anli M, Ait-el-mokhtar M, Ait-Rahou Y, Ben-Laouane R, Ben Ahmed H, Mitsui T, Baslam M, Meddich A (2022) The native arbuscular mycorrhizal fungi and vermicompost-based organic amendments enhance soil fertility, growth performance, and the drought stress tolerance of quinoa. Plants 11:393
Bernardo L, Morcia C, Carletti P, Ghizzoni R, Badeck FW, Rizza F, Lucini L, Terzi V (2017) Proteomic insight into the mitigation of wheat root drought stress by arbuscular mycorrhizae. J Proteomics 169:21–32. https://doi.org/10.1016/j.jprot.2017.03.024
Berni R, Romi M, Cantini C, Hausman JF, Guerriero G, Cai G (2019) Functional molecules in locally-adapted crops: The case study of tomatoes, onions, and sweet cherry fruits from tuscany in Italy. Front Plant Sci 9:1–8. https://doi.org/10.3389/fpls.2018.01983
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal Biochem 161:559–566. https://doi.org/10.1016/0003-2697(87)90489-1
Bonjoch NP, Tamayo PR (2001) Protein content quantification by Bradford method. In: Handbook of plant ecophysiology techniques. Springer, Dordrecht, pp 283–295
Boutasknit A, Baslam M, Ait-El-Mokhtar M, Anli M, Ben-Laouane R, Douira A, El Modafar C, Mitsui T, Wahbi S, Meddich A (2020) Arbuscular mycorrhizal fungi mediate drought tolerance and recovery in two contrasting carob (Ceratonia siliqua L.) ecotypes by regulating stomatal, water relations, and (in)organic adjustments. Plants 9:80. https://doi.org/10.3390/plants9010080
Boutasknit A, Ait-Rahou Y, Anli M, Ait-El-Mokhtar M, Ben-Laouane R, Meddich A (2021a) Improvement of garlic growth, physiology, biochemical traits, and soil fertility by rhizophagus irregularis and compost. Gesunde Pflanz 73:149–160
Boutasknit A, Baslam M, Ait-El-Mokhtar M, Anli M, Ben-Laouane R, Ait-Rahou Y, Mitsui T, Douira A, El Modafar C, Wahbi S, Meddich A (2021b) Assemblage of indigenous arbuscular mycorrhizal fungi and green waste compost enhance drought stress tolerance in carob (Ceratonia siliqua L.) trees. Sci Rep 11:1–23. https://doi.org/10.1038/s41598-021-02018-3
Boutasknit A, Baslam M, Anli M, Ait-El-Mokhtar M, Ben-Laouane R, Ait-Rahou Y, El Modafar C, Douira A, Wahbi S, Meddich A (2021c) Impact of arbuscular mycorrhizal fungi and compost on the growth, water status, and photosynthesis of carob (Ceratonia siliqua) under drought stress and recovery. Plant Biosyst. https://doi.org/10.1080/11263504.2021.1985006
Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C (2015) How tree roots respond to drought. Front Plant Sci 6:1–16. https://doi.org/10.3389/fpls.2015.00547
Chen W, Meng P, Feng H, Wang C (2020) Effects of arbuscular mycorrhizal fungi on growth and physiological performance of catalpa bungei C.A.Mey. under drought stress. Forests 11:1–29. https://doi.org/10.3390/f11101117
Cheng HQ, Zou YN, Wu QS, Kuča K (2021) Arbuscular mycorrhizal fungi alleviate drought stress in trifoliate orange by regulating H+-ATPase activity and gene expression. Front Plant Sci 12:1–9. https://doi.org/10.3389/fpls.2021.659694
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867
Corpas FJ, Del Río LA, Palma JM (2019) Plant peroxisomes at the crossroad of NO and H2O2 metabolism. J Integr Plant Biol 61:803–816
Dhalaria R, Kumar D, Kumar H, Nepovimova E, Kuča K, Torequl Islam M, Verma R (2020) Arbuscular mycorrhizal fungi as potential agents in ameliorating heavy metal stress in plants. Agronomy 10:815
Dineshkumar R, Subramanian J, Gopalsamy J, Jayasingam P, Arumugam A, Kannadasan S, Sampathkumar P (2019) The impact of using microalgae as biofertilizer in maize (Zea mays L.). Waste Biomass Valor 10:1101–1110
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Duo LA, Liu CX, Zhao SL (2018) Alleviation of drought stress in turfgrass by the combined application of nano-compost and microbes from compost. Russ J Plant Physiol 65:419–426. https://doi.org/10.1134/S102144371803010X
Ferreira THS, Tsunada MS, Bassi D, Araújo P, Mattiello L, Guidelli GV, Righetto GL, Gonçalves VR, Lakshmanan P, Menossi M (2017) Sugarcane water stress tolerance mechanisms and its implications on developing biotechnology solutions. Front Plant Sci 8:1077
Fondio L, Djidji HA, N’Gbesso F, Kone D (2013) Evaluation de neuf variétés de tomate (Solanum Lycopersicum L.) par rapport au flétrissement bactérien et à la productivité dans le Sud de la Côte d’Ivoire. Int J Biol Chem Sci 7:1078–1086
Ghanbarzadeh Z, Mohsenzadeh S, Rowshan V, Moradshahi A (2019) Evaluation of the growth, essential oil composition and antioxidant activity of Dracocephalum moldavica under water deficit stress and symbiosis with Claroideoglomus etunicatum and Micrococcus yunnanensis. Sci Hortic 256:108652. https://doi.org/10.1016/j.scienta.2019.108652
Gou J‑Y, Suo S‑Z, Shao K‑Z, Zhao Q, Yao D, Li H‑P, Zhang J‑L, Rensing C (2020) Biofertilizers with beneficial rhizobacteria improved plant growth and yield in chili (Capsicum annuum L.). World J Microbiol Biotechnol 36:86. https://doi.org/10.1007/s11274-020-02863-w
Granot D, Kelly G (2019) Evolution of guard-cell theories: the story of sugars. Trends Plant Sci 24:507–518. https://doi.org/10.1016/j.tplants.2019.02.009
Halder T, Choudhary M, Liu H, Chen Y, Yan G, Siddique KHM (2022) Wheat proteomics for abiotic stress tolerance and root system architecture: current status and future prospects. Proteomes 10:17. https://doi.org/10.3390/proteomes10020017
Hameed A, Ahmed MZ, Hussain T, Aziz I, Ahmad N, Gul B, Nielsen BL (2021) Effects of salinity stress on chloroplast structure and function. Cells 10:2023
He X, Estes L, Konar M, Tian D, Anghileri D, Baylis K, Evans TP, Sheffield J (2019) Integrated approaches to understanding and reducing drought impact on food security across scales. Curr Opin Environ Sustain 40:43–54. https://doi.org/10.1016/j.cosust.2019.09.006
Higo M, Isobe K, Matsuda Y, Ichida M, Torigoe Y (2015) Influence of sowing season and host crop identity on the community structure of arbuscular mycorrhizal fungi colonizing roots of two different gramineous and leguminous crop species. Adv Microbiol 5:107
Hirich A, Choukr-Allah R, Jacobsen S (2014) Deficit irrigation and organic compost improve growth and yield of quinoa and pea. J Agro Crop Sci 200:390–398
Hori K, Ayako W, Shibuta T (1997) NII-electronic library service. Chem Pharm Bull 57:364–370
Huang Y, Lin J, Zou J, Xu J, Wang M, Cai H, Yuan B, Ma J (2021) ABTS as an electron shuttle to accelerate the degradation of diclofenac with horseradish peroxidase-catalyzed hydrogen peroxide oxidation. Sci Total Environ 798:149276
Inculet C‑S, Mihalache G, Sellitto VM, Hlihor R‑M, Stoleru V (2019) The effects of a microorganisms-based commercial product on the morphological, biochemical and yield of tomato plants under two different water regimes. Microorganisms 7:706. https://doi.org/10.3390/microorganisms7120706
Jaggard KW, Qi A, Ober ES (2010) Possible changes to arable crop yields by 2050. Philos Trans R Soc Lond B Biol Sci 365:2835–2851
Kapoor D, Singh S, Kumar V, Romero R, Prasad R, Singh J (2019) Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 19:100182. https://doi.org/10.1016/j.plgene.2019.100182
Kasim WA, Osman ME, Omar MN, Abd El-Daim IA, Bejai S, Meijer J (2013) Control of drought stress in wheat using plant-growth-promoting bacteria. J Plant Growth Regul 32:122–130
Kijne JW, Barker R, Molden D (2003) Improving water productivity in agriculture: editors’ overview. In: Water productivity in agriculture: Limits and opportunities for improvement, pp xi–xix
Kour D, Rana KL, Kaur T, Sheikh I, Yadav AN, Kumar V, Dhaliwal HS, Saxena AK (2020) Microbe-mediated alleviation of drought stress and acquisition of phosphorus in great millet (Sorghum bicolour L.) by drought-adaptive and phosphorus-solubilizing microbes. Biocatal Agric Biotechnol 23:101501. https://doi.org/10.1016/j.bcab.2020.101501
Lahbouki S, Ben-Laouane R, Anli M, Boutasknit A, Ait-Rahou Y, Ait-El-Mokhtar M, El Gabardi S, Douira A, Wahbi S, Outzourhit A, Meddich A (2022) Arbuscular mycorrhizal fungi and/or organic amendment enhance the tolerance of prickly pear (Opuntia ficus-indica) under drought stress. J Arid Environ 199:104703. https://doi.org/10.1016/j.jaridenv.2021.104703
Lemichez S (2020) Comprendre les performances et l’adaptation de deux variétés-populations de tomate (S. lycopersicum) dans un système agroforestier via l’étude du microbiome racinaire dans le cadre d’une recherche participative
Ma J, Janoušková M, Yan Y, Yu XC, Li YS, He CX (2018) Arbuscular mycorrhizal fungi (AMF) increase carbohydrate content in cucumber subjected to low temperature stress. In: XXX International Horticultural Congress IHC2018: III International Symposium on Innovation and New Technologies in Protected 1271, pp 359–364
Mathur S, Tomar RS, Jajoo A (2019) Arbuscular Mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth Res 139:227–238. https://doi.org/10.1007/s11120-018-0538-4
Meddich A, Ait El Mokhtar M, Bourzik W, Mitsui T, Baslam M, Hafidi M (2018) Optimizing growth and tolerance of date palm (Phoenix dactylifera L.) to drought, salinity, and vascular fusarium-induced wilt (Fusarium oxysporum) by application of arbuscular mycorrhizal fungi (AMF). In: Root biology, pp 239–250 https://doi.org/10.1007/978-3-319-75910-4
Meddich A, Ait Rahou Y, Boutasknit A, Ait-El-Mokhtar M, Fakhech A, Lahbouki S, Benaffari W, Ben-Laouane R, Wahbi S (2021) Role of mycorrhizal fungi in improving the tolerance of melon (Cucumus melo) under two water deficit partial root drying and regulated deficit irrigation. Plant Biosyst 156:469–479. https://doi.org/10.1080/11263504.2021.1881644
Meena M, Swapnil P, Nandan Y, Andleeb T, Meena M, Swapnil P, Divyanshu K, Kumar S, Tripathi YN, Zehra A, Marwal A, Upadhyay RS (2020) PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J Basic Microbiol 60:828–861. https://doi.org/10.1002/jobm.202000370
Mishra A, Bruno E, Zilberman D (2021) Compound natural and human disasters: Managing drought and COVID-19 to sustain global agriculture and food sectors. Sci Total Environ 754:142210. https://doi.org/10.1016/j.scitotenv.2020.142210
Molero G, Tcherkez G, Roca R, Mauve C, Cabrera-Bosquet L, Araus JL, Nogués S, Aranjuelo I (2019) Do metabolic changes underpin physiological responses to water limitation in alfalfa (Medicago sativa) plants during a regrowth period? Agric Water Manag 212:1–11
Musolino DA, Massarutto A, de Carli A (2018) Does drought always cause economic losses in agriculture? An empirical investigation on the distributive effects of drought events in some areas of Southern Europe. Sci Total Environ 633:1560–1570
Nadal M, Flexas J (2019) Variation in photosynthetic characteristics with growth form in a water-limited scenario: Implications for assimilation rates and water use efficiency in crops. Agric Water Manag 216:457–472
Ojuederie O, Olanrewaju O, Babalola O (2019) Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: Implications for sustainable agriculture. Agronomy 9:712. https://doi.org/10.3390/agronomy9110712
Paradelo R, Basanta R, Barral MT (2019) Water-holding capacity and plant growth in compost-based substrates modified with polyacrylamide, guar gum or bentonite. Sci Hortic 243:344–349. https://doi.org/10.1016/j.scienta.2018.08.046
Paucek I, Pennisi G, Pistillo A, Appolloni E, Crepaldi A, Calegari B, Spinelli F, Cellini A, Gabarrell X, Orsini F (2020) Supplementary LED interlighting improves yield and precocity of greenhouse tomatoes in the Mediterranean. Agronomy 10:1002
Pirzadah TB, Malik B, Tahir I, Rehman RU, Hakeem KR, Alharby HF (2019) Aluminium stress modulates the osmolytes and enzyme defense system in Fagopyrum species. Plant Physiol Biochem 144:178–186
Pisoschi AM, Pop A, Iordache F, Stanca L, Predoi G, Serban AI (2021) Oxidative stress mitigation by antioxidants—An overview on their chemistry and influences on health status. Eur J Med Chem 209:112891
Sadeghi F, Samsampour D, Askari Seyahooei M, Bagheri A, Soltani J (2020) Fungal endophytes alleviate drought-induced oxidative stress in mandarin (Citrus reticulata L.): Toward regulating the ascorbate-glutathione cycle. Sci Hortic 261:108991. https://doi.org/10.1016/j.scienta.2019.108991
Saleem MH, Fahad S, Adnan M, Ali M, Rana MS, Kamran M, Ali Q, Hashem IA, Bhantana P, Ali M (2020) Foliar application of gibberellic acid endorsed phytoextraction of copper and alleviates oxidative stress in jute (Corchorus capsularis L.) plant grown in highly copper-contaminated soil of China. Environ Sci Pollut Res 27:37121–37133
Secchi F, Pagliarani C, Zwieniecki MA (2017) The functional role of xylem parenchyma cells and aquaporins during recovery from severe water stress. Plant Cell Environ 40:858–871
Shafiq S, Akram NA, Ashraf M, García-Caparrós P, Ali OM, Latef AAHA (2021) Influence of glycine betaine (natural and synthetic) on growth, metabolism and yield production of drought-stressed maize (Zea mays L.) plants. Plants 10:2540. https://doi.org/10.3390/plants10112540
Sharma S, Joshi J, Kataria S, Verma SK, Chatterjee S, Jain M, Pathak K, Rastogi A, Brestic M (2020) Regulation of the calvin cycle under abiotic stresses: an overview. In: Plant life under changing environment, pp 681–717
Silva TR, Cazetta JO, Carlin SD, Telles BR (2017) Drought-induced alterations in the uptake of nitrogen, phosphorus and potassium, and the relation with drought tolerance in sugar cane. Ciência Agrotecnologia 41:117–127. https://doi.org/10.1590/1413-70542017412029416
Somerville C, Briscoe J (2001) Genetic engineering and water. Science 292:2217
Tahiri A, Meddich A, Raklami A, Alahmad A, Bechtaoui N, Anli M, Göttfert M, Heulin T, Achouak W, Oufdou K (2022a) Assessing the potential role of compost, PGPR, and AMF in improving tomato plant growth, yield, fruit quality, and water stress tolerance. J Soil Sci Plant Nutr 22:743–764. https://doi.org/10.1007/s42729-021-00684-w
Tahiri A, Raklami A, Bechtaoui N, Anli M, Boutasknit A, Oufdou K, Meddich A (2022b) Beneficial effects of plant growth promoting rhizobacteria, arbuscular mycorrhizal fungi and compost on Lettuce (Lactuca sativa) growth under field conditions. Gesunde Pflanz 74:219–235. https://doi.org/10.1007/s10343-021-00604-z
Tejera García NA, Olivera M, Iribarne C, Lluch C (2004) Partial purification and characterization of a non-specific acid phosphatase in leaves and root nodules of Phaseolus vulgaris. Plant Physiol Biochem 42:585–591. https://doi.org/10.1016/j.plaphy.2004.04.004
Toubali S, Ait-El-Mokhtar M, Boutasknit A, Anli M, Ait-Rahou Y, Benaffari W, Ben-Ahmed H, Mitsui T, Baslam M, Meddich A (2022) Root reinforcement improved performance, productivity, and grain bioactive quality of field-droughted Quinoa (Chenopodium quinoa). Front Plant Sci. https://doi.org/10.3389/fpls.2022.860484
Trouvelot A, Kough JL, Gianinazzi V (1986) Mesure de taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In: Gianinazzi P, Gianinazzi S (eds) Physiological and genetic aspects of mycorhizical. INRA, Paris, pp 217–221
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Vurukonda SSKP, Vardharajula S, Shrivastava M, Sk ZA (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res. https://doi.org/10.1016/j.micres.2015.12.003
Wu N, Li Z, Wu F, Tang M (2016) Comparative photochemistry activity and antioxidant responses in male and female Populus cathayana cuttings inoculated with arbuscular mycorrhizal fungi under salt. Sci Rep 6:1–15. https://doi.org/10.1038/srep37663
Xie X, He Z, Chen N, Tang Z, Wang Q, Cai Y (2019) The roles of environmental factors in regulation of oxidative stress in plant. Biomed Res Int 2019:1–11
Xu H, Lu Y, Zhu X (2016) Effects of arbuscular mycorrhiza on osmotic adjustment and photosynthetic physiology of maize seedlings in black soils region of northeast China. Braz Arch Biol Technol 59:1–9. https://doi.org/10.1590/1678-4324-2016160392
Yang Y, Bi M, Nie Z, Jiang H, Liu X, Fang X, Brodribb TJ (2021) Evolution of stomatal closure to optimize water-use efficiency in response to dehydration in ferns and seed plants. New Phytol 230:2001–2010
Yaseen R, Zafar-ul-Hye M, Hussain M (2019) Integrated application of ACC-deaminase containing plant growth promoting rhizobacteria and biogas slurry improves the growth and productivity of wheat under drought stress. IJAB 21:869–878. https://doi.org/10.17957/IJAB/15.0969
Yooyongwech S, Samphumphuang T, Tisarum R, Theerawitaya C, Cha-Um S (2016) Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci Hortic 198:107–117. https://doi.org/10.1016/j.scienta.2015.11.002
Yu L, Fan J, Xu C (2019) Peroxisomal fatty acid β‑oxidation negatively impacts plant survival under salt stress. Plant Signal Behav 14:1561121
Zare M, Ordookhani K, Alizadeh O (2011) Effects of PGPR and AMF on growth of two bred cultivars of tomato. Adv Environ Biol 5:2177–2181
Zhang F, Liu M, Li Y, Che Y, Xiao Y (2019) Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci Total Environ 655:1150–1158
Zoppellari F, Malusà E, Chitarra W, Lovisolo C, Spanna F, Bardi L (2014) Improvement of drought tolerance in maize (Zea mays L.) by selected rhizospheric microorganisms. Ital J Agrometeorology 1:5–18
Acknowledgements
The authors gratefully acknowledge the research and development project supported by CNRST in the framework of the Morocco-Tunisia project. This project has also received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement N° 862555. The project “Sus-Agri-CC” was carried out under the ERA-Net Cofund FOSC (Grant N° 862555), built upon and supported by the experience from the Joint Programming Initiative on Agriculture, Food Security & Climate change (FACCE-JPI) and the ERA-Net Cofund LEAP-Agri. Also, we thank Project MARBIO (Funded by the Ministry of Environment, Morocco) for its support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
S. Lamaizi, A. Meddich, A. Boutasknit, M. Anli, S. Lahbouki, L. El Fels, Y. Ouhdouch and M. Hafidi declare that they have no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lamaizi, S., Meddich, A., Boutasknit, A. et al. Application of Olive-Mill-Wastewater-Compost in Combination with Symbiotic Microorganisms Improves the Physiological, Biochemical Performance and Tolerance of Tomato (Solanum lycopersicum) Under Drought Stress. Gesunde Pflanzen 75, 1719–1735 (2023). https://doi.org/10.1007/s10343-022-00824-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-022-00824-x