Abstract
Arbuscular mycorrhizal fungi (AMF) establish a mutualistic symbiosis with several plants and play a key role in improving plant growth, tolerance to abiotic and biotic stresses as well as the soil structure. This work aimed at elucidating the AMF temperature stress modulating impact on four pearl millet lines plant growth and soil aggregation. Experimental trials were carried out in both greenhouse and growth chamber to determine the response of the four millet lines to inoculation with two AMF strains (Rhizophagus aggregatus and Funneliformis mosseae) under heat and non-stress conditions. We first investigated the mycorrhizal colonization (MC) and the mycorrhizal growth response (MGR) of millet lines in relation with their soil aggregation potential (root adhering soil/root biomass, MAS/RB) in the greenhouse. Secondly, the four millet lines were grown in two separated growth chambers and subjected to a day/night temperature of 32/28 °C as the control treatment and 37/32 °C as the temperature stress treatment. Plant growth, mycorrhization rate and several physiological, mycorrhizal and soil parameters were measured. Results showed that the mycorrhization rates of millet lines were low and not significantly different. Funneliformis mosseae (31.39%) showed higher root colonization than Rhizophagus aggregatus (22.79%) and control (9.79%). The temperature stress reduced the mycorrhizal colonization rate, shoot and root biomass, and the soil aggregation for all tested lines. L220 and L132 showed more MC rate and MGR than the other lines under control and high-temperature treatment. The MGR was significantly better under temperature stress conditions than in the control. Under the temperature stress conditions, inoculation with R. aggregatus and F. mosseae increased chlorophyll concentration, root dry weight and shoot dry weight as compared to non-inoculated plants. AMF inoculation, particularly with F. mosseae had a positive influence on the tolerance of millet lines to temperature stress. This study demonstrates that AMF play an important role in the response of these four millet lines to temperature stress. AMF is therefore an important component in the adaptation of crops to climatic variations in Sub-Saharan Africa.
Zusammenfassung
Arbuskuläre Mykorrhizapilze (AMF) gehen mit verschiedenen Pflanzen eine wechselseitige Symbiose ein und spielen eine Schlüsselrolle bei der Verbesserung des Pflanzenwachstums, der Toleranz gegenüber abiotischen und biotischen Stressfaktoren sowie der Bodenstruktur. Ziel dieser Arbeit war es, den Einfluss von AMF auf das Pflanzenwachstum und die Bodenaggregation von vier Perlhirse-Linien zu untersuchen. Experimentelle Versuche wurden sowohl im Gewächshaus als auch in der Wachstumskammer durchgeführt, um die Reaktion der vier Hirsesorten auf die Inokulation mit zwei AMF-Stämmen (Rhizophagus aggregatus und Funneliformis mosseae) unter Hitze- und Nichtstressbedingungen zu bestimmen. Zunächst untersuchten wir im Gewächshaus die Mykorrhizabesiedlung (MC) und die Mykorrhizawachstumsreaktion (MGR) der Hirsesorten in Abhängigkeit von ihrem Bodenaggregationspotenzial (Wurzelanhaftung/Wurzelbiomasse, MAS/RB). Im Anschluss wurden die vier Hirsesorten in zwei getrennten Wachstumskammern angebaut und einer Tages‑/Nachttemperatur von 32/28 °C als Kontrollbehandlung und 37/32 °C als Temperaturstressbehandlung unterzogen. Das Pflanzenwachstum, die Mykorrhizierungsrate und verschiedene physiologische, Mykorrhizierungs- und Bodenparameter wurden gemessen. Die Ergebnisse zeigten, dass die Mykorrhizierungsraten der Hirsesorten niedrig waren und sich nicht signifikant unterschieden. Funneliformis mosseae (31,39 %) zeigte eine höhere Wurzelbesiedlung als Rhizophagus aggregatus (22,79 %) und die Kontrolle (9,79 %). Der Temperaturstress reduziert die Mykorrhizabesiedlungsrate, die Spross- und Wurzelbiomasse sowie die Bodenaggregation bei allen getesteten Linien. L220 und L132 zeigten eine höhere MC-Rate und MGR als die anderen Linien unter der Kontroll- und Hochtemperaturbehandlung. Die MGR war unter Temperaturstressbedingungen signifikant besser als in der Kontrollgruppe. Unter den Temperaturstressbedingungen erhöhte die Inokulation mit R. aggregatus und F. mosseae die Chlorophyllkonzentration, das Wurzeltrockengewicht und das Sprossen-Trockengewicht im Vergleich zu nicht inokulierten Pflanzen. Die Beimpfung mit AMF, insbesondere mit F. mosseae, hatte einen positiven Einfluss auf die Toleranz der Hirsepflanzen gegenüber Temperaturstress. Diese Studie zeigt, dass AMF eine wichtige Rolle bei der Reaktion dieser vier Hirsesorten auf Temperaturstress spielen. AMF ist daher eine wichtige Komponente bei der Anpassung von Nutzpflanzen an Klimaschwankungen in Afrika südlich der Sahara.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Sub-Saharan Africa, cereal crops play a crucial role in ensuring food security (Gaye 1994). Millet (Pennisetum glaucum L. R. Br.) is one of the cereals grown in the arid and semi-arid zones of Africa and Asia (FAO, 2009). In Senegal, it is the most cultivated cereal, occupying ~71% of the land allocated to cereals, and accounts for ~60% of total cereal production. However, despite the country’s agricultural potential, millet productivity is still low (Kane et al. 2016). This low productivity is associated with agricultural land degradation (Traoré and Bagayogo 2002), reduced fallow duration and the crop monoculture practice (Bationo et al. 1993; Samaké et al. 2005). These factors result in a decreased soil fertility, and thus, low productivity (Buresh et al., 1997). Faced with this problem, sustainable management of soil fertility is essential to increase millet yields (Gaston et al. 2016; Naoura et al. 2014).
The symbiotic association between plants and soil microorganisms plays an important role in improving plant growth and increasing the productivity of agrosystems (Dirlewanger et al. 2002). In addition to ecosystem functions, soil microorganisms are also involved in improving soil structure through the formation and stabilization of aggregates (Bethlenfalvay and Barea 2009; Rillig and Mummey 2006). The role of soil microorganisms in the soil aggregation process is influenced by plant species and activities through root architecture and rhizodeposition (Bronick and Lal 2005). However, the quantity and quality of exudates produced depend on the photosynthetic activity, the genotype and the plant growth stage (Aulakh et al. 2001). Besides, Chaney and Swift (1984) showed that plant roots and fungal mycelium are actively involved in the soil aggregation process. Alone, they control ~40% of soil aggregation.
The influence of plants on soil microbial activity and soil aggregation has been widely documented on maize (Aira et al. 2010), wheat (Kaci et al. 2005) and sunflower (Alami et al. 2000). Phenotyping of pearl millet lines for rhizospheric soil aggregation potential reveled contrasted lines. for this lines, the soil-aggregation potential was correlated to the diversity and activity of rhizospheric bacterial communities producing exopolysaccharides (Ndour et al. 2017). However, several studies have reported that arbuscular mycorrhizal fungi affect microbial community structure and rhizospheric soil aggregation (Marschner and Baumann 2003). in addition, the formation and function of mycorrhizal relationships are affected by edaphic conditions such as soil composition, moisture, temperature, pH, Cation Exchange Capacity (CEC), and anthropogenic stressors (Entry et al. 2002). There is limited information of the effect of temperature on the functioning of mycorrhizal symbiosis on millet and its effect on soil aggregation. It was reported that the rise of the temperature can induce an increase the AMFs’ internal and external structures (Heinemeyer et al. 2004; Compant et al. 2010; Zhu et al. 2011).
In this study, we investigate the mycorrhization capacity of four millet lines and assess the effects of temperature stress on physiological parameters, mycorrhizal colonization and the mycorrhizal growth response of millet lines. We evaluated also the effect of high temperature treatments on the rhizosphere soil aggregation. We hypothesized that AMF inoculation could alleviate negative effects of temperature stresses on millet lines.
Materials and Methods
Biological Materials
The plant material consisted of four millet lines with contrasting potential for the soil aggregation trait. These include L220 and L3 (low soil aggregation potential) and L132 and L253 (high soil aggregation potential). They are isogenic lines phenotyped based on their soil aggregation potential (Ndour et al. 2017). The seeds were produced by the International Crops Research Institute for the Semi-Arid Tropics (ICRISAT) and obtained from the “Institut de Recherche pour le Développement (IRD)” research center in Senegal throughout the “Laboratoire d’Ecologie Microbienne des Sols et Agro Systèmes Tropicaux (LEMSAT)”. The fungal material consisted of AMF strains from the “Laboratoire Commun de Microbiologie” (LCM)’s collection. Two strains including Rhizophagus aggregatus and Funneliformis mosseae were selected and multiplied separately in the greenhouse on maize (Zea mays L) as a host plants (Tian et al. 2013; Ndeko et al. 2019). These were grown 4 months on sterilized sandy soil (autoclaved at 120 °C for 20 min) from Bambey (Table 1). These strains were selected based on their high effectiveness in improving growth, nutrition and drought tolerance on many plant species (Wright et al. 2005; Tian et al. 2013). The inoculum produced was a mixture of infected root fragments, sand, spores and mycelia from the trap cultures (crops) and contained ~40 and 55 spores g−1 for R. aggregatus and F. mosseae, respectively. After production, the inoculum of AMF strains were stored at 4 °C in a cold storage chamber for 20 days before use.
Experimental Design and Growth Conditions
Two independent experiments were carried out in both the greenhouse and culture chamber to compare the ability of four pearl millet lines to mycorrhizal inoculation and assess the effect of temperature stress on mycorrhization and soil aggregation on pearl millet lines, respectively. The greenhouse experiment was conducted from January to April 2018, while the culture chamber experiment was conducted from April to August 2018 at the “Laboratoire Commun de Microbiologie” (IRD/ISRA/UCAD) located at BelAir, Senegal. The soil used in these experiments was collected from the experimental site of the National Centre for Agricultural Research (CNRA/ISRA) at Bambey region (14◦42′38.7″N and 16◦28′47.2″W), located in the “Bassin Arachidier” of Senegal where millet is widely cultivated. The soil was an aerosol type (FAO 2006) whose physical and chemical compositions are presented in Table 1. Physicochemical and microbiological analyses of the soil samples were carried out respectively in the “Laboratoire des moyens analytiques” (LAMA) and in the Common Microbiology Laboratory (IRD/ISRA/UCAD) located in the IRD/ISRA/UCAD research center at Bel-Air, in Dakar, Senegal.
Experiment 1: Response of Four Pearl Millet Lines to Mycorrhizal Inoculation
Experimental Design
This experiment was conducted in greenhouse conditions using a two-factor completely randomized block design. The factors included: (a) mycorrhizal inoculation (R. aggregatus, F. mosseae and the control treatment without inoculation) and (b) genotype with 4 millet lines (L3, L220, L132 and L253). The combination of factors gave a total of 12 treatments (Inoculation × Lines). Ten replications (equivalent to 10 plants) were used for each treatment. The 12 treatments were as follows: (1) L3-Non-AMF plants, (2) L3 + R. aggregatus, (3) L3 + F. mosseae, (4) L220-Non-AMF plants, (5) L220 + R. aggregatus, (6) L220 + F. mosseae, (7) L132-Non-AMF plants, (8) L132 + R. aggregatus, (9) L132 + F. mosseae, (10) L253-Non-AMF plants, (11) L253 + R. aggregatus, and (12) L253 + F. mosseae. The choice of AMF strains was made on the basis of their performance in previous studies. In order to properly measure soil aggregation, plants were grown in special bottomless pots made of two parts that could be easily detached without altering the plant root system. Each pot was filled with 1.5 kg of soil and inoculated before sowing on non-sterilized soil. Five seeds were sown per pot and thinned to one plant 10 days after seedling emergence. Used seeds were disinfected with a sodium hypochlorite solution (8% active chloride, 15 min) and rinsed three times with sterilized (at 121 °C for 15 min) deionized water (for 10 min). In total, the experiment consisted of 10 non-AMF plants, 10 plants inoculated with R. aggregatus, and 10 plants inoculated with F. mosseae for each millet line. For each AMF strain, 30 g of inoculum were used for AM inoculation in pots, while plants without AM inoculation were supplemented with 30 g of autoclaved (dead) inoculum. The inoculum was placed at ~4 cm deep in contact with seeds. Pots were placed 20 cm apart on the greenhouse bench to prevent contamination. Plants were watered daily as needed at the 80% of the field capacity with distillated water throughout the whole period of the experiment. Plants were grown until physiological maturity before they were harvested.
Data Collection
The above ground (shoot) and underground (root) biomasses, mycorrhizal colonization and soil aggregation were measured at the 60th day after sowing. At harvest, roots were carefully removed from the soil and washed under running water and separated from the aerial parts. The dry weight of above and underground biomasses was determined after drying in an oven at 65 °C for 72 h. In addition, the total biomass including, the above- and below-ground biomasses, was determined using a weighing balance. Some root samples (2 g) were collected to assess the levels of mycorrhizal colonization of millet lines.
The assessment of the mycorrhizal colonization rate of a millet line was performed on root samples taken from inoculated and non-inoculated plants. At harvest, root samples were rinsed with distilled water, taken and preserved in 70% ethanol before staining (Heinemeyer et al. 2004). The collected roots were placed in tubes and stained according to Pierre et al. (2020). The root samples were then placed in a 10% KOH solution (W/V) and incubated in a 90 °C water bath for 1 h. Roots were then soaked in a 0.05% Trypan blue solution and the tubes were further incubated in a water bath at 80 °C for 30 min. Measurement of the AMF root colonization rate was performed using the root intersection microscopy method with a compound stereo microscope as reported by Jerbi et al. (2020). Finally, the response of millet lines to inoculation with different arbuscular mycorrhizal fungus was determined by the calculation of the Mycorrhizal Growth Response (MGR) according to Raya-Hernández et al. (2020). The following formula was used:
With MGR: Mycorrhizal Growth Response, TB (Myc): the total biomass of inoculated plants and TB (Non Myc): the total biomass of uninoculated plants.
Soil Aggregation Measurement
At harvest, the two parts of the pot were detached and the mass of adhering soil to the roots was determined. The plants were attached to an electric agitator (S50, CAT, Staufen, Germany) and shaken at a constant speed (1100 rpm/min) for one minute to separate the mass of non-adhering from adhering soil (see Fig. SM1 in Supplementary Material). The root adhering soils were collected on cups with sterile distilled water and dried at 105 °C for 72 h and then weighed. Soil aggregation was determined by the ratio of the mass of adhering soil (MAS) and the root biomass (MAS/RB).
Experiment 2: Assessment of the Effect of Temperature Stress On Mycorrhization and Soil Aggregation in Millet Lines
Experimental Design
This experiment were conducted using two controlled growth chambers, model E15 with a comp 3244 controller (Conviron, Controlled Environments Ltd., Manitoba, Canada), to evaluate the effect of temperature stress and AMF inoculation on millet lines growth, mycorrhizal colonization, MGR and soil aggregation. The experiment was a three-factor completely randomized block design. The factors included: (a) millet lines (L3, L220, L132 and L253), (b) mycorrhizal inoculation (R. aggregatus, F. mosseae and control treatment without inoculum) and (c) temperature treatment (high temperature treatment: 37/33 °C and control treatment: 32/28 °C). The combination of factors generated a total of 24 treatments (Lines × Inoculation × Temperature stress) conducted with 10 replications (equivalent to ten plants per treatment).
The millet plants were grown in WM pots of 2.0-litre volume capacity filled with the soil from Bambey (Table 1). Five seeds per pot were sown, followed by thinning to one plant per pot 10 days after sowing (DAS). The conditions within the control chamber were maintained at optimum temperature: 32/28 °C (day/night temperature). The photoperiod was 16 h, and the photosynthetic photon flux density (PPFD) was ~300 μmol m−2 s−1. On the other hand, the temperature stress chamber as set at 37/28 °C (day/night temperature) while other parameters were maintained as in the control treatment. The relative humidity (RH) in both chambers was between 40 and 65%. All plants were grown in the control and high temperature chambers for 60 days. In both chambers, pots were randomly moved every week to avoid the environmental effects within the chambers.
Data Collection
All plants per treatment were harvested 60 days after sowing. Growth (plant high, shoot biomass, root biomass, leaf area), physiological (chlorophyll concentration) and mycorrhization (mycorrhizal colonization rate, MC) parameters were measured. The MGR of millet lines and the rhizospheric soil aggregation (Masse of adhering soil/Root biomass, MAS/RB) were also determined. The whole plant was harvested followed by separation of the above-ground part and the root system. Dry weights were recorded after drying the plant parts at 65 °C for 72 h to determine the shoot and root biomasses. Plant height and the leaf area were recorded before harvesting. The leaf area was measured by the “Length-Width” method which considered the number of leaves N and the average of the “Length × width” products of the selected leaves (3 leaves per plant) according to (Persaud et al. 1993). Finally, chlorophyll concentration was measured before harvesting using the SPAD-502Plus chlorophyll meter (Uddling et al. 2007). The root samples were washed in tap water, stored in ethanol (70%) and maintained at 4 ˚C before measuring the AMF colonization.
Data Treatment and Analysis
The statistical analysis was carried out using R 3.6.1 and Microsoft excel® XLSTAT package (XLSTAT paris, 2017). For the first experiment, plant growth, soil and mycorrhizal parameters (shoot dry weight, root dry weight, mycorrhizal colonization and mycorrhizal growth response) were analyzed by a two-way ANOVA (p < 0.05). For the second experiment, physiological, growth, soil and mycorrhizal parameters (root dry weight, shoot dry weight, plant height, leaf area, chlorophyll concentration, mycorrhizal colonization, MGR and soil aggregation) were analyzed by a three-way ANOVA under a randomized complete block design. Differences among the treatments’ means were separated with the Turkey HSD test at 5% threshold.
Results
Experiment 1: Mycorrhizal Colonization (MC) and the Mycorrhizal Growth Response (MGR) of Millet Lines Under Greenhouse Conditions
Mycorrhizal Colonization of Millet Lines as Influenced by AMF Strains
Root microscopic examination revealed that mycorrhizal colonization rate was generally low (< 50%) and not significantly different (p = 0.059) among the tested lines (Fig. 1a). AMF significantly affected (p = 0.045) the mycorrhizal colonization rate of millet lines. F. mosseae (32.9%) showed high percentage of root colonization compared to R. aggregatus (22.8%) and non-inoculated (Control) (9.8%). The use of non-sterile soil in this study, and non-inoculated plants also was also colonized by AMF due to the presence of some local soil-native AM species.
Arbuscular mycorrhizal fungal colonization (a), plant shoot biomass (b) and plant root biomass (c) of pearl millet lines as affected by AMF strains inoculation (Rhizophagus aggregatus, RA, and Funneliformis mosseae, FM). Values are means (±SE) of six replicates. Difference between treatments were assessed by the two-way ANOVA (P < 0.05)
Growth and Physiological Traits of Millet Lines Under Greenhouse Conditions
There was a significant difference in the shoot biomass (p = 0.005). However, the root biomass was not affected by lines L220 accumulated more shoot biomass than other lines. There were no significant effects (Table 2) of AMF inoculation on the shoot and root biomasses (shoot and root dry weight). The interaction between the tested lines and the AMF inoculation was not significant for the shoot and root dry weights (Fig. 2). This result suggest that the AMF colonization rate was an indicator of the activity of the mycorrhizae in pearl millet lines.
Mycorrhizal growth response (MGR, (a)) and mass of adhering soil/root biomass (soil aggregation, (b)) of millet lines as a function of AMF strains inoculation (Rhizophagus aggregatus, RA, and Funneliformis mosseae, FM) under greenhouse conditions. Values are means (±SE) of six replicates. Difference between treatments were assessed by the two-way ANOVA (P < 0.05)
Mycorrhizal Responsiveness of Millet Lines Under Greenhouse Conditions
The pearl millet lines responded differently to inoculation with AMF strains. Shoot growth responsiveness differed among lines when the plant roots are colonized by AMF. Overall, the mycorrhizal growth response (MGR %) of millet lines ranged from +57% to −84% (Fig. 3). The results showed a significant difference (P = 0.003) in the mycorrhizal growth response among the millet lines. L220 and L253 had highest and positive MGR values when colonized by R. aggregatus (+57%) and F. mosseae (47.4%). L220 recorded highest MGR response values compared to other genotypes (Table 2).
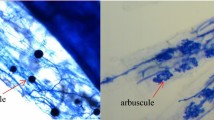
Mycorrhizal root colonization of pearl millet lines by Funneliformis mosseae (a, b, c, e, f, g) and Rhizophagus aggregatus (d, h) under control and high temperature treatments in culture chamber. a L253 at 32–28 °C (day-night temperature), b L220 at 32–28 °C, c L132 at 32–28 °C, d L3 at 32–28 °C, e L253 at 37–32 °C, f L220 at 37–32 °C, g L132 at 37–32 °C, h L3 at 37–32 °C. Arrow: vesicles; triangle: hyphae; circle: intraradical spore
Effects of AMF Inoculation On Rhizospheric Soil Aggregation (MAS/RB) of Millet Lines
Soil aggregation varied widely among millet lines (p < 0.0001). L132 and L253 had high than L3 and L220 (Fig. 4). In L253, control plants showed low MAS/RB values compared to plants inoculated with R. aggregatus and F. mosseae, which showed a positive effect of inoculation on soil aggregation.
Experiment 2: Effect of Temperature Stress On Mycorrhizal Growth Response of Pearl Millet Lines
Effect of Temperature Stress and AMF Inoculation On Growth and Physiological Traits of Pearl Millet Lines
Pearl millet plant height decreased by ~12% under the high-temperature treatment (p < 0.001) and varied significantly among lines (p < 0.001) (Table 3). L3, L220 and L253 showed high plant heights in both high (37/32 °C) and normal temperature (32/28 °C). Leaf area increased significantly in the high-temperature treatment but not different among lines. Chlorophyll concentration was reduced significantly (p < 0.001) with the high-temperature and among lines. L3 exhibited highest chlorophyll concentration while L253 and L132 had lowest levels. Root biomass (root dry weight) decreased by 39.1% (L132), 27% (L220), 37.8% (L3), and 58.6% (L253) under high-temperature treatment. A significant interaction was observed between lines and treatments for root biomass (p = 0.044). L253 had accumulated highest root biomass under non-stress conditions. The rising of temperature decreased root biomasses in L3, L132 and L253. For L220, however, no significant difference between the control (32/28 °C) and high-temperature treatments (37/32 °C) was observed (Table 3). Shoot biomass (shoot dry weight) also decreased significantly under temperature stress conditions (p = 0.046), but no differences were founded between lines (Table 3). The decline in shoot dry weight was more pronounced on L253 (34.9%) and L132 (32.7%) under temperature stress conditions than on L3 (11.2) and L220 (20.3%).
The effect of AMF inoculation on plant height, leaf area, chlorophyll concentration, shoot biomass and root biomass varied with millet lines under temperature treatments (Table 3). For all lines, plant height was unaffected by either of AM inoculation when plant was exposed to optimal (control) temperature. At high temperature treatment, lines showed increased plant height with AMF inoculation, but there were no significant differences between F. mosseae and R. aggregatus. On L3, L132 and L253, leaf area significantly increased with AMF inoculation under high temperature treatment only. On L220, AMF inoculation affected leaf area in both high and control treatments. Plants inoculated with F. mosseae showed increased leaf area than R. aggregatus and uninoculated plants. Chlorophyll concentration differed between AM-inoculated and non-inoculated plants in L220 under both high and control temperature treatments. Chlorophyll concentration increased with AM inoculation but the statistical analysis displayed no significant differences between R. aggregatus and F. mosseae. On L3 and L253, chlorophyll concentration significantly increased with AMF inoculation only under high temperature treatment. On L132, AMF inoculation did not affect the chlorophyll concentration in both high and control temperature treatments. Shoot biomass did not vary among millet lines but significantly increased with AM inoculation (p = 0.049) in L220 and L253 regardless of the thermal regime. On the other hand, AMF applications did not increase significantly the root and shoot dry weights under control treatment (non-stress conditions) for L3 and L132 lines. However, when exposed to temperature stress, plants inoculated by R. aggregatus and F. mosseae showed a significant rise in shoot biomass as compared to uninoculated plants. AMF inoculation affected significantly the root biomass of millet lines (p = 0.04). At high temperature treatment, only L220, L132 and L3 had increased root biomass when inoculated with AMF strains. However, for L253, inoculation only had a significant effect under non-stress conditions.
Effect of Temperature Stress On Mycorrhizal Colonization Rate and Mycorrhizal Growth Response (MGR) and Soil Aggregation
This study showed a significant difference (p < 0.001) in the percentage of root colonization between control and high-temperature treatments. Mycorrhizal colonization significantly decreased in high-temperature treatment (Fig. 3 and Table 4). A significant difference (p = 0.003) in mycorrhizal colonization was also observed between the millet lines. L220 and L132 (47.7% and 44.2%, respectively) showed more mycorrhizal colonization as compared to other genotypes under control and high-temperature treatments. No significant interaction between genotype and heat treatment was detected. The mycorrhizal growth response significantly (p = 0.005) increased with high-temperature treatment and significantly differed (p = 0.02) among millet genotypes. Results showed that the greatest and most positive mycorrhizal growth response was from L220 and L132 compared to other genotypes in high-temperature treatment (Table 4). Negative MGR values were observed on L132, L3, and L253 in the control treatment (32/28 °C). In general, we observed that MGR was significantly better under temperature stress than in the control treatment and varied among lines. AMF mycorrhizal inoculation significantly affected root colonization and the mycorrhizal growth response. We also observed significant interaction between lines, temperature stress and mycorrhizal inoculation (p = 0.018). Regardless of the thermal regime applied, AMF inoculation significantly improved the mycorrhization rate of all lines. High mycorrhizal colonization and mycorrhizal response were obtained by using F. mosseae.
Effect of Temperature Stress On the Soil Aggregation (MAS/RB) in the Rhizosphere of Pearl Millet Lines
Results from this study (Table 4) showed that the soil aggregation was reduced significantly (p < 0.001) under high-temperature treatment and was also significantly different (p < 0.001) among genotypes. L132 and L253 recorded the highest MAS/RB ratio in both control and high temperature treatments while L3 and L220 had lowest MAS/RB. The inoculation did not significantly influence the MAS/RB ratio regardless the heat treatment. There were no significant effects of the interaction between AMF inoculation and temperature treatments for the soil aggregation. The differences observed in soil aggregation among lines could be explained by line-specific traits for this variable (Table 4). However, the inoculation seemed to positively influence soil aggregation regardless of the temperature treatment. For L220, soil aggregation increased in high-temperature treatment when root was colonized by F. mosseae (Fig. 4). Whereas, for L132, a depressive effect of inoculation on soil aggregation was observed and we had no visible effect on L3.
The Pearson’s correlation analysis was used to assess the relationship between AMF colonization and all growth and physiological parameters (Fig. 5). Under the control treatment, the results indicate that no significant correlation was observed between mycorrhizal colonization and the growth parameters of the millet lines. In contrast, under the temperature stress treatment, mycorrhizal colonization of millet lines was highly and positively correlated with plant height (r = 0.46), chlorophyll concentration (r = 0.69), shoot biomass (r = 0.67), root biomass (r = 0.53) and MGR (0.44). There were no significant relationships between number of leaves, leaf area, MAS/RB (soil aggregation) and AMF colonization.
Pearson’s correlation matrix between growth, physiological parameters, soil aggregation and AMF root colonization. Plant height (Pheight), number of leaves (Nleaves), leaf area (Larea), hhlorophyll concentration (Chl), shoot biomass (SB), root biomass (RB), mycorrhizal colonization rate (MC), mycorrhizal growth response (MGR) and mass of adhering soil/root biomass (MAS/RB)
Discussion
Arbuscular Mycorrhizal Fungal Colonization of Pearl Millet Lines
The symbiotic relationship between plants and AMF is the oldest one existing in terrestrial ecosystems and involved in enhancing plant growth, mineral nutrition and stress tolerances (Fahey et al. 2016; Begum et al. 2019). Its role in enhancing stress tolerance was reported by other studies but they did not consider the effect of plant genotypes. This study highlighted differences in responses of millet lines to inoculation with two AMF strains and the significant effect of temperature stress on mycorrhization, plant growth and soil aggregation. The result reveals that, only L220 and L253 responded positively to all inoculated AMF strains. This response would be the result of an improvement in the nutritional status of the plants ensured by developing a network of extraradicial hyphae of the fungus and by the extension of the soil volume explored by the roots (Benjelloun et al. 2014). Early reports showed that cultivars react differently to an AMF strain (Seifi et al. 2014). Similar effects on mycorrhization have been observed in fonio (Ndoye et al. 2016), cowpea (Diop et al. 2013), tamarind (Bourou et al. 2011), and maize (Miller and Yastrow 2000). In date palms, Zougari-Elwedi et al. (2012) observed an increase in biomass (+45%) and in the levels of phosphorus, nitrogen, potassium, copper and zinc in inoculated young plants. This is in line with Plenchette et al. (1999) who concluded that millet have a low mycorrhizal dependency although growth stimuli are observed (El Mrabet et al. 2017). Higher rates of mycorrhizal colonization have been observed in other cereals in controlled conditions (Nouaim and Chaussod 1996). This suggests that there is a high degree of variability between different millet lines in their response to mycorrhizal inoculation. Several factors can affect mycorrhizal colonization and the response of plants to mycorrhizal inoculation. According to Sensoy et al. (2007), the genotype of the host plant is an important determinant of the response to mycorrhizal inoculation.
Arbuscular Mycorrhizal Fungal Colonization of Pearl Millet Lines in Relation to the Soil Aggregation
Soil aggregation is an important aspect of ecosystem functioning in terrestrial ecosystems. Arbuscular mycorrhizal fungi (AMF) play a key role in soil aggregate formation and stabilization (Rillig and Mummey 2006). In the present study we find that millet lines maintained their soil aggregation potential regardless of whether or not mycorrhizal fungi are applied. Lines 132 and 253 had high MAS/RB values while lines 3 and 220 had low values. Inoculation with AMF strains did not significantly influence the MAS/RB ratio of millet lines. The contribution of AMFs to soil aggregation and aggregate stabilization depends largely on the textural characteristics of the soil under consideration (Miller and Yastrow 2000). Inoculation with an AMF strain has different effects on aggregation depending on the soil type. Under these conditions, sandy soil and loamy soils have very poor aggregation compared to organic soil (Bethlenfalvay and Barea 2009). In our context, the characteristics of the soil and plant genotypes used could have an impact on mycorrhiza formation and on the production of glomalin, a glycoprotein produced by the AMF and involved in the soil aggregation process (Wright et al., 2007). In fact, mycorrhizal establishment produce an increase in glomalin concentration and there is a positive correlation between Glomalin concentration, soil aggregation and AMF root colonization (Bedini et al. 2009). Early reports showed soil that structure determined the level of soil aggregation. The same results in pea, inoculation of pea plants with Funneliformis mosseae increased the level of soil aggregation more significantly in a silty soil (400%) than in a clay soil (50%). On the other hand, the root systems of plants and their rhizospheres have a great influence on soil aggregation. The mechanisms involved include root penetration, modification of the soil water regime, root exudation, decomposition of dead roots and root entanglement, which vary widely between plant genotypes (Bronick and Lal 2005). The influence of mycorrhizal fungi on soil aggregation is, therefore, only possible when carbohydrate production is not limited by other external factors, which would also limit the proper functioning of mycorrhizal symbiosis (Singh et al. 2014).
Effect of Temperature Stress and Arbuscular Mycorrhizal Fungal Inoculation On Millet Line Development and Soil Aggregation
In response to temperature stress, results showed that the mycorrhizal colonization of millet genotypes significantly decreased in high temperature treatment than in control treatment. The increase in temperature induced a reduction in mycorrhizal colonization in all millet lines. Extreme environmental conditions can negatively affect some beneficial microorganisms such as Arbuscular Mycorrhizal Fungi (Jerbi et al., 2020). Indeed, the effect of temperature stress on mycorrhization depends on the plant species and inoculated AMF strains (Heinemeyer and Fitter 2004). The effects of increased temperature on beneficial plant-associated microorganisms is more variable, positive, neutral, and negative effects can be observed, but varies according to the study system and the temperature range investigated. Temperature can also significantly alter the hyphal network structure of AMF and induce a reduction in vesicle and hyphae (Compant et al. 2010). Under high temperature treatment, the reduction of host plant’s photosynthetic capacity resulting in a low allocation of carbon to the fungus, could thus reduce the AMF colonization (Heinemeyer et al. 2006). These results disagree with those of Zhu et al. (2011) on maize grown at 25, 35, and 40 °C. Indeed, the temperature rise did not significantly reduce the mycorrhizal colonization rate of maize plants inoculated with Glomus etunicatum. However, Gavito et al. (2005) showed that a temperature increase of up to 30 °C can promote the development of AMF. In addition, Lekberg and Koide (2008) observed a reduction in the mycorrhization rate in sorghum plants inoculated with Glomus etunicatum and Funneliformis mosseae under the same conditions.
No significant effect of AMF inoculation on growth and physiological parameters was found under non-stress conditions (32/28 °C), but when exposed to temperature stress, plants inoculated by F. mosseae and R. aggregatus showed a significant rise in shoot dry weight, root dry weight, chlorophyll concentration, mycorrhizal colonization, mycorrhizal growth response and the soil aggregation. Early reports showed that AM colonization could enhance plant biomass and mineral accumulation under temperature stress conditions. Mycorrhizal colonization could enhance photosynthetic activity and plant biomass under temperature stress conditions (Heinemeyer et al. 2006). Fahey et al. (2016) found that AMF inoculation increased root, and shoot biomass and respiration rate under temperature stress, whereas the AMF effect varied among species. Our results confirm a previous study concerning the effects of different AM fungal taxa on plant growth under environmental stress (Duc et al. 2018).
Our results also showed the significant influence of temperature stress on the rhizospheric soil aggregation of millet lines. An increase in temperature significantly decreased the MAS/BR ratio in millet genotypes. In the present study, we observed that chlorophyll concentration decreased significantly under temperature stress in uninoculated treatment. Root and shoot biomass also decreased in the same conditions, implying low photosynthetic activity and reduced carbon allocation to the root zone. But the reduced carbon allocation could significantly impact root exudation and soil microbial activity, which is actively involved in the soil aggregation process (Duc et al. 2018). These authors showed that AM symbiosis considerably elevated chlorophyll concentration in tomato plants, particularly when inoculated with Septoglomus constrictum. Though, in contrast with our result, they have more effect under combined drought and temperature stress. Indeed, it is recognized that climatic parameters such as temperature and precipitation have a strong direct or indirect influence on the soil aggregation (Bronick and Lal 2005). Their influence on soil organic carbon determines the level of soil aggregation (Albrecht et al. 1998). In particular, temperature affects soil aggregation by influencing the nature of microbial populations involved in the decomposition of soil organic matter and by determining the rate of decomposition (Mataix-Solera et al. 2011). The maintenance of the level of soil aggregation in L132 and L220 under temperature stress conditions (37/32 °C) could be related to their ability to associate with AMF under these conditions. Bunn et al. (2009) obtained similar results on two thermopylic plants (Chanthelium lanuginosum and Eragrostis scabra). They suggested that the mycorrhization improves the growth of thermophilic plants under temperature stress conditions. In addition, Six et al. (2004) established a link between the root system development and soil aggregation. Plant roots and their rhizosphere have a great influence on aggregation through rhizodeposition, decomposition of dead roots and root entanglement. The results of this work suggest that mycorrhization could allow millet genotypes to maintain and improve the soil aggregation level under heat-stress conditions.
Conclusion
The present study investigated the influence of temperature stress and AMF application on mycorrhizal colonization and rhizospheric soil aggregation of millet lines. Results showed that root colonization rate with AMF was low and not different among millet lines. AMF inoculation significantly increased root colonization; Funneliformis mosseae showed the highest percentage of root colonization. Millet genotypes responded differently to inoculation with R. aggregatus and F. mosseae. However, the AMF inoculation did not significantly effect on the rhizospheric soil aggregation of the lines (MAS/RB). As observed in this study, temperature stress significantly affected all the growth and physiological parameters, and had significantly reduced the mycorrhizal colonization rate and the rhizospheric soil aggregation. AMF inoculation had no effect on plant growth under non-stress conditions, however under high temperature treatment; AMF influenced significantly plant growth, mycorrhizal colonization and the mycorrhizal growth response of millet lines. This study showed that the effect of AMF colonization on millet development differs among millet lines. L132 and L220 maintained their soil aggregation level in control and R. aggregatus and F. mosseae treatments, respectively, because of their mycorrhizal colonization rate and a positive mycorrhizal growth response under high-temperature treatment compared to other lines.
References
Aira M, Gómez-Brandón M, Lazcano C, Bååth E, Domínguez J (2010) Plant genotype strongly modifies the structure and growth of maize rhizosphere microbial communities. Soil Biol Biochem 42(12):2276–2281. https://doi.org/10.1016/j.soilbio.2010.08.029
Alami Y, Achouak W, Marol C, Heulin T (2000) Rhizosphere soil aggregation and plant growth promotion of sunflowers by an exopolysaccharide-producing Rhizobium sp. Strain isolated from sunflower roots. Appl Environ Microbiol 66(8):3393–3398
Albrecht A, Angers DA, Beare MH, Blanchart E (1998) Déterminants organiques et biologiques de l’agrégation: implications pour la recapitalisation de la fertilité physique des sols tropicaux. Agricultures 7(5):357–363
Aulakh MS, Wassmann R, Bueno C, Kreuzwieser J, Rennenberg H (2001) Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol 3(2):139–148. https://doi.org/10.1055/s-2001-12905
Bationo A, Christianson CB, Klaij MC (1993) The effect of crop residue and fertilizer use on pearl millet yields in Niger. Fertil Res 34(3):251–258
Bedini S, Pellegrino E, Avio L, Pellegrini S, Bazzoffi P, Argese E, Giovannetti M (2009) Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol Biochem 41(7):1491–1496
Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M, Ahmed N, Zhang L (2019) Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front Plant Sci 10:1068
Benjelloun S, El Harchli EH, Amrani JK, El Ghachtouli N, Fikri BK, El Yamani J (2014) Etude de l’importance de la mycorhization dans la synthèse des composés phénoliques chez le maïs ( Zea mays L.) en condition de stress hydrique. Int J Eng Sci 04(12):43–49
Bethlenfalvay GJ, Barea J‑M (2009) Mycorrhizae in sustainable agriculture. I. Effects on seed yield and soil aggregation. Am J Altern Agric 9(04):157. https://doi.org/10.1017/S0889189300005919
Bourou S, Ndiaye F, Diouf M, Van Damme P (2011) Effets de l ’ inoculation mycorhizienne sur le comportement agro-physiologique des écotypes du tamarinier ( Tamarindus indica L .) au Sénégal. J Appl Biosci 46:3093–3102
Bronick CJ, Lal R (2005) Soil structure and management: a review. Geoderma 124(1–2):3–22. https://doi.org/10.1016/j.geoderma.2004.03.005
Bunn R, Lekberg Y, Zabinski C (2009) Arbuscular mycorhizal fungi ameliorate temperature stress in thermophilic plants. Ecol Soc Am 90(5):1378–1388
Buresh RJ, Smithson PC, Hellums DT (1997) Building soil phosphorus capital in Africa. Replenishing soil fertility in Africa 51:111–149
Chaney K, Swift RS (1984) The influence of organic matter on aggregate stability in some British soils. J Soil Sci 35(2):223–230. https://doi.org/10.1111/j.1365-2389.1984.tb00278.x
Compant S, Van Der Heijden MGA, Sessitsch A (2010) Climate change effects on beneficial plant-microorganism interactions. FEMS Microbiol Ecol 73(2):197–214. https://doi.org/10.1111/j.1574-6941.2010.00900.x
Diop I, Kane A, Wade TK, Sanon KB, Neyra M, Noba K (2013) Impacts des conditions pédoclimatiques et du mode cultural sur la réponse du niébé ( Vigna unguiculata L . Walp .) à l’inoculation endomycorhizienne avec Rhizophagus irregularis. J Appl Biosci 69:5465–5474
Dirlewanger E, Cosson P, Tavaud M, Aranzana MJ, Poizat C, Zanetto A, Arús P, Laigret F (2002) Development of microsatellite markers in peach [Prunus persica (L.) Batsch] and their use in genetic diversity analysis in peach and sweet cherry (Prunus avium L.). Theor Appl Genet 105(1):127–138. https://doi.org/10.1007/s00122-002-0867-7
Duc NH, Csintalan Z, Posta K (2018) Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Phys Biochem 132:297–307
El Mrabet S, Msanda F, El Mousadik A, Ouahmane L (2017) Evaluation du pouvoir mycorhizien des sols rhizosphériques de Chamaecytisus albidus et Ononis natrix dans la production de plants de qualité d’Argania spinosa (L.) Skeels. Am J Innov Res Appl Sci 4(2):44–51.
Entry J, Rygiewicz P, Watrud L, Donnelly P (2002) Influence of adverse soil conditions on the formation and function of Arbuscular mycorrhizas. Adv Environ Res 7(1):123–138. https://doi.org/10.1016/S1093-0191(01)00109-5
Fahey C, Winter K, Slot M, Kitajima K (2016) Influence of arbuscular mycorrhizal colonization on whole‐plant respiration and thermal acclimation of tropical tree seedlings. Ecol Evol 6(3):859–870
FAO (2006) World Reference Base for Soil Resources. A Framework for International Classification, Correlation and Communication. Food and Agriculture Organization, Rome, 103 pages. Available online at: http://www.fao.org/3/a-a0510e.pdf. Accessed 20 Oct 2020
FAO W (2009) Principles and methods for the risk assessment of chemicals in food. Environ Health Criteria 240
Gaston S, Dahiratou ID, Moussa B, Dougbedji F (2016) Contribution of previous legumes to soil fertility and millet yields in West African Sahel. Afr J Agric Res 11(28):2486–2498. https://doi.org/10.5897/AJAR2016.11156
Gavito ME, Olsson PA, Rouhier H, Medina-Peñafiel A, Jakobsen I, Bago A, Azcón-Aguilar C (2005) Temperature constraints on the growth and functioning of root organ cultures with arbuscular mycorrhizal fungi. New Phytol 168(1):179–188. https://doi.org/10.1111/j.1469-8137.2005.01481.x
Gaye M (1994) Cultures céréalières dans le bassin arachidier: motivations et contraintes chez les producteurs. Institut Senegalais de Recherches Agricoles (ISRRA), Sénegal, Unival 5(2):26
Heinemeyer A, Fitter AH (2004) Impact of temperature on the arbuscular mycorrhizal (AM) symbiosis: growth responses of the host plant and its AM fungal partner. J Exp Bot 55(396):525–534. https://doi.org/10.1093/jxb/erh049
Heinemeyer A, Ridgway KP, Edwards EJ, Benham DG, Young JPW, Fitter AH (2004) Impact of soil warming and shading on colonization and community structure of arbuscular mycorrhizal fungi in roots of a native grassland community. Glob Change Biol 10(1):52–64
Heinemeyer A, Ineson P, Ostle N, Fitter AH (2006) Respiration of the external mycelium in the arbuscular mycorrhizal symbiosis shows strong dependence on recent photosynthates and acclimation to temperature. New Phytol 171(1):159–170
Jerbi M, Labidi S, Lounès-Hadj Sahraoui A, Chaar H, Ben Jeddi F (2020) Higher temperatures and lower annual rainfall do not restrict, directly or indirectly, the mycorrhizal colonization of barley (Hordeum vulgare L.) under rainfed conditions. PloS one 15(11):e0241794
Kaci Y, Heyraud A, Barakat M, Heulin T (2005) Isolation and identification of an EPS-producing Rhizobium strain from arid soil (Algeria): characterization of its EPS and the effect of inoculation on wheat rhizosphere soil structure. Res Microbiol 156(4):522–531. https://doi.org/10.1016/j.resmic.2005.01.012
Kane A, Diop D, Sylla SN, Noba K (2016) Date et densité optimales de semis du niébé [ Vigna unguiculata ( L .) Walp .] en association avec le mil [ Pennisetum glaucum ( L ... Date et densité optimales de semis du niébé [ Vigna unguiculata ( L .) Walp .] en association avec le mil [ Pennisetum g. J Appl Biosci. https://doi.org/10.4314/jab.v76i1.4
Lekberg Y, Koide RT (2008) Effect of soil moisture and temperature during fallow on survival of contrasting isolates of arbuscular mycorrhizal fungi. Botany 86(10):1117–1124. https://doi.org/10.1139/B08-077
Marschner P, Baumann K (2003) Changes in bacterial community structure induced by mycorrhizal colonisation in split-root maize. Plant Soil 251(2):279–289
Mataix-Solera J, Cerdà A, Arcenegui V, Jordán A, Zavala LM (2011) Fire effects on soil aggregation: a review. Earth Sci Rev 109(1):44–60. https://doi.org/10.1016/j.earscirev.2011.08.002
Miller RM, Yastrow JD (2000) Mycorrhizal fungi influence soil structure. In: Arbuscular mycorrhizas: physiology and function, pp 3–18 https://doi.org/10.1007/978-94-017-0776-3
Naoura G, Nebie B, Nanema RK, Kando PB, Traore ER, Sawadogo M, Zongo J (2014) Caractérisation de quelques écotypes performants de sorghos Burkinabés. Int J Biol Chem Sci 8(5):2109–2118
Ndeko AB, Basimine GC, Bagula EM, Mugumaarhahama Y, Ndusha BN, Rehema P, Nachigera GM (2019) Comparative effect of Rhizophagus irregularis strain on cassava root development and Phosphorus uptake under acidic soils conditions of Walungu territory, Eastern DR Congo. J Appl Biosci. https://doi.org/10.35759/JABs.148.1
Ndour PMS, Gueye M, Barakat M, Ortet P, Bertrand-Huleux M, Pablo A‑L, Dezette D, Chapuis-Lardy L, Assigbetsé K, Kane NA, Vigouroux Y, Achouak W, Ndoye I, Heulin T, Cournac L (2017) Pearl millet genetic traits shape Rhizobacterial diversity and modulate Rhizosphere aggregation. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01288
Ndoye F, Diedhiou AG, Gueye M, Fall D, Barnaud A, Sy MO et al (2016) Réponse du fonio blanc (Digitaria exilis Stapf) à l’inoculation avec des champignons mycorhiziens à arbuscules en conditions semi-contrôlées. J Appl Biosci 103:9784–9799
Nouaim R, Chaussod R (1996) Rôle des mycorhizes dans l’alimentation hydrique et minérale des plantes, notamment des ligneux de zones arides. Cah Options Mediterr 20:9–26
Persaud N, Gandah M, Ouattara M, Mokete N (1993) Estimating Leaf Area of Pearl Millet from Linear Measurements. Agron J 85(1):10. https://doi.org/10.2134/agronj1993.00021962008500010002x
Pierre S, Litton CM, Giardina CP, Sparks J P, Fahey TJ (2020) Mean annual temperature influences local fine root proliferation and arbuscular mycorrhizal colonization in a tropical wet forest. Ecol Evol 10(18):9635–9646.
Plenchette C, Bois J‑F, Cadet P, Duponnois R (1999) La mycorhization ( Glomus aggregatum ) du mil ( Pennisetum glaucum ). Gestion 7:379–384
Raya-Hernández AI, Jaramillo-López PF, López-Carmona DA, Díaz T, Carrera-Vltierra JA, Larsen J (2020) Field evidence for maize-mycorrhiza interactions in agroecosystems with low and high P soils under mineral and organic fertilization. Appl Soil Ecol 149:103511. https://doi.org/10.1016/j.apsoil.2020.103511
Rillig MC, Mummey DL (2006) Mycorrhizas and soil structure. New Phytol 171(1):41–53. https://doi.org/10.1111/j.1469-8137.2006.01750.x
Samaké O, Smaling EMA, Kropff MJ, Stomph TJ, Kodio A (2005) Effects of cultivation practices on spatial variation of soil fertility and millet yields in the Sahel of Mali. Agric Ecosyst Environ 109(3):335–345
Seifi E, Teymoor YS, Alizadeh M, Fereydooni H (2014) Olive mycorrhization: influences of genotype, mycorrhiza, and growing periods. Sci Hortic 180:214–219
Sensoy S, Demir S, Turkmen O, Erdinc C, Savur OB (2007) Responses of some different pepper (Capsicum annuum L.) genotypes to inoculation with two different arbuscular mycorrhizal fungi. Sci Hortic 113(1):92–95.
Singh KP, Kumar KR, Prasad KV, Pal M, Raju DVS (2014) Influence of VAM inoculation on root colonization, survival, physiological and biochemical characteristics of chrysanthemum plantlets Influence of VAM inoculation on root colonization, survival, physiological and biochemical characteristics of chrysan. Indian J Hort 65(4):461–465
Six J, Bossuyt H, Degryze S, Denef K (2004) A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res 79(1):7–31. https://doi.org/10.1016/j.still.2004.03.008
Tian H, Drijber RA, Li X, Miller DN, Wienhold BJ (2013). Arbuscular mycorrhizal fungi differ in their ability to regulate the expression of phosphate transporters in maize (Zea mays L.). Mycorrhiza 23(6):507–514
Traoré S, Bagayoko M, Coulibaly BS, Coulibaly A (2002) Amélioration de la gestion de la fertilité des sols et celle des cultures dans les zones sahéliennes de l’Afrique de l’Ouest: une condition sine qua none pour l’augmentation de la productivité et de la durabilité des systèmes de culture à base de mil. Syngenta foundation for sustainaible agriculture http://www.syngentafoundation.org.
Uddling J, Gelang-Alfredsson J, Piikki K, Pleijel H (2007) Evaluating the relationship between leaf chlorophyll concentration and SPAD-502 chlorophyll meter readings. Photosynth Res 91(1):37–46
Wright DP, Scholes JD, Read DJ, Rolfe SA (2005). European and African maize cultivars differ in their physiological and molecular responses to mycorrhizal infection. New Phytol 167(3):881–896
Wright SF, Green VS, Cavigelli MA (2007) Glomalin in aggregate size classes from three different farming systems. Soil Tillage Res 94(2):546–549
Zhu X‑C, Song F‑B, Liu S‑Q, Liu T‑D (2011) Effects of arbuscular mycorrhizal fungus on photosynthesis and water status of maize under high temperature stress. Plant Soil 346(1–2):189–199. https://doi.org/10.1007/s11104-011-0809-8
Zougari-Elwedi B, Sanaa M, Sahraoui AL-HS (2012) Évaluation de l’impact de la mycorhization arbusculaire sur la nutrition minérale des plantules de palmier dattier (Phœnix dactylifera L . var. Deglet Nour). Etude Et Gestion Des Sols 19(3+4):193–202
Acknowledgements
The authors would like to acknowledge the Laboratoire Commun de Microbiologie, LCM, and the Laboratoire d’Ecologie Microbienne des Sol d’Afrique Tropical, LEMSAT, for providing all the necessary resources to carry out this research. We also acknowledge the German Academic Exchange Service, DAAD, for awarding a scholarship to the first author to facilitate the completion of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.B. Ndeko, H. Founoune-Mboup, A. Kane and L. Cournac declare that they have no competing interests.
Supplementary Information
10343_2021_588_MOESM1_ESM.pdf
Fig SM1: Image of pearl millet lines in the field (A), soil aggregation measurement procedure (B) and pearl millet plant after de-potting (C)
Rights and permissions
About this article
Cite this article
Ndeko, A.B., Founoune-Mboup, H., Kane, A. et al. Arbuscular Mycorrhizal Fungi Alleviate the Negative Effect of Temperature Stress in Millet Lines with Contrasting Soil Aggregation Potential. Gesunde Pflanzen 74, 53–67 (2022). https://doi.org/10.1007/s10343-021-00588-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-021-00588-w