Abstract
Following wildfires, salvage logging is applied for silvicultural, safety or even aesthetic reasons. Such operations impact on biological legacies, impair natural vegetation recovery and may affect several animal species that depend on vegetation structure and specific plant resources. Rodents, such as wood and Algerian mice, use vegetation cover as protection against predators and are important prey, moderately effective seed dispersers and efficient seed predators. Different post-fire management strategies may either promote rodent abundance, enhancing their key biological roles, or result in low rodent abundance, creating a low seed predation window of opportunity when assisted regeneration of burnt forests is required. In order to evaluate the effects of post-fire salvage logging on plant-animal interactions, we compared plant regeneration, the availability of trophic resources (seeds and fleshy fruits), rodent foraging activity and rodent relative abundance between unlogged and logged burnt pine forests in the north-eastern Iberian Peninsula at different distances (up to 700 m) from the burnt area perimeter. The results show that vegetation recovered more slowly in salvage logged than in unlogged areas. Foraging activity of rodents increased both with the volume of woody debris, mainly derived from salvage logging, and with increasing foliage cover. Management strategies aimed at promoting the presence of rodents and associated biodiversity can, however, hamper assisted regeneration by seed sowing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wildfire is an increasingly important factor for biodiversity conservation and natural resource management (Kelly and Brotons 2017). After a forest fire, burnt trees are usually removed in a process called salvage logging, due to silvicultural, safety or even aesthetic reasons (Mavsar et al. 2012; Müller et al. 2018). The ecological consequences of post-fire salvage logging have been the subject of intense discussion in the last decade (Lindenmayer et al. 2004; Donato et al. 2006; Hutto 2006; Thorn et al. 2018). This logging results in the structural simplification of habitats and may slow down vegetation regeneration, reduce the diversity of plant and animal communities and increase soil erosion (Thorn et al. 2018). Consequently, less severe management options, even including non-intervention, are being recommended, under the assumption that snags and decaying burnt wood are biological legacies that promote ecosystem recovery and diversity (Dellasala et al. 2006). In the Mediterranean Basin, however, the magnitude of post-fire salvage logging, in terms of area affected or biomass extracted, has rarely been quantified, although it is widespread (Pons and Rost 2017; Cervera et al. 2019).
Many species depend on the structural components of vegetation for foraging, breeding, or finding refuge (Jędrzejewska and Jędrzejewski 1990; Longland and Price 1991; Müller et al. 2007) and may thus be affected by salvage logging, both directly and through its influence on post-fire succession. Salvage logging has the potential to impact rodent populations by simplifying the structure and altering resource availability of recently burnt forests (Haim and Izhaki 1994). Rodents are a fundamental part of the diet of many predators (Ballesteros et al. 2000; Díaz-Ruiz et al. 2013) and provide valuable ecosystem services as they are both seed predators and short-distance seed dispersers (Gómez et al. 2008; Puerta-Piñero et al. 2010). The ecological processes in which they are involved can therefore be affected. Alternative management strategies may also favour rodent populations in burnt areas (Manning and Edge 2008), being compatible with the preservation of ecosystem services provided by rodents. This is the case of the in situ retention of coarse woody debris (e.g. piled up of branches), which also provides functions that are essential to maintaining biodiversity and long-term ecosystem productivity, e.g. nutrient and water reserves, microsites and substrates for seedlings, and quality of habitat for a wide range of wildlife species (McComb 2003; Rost et al. 2010; Santos and Poquet 2010; Rollan and Real 2011; Sullivan et al. 2012).
Post-fire management practices that affect rodent populations may have consequences for the assisted regeneration of burnt areas, such as seed sowing (Pulido and Díaz 2005; Broncano et al. 2008; Limousin et al. 2009). Rodents predate most of the seeds they handle (Perea et al. 2011) and assisted regeneration strategies have sometimes been discarded due to high seed loss by rodents (Tyler et al. 2006) that can be as high an 87% after 90 days (Leverkus et al. 2013). However, if seed predation is maintained at lower levels, assisted regeneration may be an effective and cost-efficient method of habitat restoration (Gómez 2004; Leverkus et al. 2013; Martelletti et al. 2018).
Understanding how rodents respond to habitat modification, due to forest management after a fire, is thus important for decision-making in restoration and for improving forestry practices. With this aim, we studied how management practices affected plant regeneration, trophic resources (availability of seeds and fleshy fruits), rodent populations and their seed removal activity in burnt pine forest in north-eastern Iberian Peninsula, while controlling for several fire-related variables. We hypothesized that post-fire salvage logging would: (1) slow down the recovery of plant cover and the availability of trophic resources for rodents, and, consequently, (2) negatively affect foraging activity by rodents, while (3) piled up woody debris would promote rodent populations and their foraging activity in the short term.
Material and methods
Study context
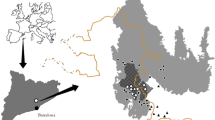
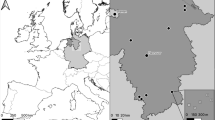
We studied three recently burnt areas in Catalonia (NE Iberian Peninsula), where burnt pine forests had been logged: La Jonquera, Viu de Llevata and Ger (Table 1). Currently, 31% of the Catalan territory is covered by forest, 60% of which are conifer forests, with agricultural lands (33%) and evergreen shrublands (29%) being the other main land uses (Vallecillo et al. 2013). It is estimated that about 25% of the wildland area (forest and shrubland) burnt between 1975 and 2006 (González and Pukkala 2007). Most burnt pine forests in Catalonia are currently salvage logged, and the resulting woody debris may be completely removed, left scattered on the ground or piled up (Pons and Rost 2017; Cervera et al. 2019).
In La Jonquera burnt Aleppo pine forests (Pinus halepensis Miller 1768), whole trees were harvested (i.e. full trees were removed), leaving few woody debris on site. Viu de Llevata burnt Scots pine (Pinus sylvestris Linnaeus, 1753) were stem-only harvested, leaving woody debris scattered on the ground. In Ger, burnt forests were stem-only harvested, with Scots and Mountain pines (Pinus mugo Turra, 1764) woody debris being treated differentially. While the former was piled up in barriers to prevent erosion, the latter were left scattered on the ground. In the three areas, burned and severely damaged pine trees were cut down; however, some partially burnt or unburnt trees were also logged. Forwarders and caterpillars with a grapple were used for hauling. Management tasks in Ger began a week after fire, in La Jonquera 2 weeks after fire, while in Viu de Llevata began 15 weeks after fire (Table 1).
Sampling design
We set 24 transects distributed among three study areas (Table 1) for 2 years and a half. Transects were perpendicular to the burnt area perimeter (Fig. 1), located across straight borders to avoid influences of border geometry on edge effects (Fernández et al. 2002) and separated from each other by at least 50 m, with median distance between adjacent transects exceeding 200 m (Puig-Gironès et al. 2018). Each transect contained seven sampling stations distributed at increasing distances from the nearest point of the burnt area perimeter to about 700 m (mean ± standard error; 0 ± 0 m; 26.1 ± 6.4 m; 48.9 ± 8.9 m; 101.7 ± 16.7 m; 186.8 ± 13.9 m; 335.9 ± 59.9 m and 680.7 ± 104.6 m) (Fig. 1). Each transect was sampled regularly and for 3 nights on each sampling event. In La Jonquera and Ger, sampling frequency was monthly in the first 3 months, bimonthly from 7 to 24 months and every 3 months thereafter until 30 months had passed after fire. Viu de Llevata was sampled with a three-month frequency from the beginning of sampling, i.e. 23 weeks after fire (Table 1). This sampling frequency variation was aimed at capturing the most relevant information from immediate rodent recolonization to 2.5 years after fire. Some of the stations within transects were logged immediately before the start of sampling, although this was not common. Usually, salvage logging operations began after sampling had already started. As a result, 45% of stations changed from unlogged to logged at some point during sampling. Thus, the number of salvage-logged stations gradually increased during our study period (Table 1).
Schematic representation of disturbances studied and methods used in this study. Linear transects perpendicular to the burnt area perimeter with seven sampling stations distributed at increasing distances from the burnt area perimeter to about 700 m from it were used to detect how the habitat simplification resulting from wildfire and salvage logging, affects vegetation recovery, trophic resources availability (seeds and fleshy fruits), rodent population and their foraging activity. In each sampling station of 5 m diameter, we measured vegetation structure, the production of trophic resources, the relative abundance of small mammals (with a Sherman live trap) and their foraging activity (with an acorn device)
Sampling stations consisted of a 5-m diameter area with a device offering acorns (henceforth, acorn device) and a Sherman live trap for small mammals set in its centre at different moments. Acorn devices and Sherman traps were consecutively installed at sampling stations to avoid interferences between devices. Acorn devices were checked the first and third day after installation, while Sherman traps were checked daily for the 3 days following their installation. Thereby, each sampling station was active for 6 days on each sampling event.
Acorn devices, used to evaluate seed removal by rodents, consisted of a 50 × 50 cm meshed cage (1.27 cm2 of mesh size) containing 20 acorns from cork oaks (Quercus suber Linnaeus, 1753). They had four entrances (5 × 5 cm) that prevented the jay (Garrulus glandarius Linnaeus, 1758) and other birds from reaching the acorns. All cork oak acorns used had similar sizes (length of 2.5 ± 0.5 cm; mean ± SE), in order to avoid an effect of acorn size on removal probability, and were collected from the cork oak forests close to the La Jonquera study area. Acorns were chosen to evaluate seed removal by rodents because they are easy to collect, naturally available in and/or near our study areas and positively selected by rodents.
Sherman traps were baited with a mixture of tuna, flour, oil and a piece of apple (Torre and Díaz 2004). Cotton was used for thermal protection and to minimize mortality. Recorded mortality was 2.1% (11 out of 503 individuals captured), a lower rate than that recorded in the Catalan small-mammal monitoring program (Torre et al. 2018). Small mammals caught were identified at species level, sexed and marked with numbered ear tags (National Band Co. USA), following the ASM Care & Use guidelines (Sikes et al. 2011). We trapped three small-rodent species: wood mouse (Apodemus sylvaticus Linnaeus, 1758) and Algerian mouse (Mus spretus Lataste, 1883), which are abundant rodents in recently burnt areas in the Mediterranean region (Torre and Díaz 2004; Puig-Gironès et al. 2018), and common vole (Microtus arvalis Pallas, 1778) which was only captured in Ger study area.
Vegetation characteristics and trophic resources availability
Live foliage cover (in %), an indicator of plant recovery, was estimated in each sampling station by comparison with a reference chart (Prodon and Lebreton 1981) for six vegetation height layers: 0–0.25 m (C0, the “C” denotes cover), 0.25–0.5 m (C25), 0.50–1 m (C50), 1–2 m (C100), 2–4 m (C200) and more than 4 m (C400). A principal component analysis (PCA) was then used to summarize the information obtained from the six vegetation layers, after arcsine-transforming the percentage cover values. The first component (PC1, Plant cover, which explained 53% of the variance of the original dataset) corresponded to the magnitude of plant cover, mainly understory, up to 2 m, and the second (PC2, Height of vegetation, 24% of explained variance) ordered stations in terms of the maximal height of vegetation in sampling stations (Supplementary Fig. S1).
The availability of trophic resources for rodents at sampling stations was estimated by quantifying seeds (mainly acorns) and fleshy fruits, using two methods. Firstly, we counted the number of seeds and fleshy fruits at three randomly selected branches of a plant (mainly shrubs and trees). Then we extrapolated these values to the total volume of the individual plant. We repeated this procedure for each plant holding fruits within the station. Branch and shrub volumes were calculated using the formula of the cone volume (\(V = \frac{1}{3} \pi r^{2} \, \cdot \,h\)), where r is the radius and h is the height of the cone. Secondly, we counted the trophic resources (seeds and fruits) found on the ground inside a 25 × 40 cm rectangle thrown in random directions 15 times within the station and extrapolated the mean figures to the area of the station (78.5 m2). These two indicators of the availability of trophic resources were then summed up to build an estimation of Trophic resources availability for each station (Puig-Gironès et al. 2018). Main plant species with fleshy fruits in the study areas were: Amelanchier ovalis, Arbutus unedo, Arctostaphylos uva‐ursi, Coriaria myrtifolia, Crataegus monogyna, Juniperus communis, J.oxycedrus, J.phoenicea, Myrtus communis, Pistacia lentiscus, Prunus spinosa, Rhamnus alaternus, Ribes rubrum, Rosa sp., Rubus sp., Sambucus nigra, Smilax aspera, Solanum nigrum, Sorbus aria, S.aucuparia and Vaccinium myrtillus, while the dry seeds abundance was estimated mainly from Quercus suber, Q. ilex and Q. coccifera.
Environmental and management variables
Each of the three study areas was framed within a bioclimatic region, either Mediterranean (La Jonquera) or Pyrenees (Ger and Viu de Llevata). We used astronomical seasons (i.e. limited equinoxes and solstices) to classify sampling events regarding to Season (i.e. winter, spring, summer and autumn). We measured time-since-fire as the number of weeks elapsed since fire (first week = 1, ranging from 1 to 141) and Distance from the burnt area perimeter as the distance from the centre of each station to the closest point of the perimeter (this variable was square root transformed prior to analyses). We also estimated Fire severity using a categorical scale going from 1 (unburnt) to 5 (crown fire) following the methodology described by (Keeley 2009).
Finally, we defined a series of variables related to the management of the burnt pine forest. Treatment was a dichotomous variable that identified unlogged and logged sampling stations. Because two of the four forest management strategies were only used in a single study area (Table 1), the volume of debris was used as indicator of the abundance of woody debris and, consequently, as a surrogate of management. Thus, we calculated the volume of debris (in m3) per station, both scattered on the ground (area occupied within the station, and average height of the woody debris over the ground) and piled up (assimilating the pile as a hexahedron). On each sampling event the volume of debris was measured to detect possible variations over time.
Statistical analyses
The proportion of the maximum number of acorns that can potentially be removed (limited to 20 acorns per sampling event) on three consecutive nights were used as surrogates of acorns removed by rodents (from 0 to 1). Since three rodent individuals was the maximum number that could be trapped per sampling event, we also used the proportion of the number of rodents caught in relation to three as surrogate of rodent abundance (Slade and Blair 2000; Torre and Arrizabalaga 2008). When the same individual was trapped on different nights within a sampling event, it was considered as a single capture. Henceforth, “relative abundance” is used to refer to the proportion of traps occupied by rodents (0, 0.33, 0.66, 1). These numbers are related to a sampling effort per transect (i.e. number of traps set during a capture occasion) and cannot be attributed to a specific area (m2). This sampling assumption is adequate with low rodent abundance, but captures would be saturated with high abundance given that each sampling station can only provide up to 3 captures per session. Therefore, relative abundance values may be lower than those expected from actual mice abundance. This bias would be higher when rodent species are simultaneously abundant around a sampling station, since competition for traps would increase. Only 12 out of 1564 sampling times among all stations (i.e., 0.8%) corresponded to stations with three captures per session and, therefore, this bias was assumed to be negligible. In the same way, 303 and 82 stations (i.e., 19.8 and 5.2%) produced one and two captures per session, respectively. Transect-based design is appropriate to test the differences between treatments, because it increases the number of spatial replicates that can be performed simultaneously, and to study community composition when rodent abundance is low (Pearson and Ruggiero 2003). However, our transect design may not be appropriate to obtain an accurate population estimate.
We use mixed models to assess the importance of management, environmental and temporal variables on habitat structure, trophic resources and rodent abundance and its foraging activity. Linear mixed models (LMM) with Gaussian error structure were used to analyse the effects of explanatory variables on the habitat structure variables (Plant cover, Height of vegetation), and generalized linear mixed models (GLMM) with a Poisson error structure and logit link function were used to analyse trophic resources availability. Transect nested within Bioclimatic region was included as a random factor in order to control temporal pseudoreplication. First, an exploratory analysis was used to assess the temporal influence of the dependent variables. This analysis included Time-since-fire and its quadratic term to consider possible unimodal temporal patterns. Whenever the Time-since-fire quadratic term was significant, it was used in subsequent models (Supplementary Table S1). Second, we assessed the influence of the explanatory variables Time-since-fire, Quadratic-time-since-fire (if it has been selected in the first step), Study area, Fire severity, Treatment and Volume of debris on habitat structure and trophic resources. The interaction between Time-since-fire and Treatment was used in analyses to explore if vegetation recovery and trophic resources availability patterns differed between logged and unlogged stations. The models that incorporated this interaction (Time-since-fire and Treatment) never presented the Quadratic-time-since-fire variable in the model, and vice versa. All possible combinations of the fixed variables were compared.
To assess the influence of explanatory variables on foraging activity by rodents and relative abundance of wood mouse, we used GLMMs with binomial error structure and logit link functions. GLMMs with negative binomial error structure and logit link functions were used for Algerian mouse and common vole, because their variance was greater than the mean (i.e. they showed overdispersion), due to excess of zeros (Martin et al. 2005). All analyses were developed following the two-step procedure described for acorn removal. The random structure was also the same, except for Algerian mouse and common vole that had Transect as the single random factor. Season, Time-since-fire and its quadratic term (if it has been selected; Supplementary Table S1), Study area, Distance from the burnt area perimeter, Fire severity, Treatment, Volume of debris, interaction between Time-since-fire and Treatment, Plant cover, Height of vegetation and Trophic resources availability were used as fixed explanatory variables. Total rodent relative abundance was also included as fixed factor on foraging activity analysis, while Wood mouse relative abundance was included on common vole analysis to test for possible species competition.
The information-theoretic framework (IT) was used to obtain the final model for each dependent variable (plant cover (PC1), height of vegetation (PC2), trophic resources availability, acorn removal, wood mouse relative abundance, Algerian mouse and common vole). The model selection was based on the Akaike Information Criteria corrected for small samples (AICc) (Burnham and Anderson 2002) and followed a series of hierarchical steps. (1) Multicollinearity diagnostics (Zuur et al. 2009) were performed by quantifying variance-inflation factors (VIF) and generalized variance-inflation factors (GVIF[1/(2df)] with several categorical predictor variables with multiple degrees of freedom) were calculated for each fixed factor (Fox and Monette 1992), where large VIF or GVIF values (arbitrary threshold of ≤ 2.5 suggesting collinearity) were sequentially dropped from further analysis (Zuur et al. 2010). (2) From the set of variables selected, all the possible combinations of the predictor variables (management, environmental and temporal variables, described at previous paragraphs), generating different biologically meaningful models, were explored. (3) Normality and homoscedasticity were checked by visually inspecting the plots of residuals against fitted values. (4) Overdispersion was checked for Poisson error structure models (Hilbe 2014). (5) For each model, the AIC weight (AICω) was calculated (total AICω adds 1) (Wagenmakers and Farrell 2004), furthermore, if there was no clearly most parsimonious model (one or more models showed a difference in AIC less than 2 from the best model), we proceed to estimate the average final model from all those models (Burnham and Anderson 2002). (6) To interpret the weight of each variable on the average final model, the AIC weight (ω+) was calculated (Supplementary Table S2); besides, if the standard errors (SE) were large (1.96 * SE > parameter appreciation, for the 95% confidence intervals) the estimate of the parameter was considered imprecise. To perform these analyses, we used the statistical programming software R (R Development Core Team 2017) with the car (Fox and Weisberg 2011), lme4 (Bates et al. 2015), MuMIn (Bartoń 2016), psych (Revelle 2017) and sjPlot (Lüdecke 2017) packages.
Results
Effect of post-fire salvage logging on rodent resources
Mixed models showed that time-since-fire presented linear positive relationship with Plant cover, and quadratic negative temporal relationships with height of vegetation and trophic resources availability (Table 2), i.e., both variables increased after fire until they stabilized or decreased over time (Fig. 2). Plant cover and trophic resources availability were different between study areas. Although Viu de Llevata had lower plant cover and higher trophic resources availability than Ger, both variables were greater in La Jonquera than in Ger and Viu de Llevata. Fire severity negatively affected plant cover and trophic resources availability, but was positively related to height of vegetation foliage cover. Both habitat structure variables had higher values in logged than in unlogged burnt areas, contrary to the trend of trophic resources availability. Although post-logging vegetation presented greater height at the start of the study, soon after fire (Fig. 2), the interaction between time-since-fire and the treatment factor showed that recovery was quicker in unlogged than in logged stations (Table 2). Volume of debris, a variable mostly derived from forest management in the first years, benefited the trophic resources availability.
Vegetation components trends between logged and unlogged stations. Comparison of plant cover (a), height of vegetation (b) and trophic resources availability (c) between management strategies (unlogged, whole-tree harvesting, scattered debris and piled debris) through time-since-fire. Time-since-fire was grouped into seven categories according to the sampling frequency variation in order to obtain similar sample sizes even if the time interval was uneven (0–10; 11–20; 21–30; 31–60; 61–90; 91–121 and from 121–150 weeks). In logged stations trees were felled from 2 weeks after fire to a year after fire. Boxes represent quartiles 25 and 50 of the data, while whiskers represent 0 and 75 (down and up, respectively). Start points show the maximum and minimum output values
Effects of post-fire salvage logging on foraging activity by rodents
Throughout the study, 7717 acorns (24.7% of the total number offered) were removed by rodents. Mixed models showed than acorn removal was higher in areas closer to the fire perimeter (Table 3), where there was also higher rodent relative abundance, denser vegetation and greater volume of debris (Fig. 3). Acorn removal also varied seasonally, diminishing from spring to winter. This season variability was coincident with seasonal patterns in rodent abundance, although there were particularities among the three fires, since in La Jonquera the maximum population was usually found in spring, while in Ger it was usually in autumn, whereas Viu de Llevata did not present any clear pattern (Supplementary Fig. S2). Acorn removal was lower in Viu de Llevata than in the other two studied areas; it also showed positive quadratic relationships with time-since-fire, i.e., soon after wildfire the removal rate was high, decreased after 15 weeks, to grow back towards 60 weeks after fire (Fig. 4). However, acorn removal was not affected significantly by trophic resources availability.
Rodent relative abundances and acorn removal trends between treatments. Comparison of a acorn removal and b total rodent relative abundances between management strategies (unlogged, whole-tree harvesting, scattered debris and piled debris) through time-since-fire. Time-since-fire was grouped into seven categories according to the sampling frequency variation. Boxes represent quartiles 25 and 50 of the data, while whiskers represent 0 and 75 (down and up, respectively). Start points show the maximum and minimum output values, while the unfilled circles show the mean
Model predictions of acorn removal by rodents. Marginal effects (measure the instantaneous rate of change) of the model predictors on acorn removal according to each fixed factor. Trend line and standard error shown were obtained from GLMM model estimates. Standard error bars are shown for sampling season factor
Rodent relative abundances after fire and logging
Throughout the study, a trapping success (frequency of captures of small mammals) of 10.7% provided 503 captures of small mammals. From the total number of captures, 68.8% were wood mice, 14.1% were greater white-toothed shrews (Crocidura russula Hermann, 1780), 13.1% were Algerian mice, and 4.0% were common voles; i.e., 81.9% were rodent species, which are responsible for the bulk of acorn removal. In total, 437 individuals were captured only once and 23 individuals (providing 66 captures) were recaptured from one to five times (12 individuals were captured twice, 4 three times, 5 four times and 2 five times). Of these individuals that were recaptured, 52.2% corresponded to recaptures in the same sampling station; 43.5% were recaptured in different stations of the same transect, while the remaining 4.3% corresponded to individuals recaptured in two different transects.
Mixed models show than wood mice were caught more often with low fire severity and denser vegetation (Table 3). Although no significant differences in the total number of captures were found between logged and unlogged areas, the presence of debris increased the presence of the wood mouse (Fig. 3). Wood mice tended to be trapped more often in areas closest to the perimeter and with low trophic resources availability; however, distance to the perimeter and resources availability were non-significant in the final model. Algerian mouse abundance increased with time-since-fire, diminished from spring to winter and was positively associated with height of vegetation. Trophic resources availability and logging treatment showed positive effects on Algerian mice, but were non-significant. Common vole only appeared in logged areas, where the volume of debris—mainly piled up—, plant cover and trophic resources availability increased its abundance. The distance from the burnt area perimeter, high fire severity and logging treatment were selected but non-significant in the final model. The presence of wood mice did not seem to be detrimental to the presence of the common vole.
Discussion
Effects of post-fire salvage logging on rodent resources
The recovery process of the vegetation was not significantly different between logged and unlogged areas through the 2.5 years of this study. Although post-fire vegetation recovery started from nearly zero after fires, some regenerated cover was already present at the time of logging. However, the speed of plant regeneration seems to decrease after logging (Supplementary Fig. S3), as has been recorded in other regions (Donato et al. 2006). Sampling stations with greater fire severity had less trophic resources availability and lower plant cover, due to its negative effect on vegetation recovery (Keeley 2009). The high production of trophic resources shortly after fire may be related to the vigorous plant regeneration strategies and the increase in short-lived opportunistic plant species, such as annuals (Buhk et al. 2007; Puig-Gironès et al. 2017). In this sense, our results align with studies that show that logging burnt forests negatively affects short-term plant regeneration and the availability of seeds and fleshy fruits.
Trophic resources were more available in areas with larger volume of debris left on site. Although several studies show that logging and hauling of burnt trees may hamper the regeneration of the plant community (Beschta et al. 2004), seedling survival is highest in those areas where logs and branches are scattered on the ground, associated with the protection from herbivory and the improvement of microclimatic conditions created by dead wood structures (Castro et al. 2011; Marzano et al. 2013), thus enhancing the production of trophic resources.
Patterns of rodent foraging activity and relative abundance
Volume of woody debris, either scattered on the ground or piled up, affected foraging activity by rodents, likely thanks to the shelter provided by the woody debris and to a higher food availability. Debris can therefore facilitate the recolonization of the burnt area by rodents. The source population may include animals originating from the unburnt matrix that take advantage of the vacant burnt territory, but also individuals living in burnt areas close to the external perimeter, where the lower fire severity and more complex habitat structure allow higher rodent abundance (Puig-Gironès et al. 2018).
Our results show that rodent responses to post-fire salvage logging are species-specific and seem to be related to the habitat preferences of each species. Wood mouse seems especially resilient to habitat modification (Sainz-Elipe et al. 2012) and post-fire salvage logging did not appear to delay wood mouse reoccupation, although it negatively affected foliage cover and the availability of trophic resources. This may be because wood mouse populations were already immersed in a recovery process after fire when this second disturbance took place (Puig-Gironès et al., 2018). Wood mouse avoided open areas and selected those with higher cover. Larger woody debris volume provides shelter against predators and, thus, wood mouse may still find enough cover immediately after logging if debris is left on site (Longland and Price 1991). In unlogged areas, however, there will be more woody debris on the ground, falling down gradually from the dead trees (Peterson et al. 2015) and, therefore, resources for the wood mouse should increase in these areas in the mid- and long term after fire, depending on tree species and environmental conditions. Contrastingly, open habitat generated by salvage logging may favour the presence of the Algerian mouse. This early-successional species (Sainz-Elipe et al. 2012) often attains larger abundances in burnt than in unburnt areas (Torre and Díaz 2004; Puig-Gironès et al. 2018).
A few authors have studied the effect of clear-cutting on the populations of common vole in Europe (Bogdziewicz and Zwolak 2014). However, this is, to our knowledge, the first study of the combined effect of fire and salvage logging in this species. Although the sample size was low (20 captures) and may be insufficient to extract consistent conclusions, our results showed that the common vole colonized the logged area, selecting areas with greater plant cover, higher trophic resources and higher volume of debris. The common vole may find refuge in wood piles, which can play a similar role to that of shrubs in meadows.
Management implications
The rapid recolonization of rodents after fire shows the capacity of populations to recover from disturbances, but this capacity seems to be modulated by the distance to the burnt area perimeter and habitat structure. The retention of non-commercial woody debris in the logged stands seems fundamental to attract and retain seed predators and dispersers, like rodents, if vegetation regrowth has not started, facilitating population connectivity between burnt and unburnt areas, and providing a refuge from predation (Castro et al. 2012) as an alternative to plant cover (Smit et al. 2008; Seip et al. 2018). Although the management of burnt wood may limit the supply of recruits for the early stages of population recovery, alternative management recommendations are possible regarding the two strategic goals, namely promoting the presence of rodents for their ecological functions, or reducing them to increase the likelihood of success in the restoration of burnt forests.
Non-intervention is a suitable alternative to conventional salvage logging, allowing quicker vegetation recovery and benefiting the rodent community. If the burnt area is logged, the flux of dispersing rodents, both from the burnt area perimeter and from unburnt or unlogged patches within the burnt area (Puig-Gironès et al., 2018), should be preserved. Building piles of wood debris at different distances from the perimeter in burnt and logged areas can help this connectivity (Banks et al. 2011; Zaitsev et al. 2014; Seip et al. 2018). On the other hand, environmental impacts would be mitigated by leaving burnt wood on site, particularly branches and other non-commercial coarse woody debris in piles, as these structures act as refuge (Rost et al. 2010; Sullivan et al. 2012). On the contrary, for the assisted regeneration of burnt forests with seed sowing, salvage logging offers no clear advantage against seed predators, if coarse woody debris is left on site. The logged areas may be kept without woody debris on the ground to avoid generating a favourable habitat for rodents. However, if the activity of ungulates, such as wild boar, is a main problem, then woody debris could be left scattered on the ground to prevent the activity of ungulates in logged areas (Puerta-Piñero et al. 2010; Leverkus et al. 2013). Taking into account seed predators, seed sowing is recommended immediately after fire (up to 6–12 months after) and at a minimum of 100 m away from unburnt areas (Leverkus et al. 2013; Puig-Gironès et al. 2018). Before using one or another recommendation, managers should consider their effects on biodiversity and assess the costs and benefits of the interventions. Current knowledge shows that promoting habitat complexity is associated with greater biodiversity (Stein et al. 2014; Kelly et al. 2017). Ideally, a balance between dispersion and predation of seeds in recently burnt and logged areas would be positive for the entire ecosystem in succession. For that reason, in order to enhance dispersion in burnt and logged areas, the factors regulating rodent population dynamics immediately after wildfire must be considered in post-fire forest planning. Furthermore, the interactions between vegetation, fauna and fire should be taken into account through an integrated and multidisciplinary post-fire management that favours biodiversity in areas affected by fire (Mauri and Pons 2019).
References
Ballesteros T, Degollada A, Plaza V (2000) Dieta de la fagina al Parc Natural de Sant Llorenç del Munt i l’Obac. In: Xarxa de Parcs Naturals (ed) IV Trobada d’Estudiosos de Sant Llorenç Munt i l’Obac, vol 29. Monografies. Diputació de Barcelona, Barcelona, pp 119–122
Banks SC, Dujardin M, McBurney L, Blair D, Barker M, Lindenmayer DB (2011) Starting points for small mammal population recovery after wildfire: recolonisation or residual populations? Oikos 120:26–37. https://doi.org/10.1111/j.1600-0706.2010.18765.x
Bartoń K (2016) Multi-model inference (MuMIn). R package version 1.15.6. Vienna, Austria
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Beschta RL et al (2004) Postfire management on forested public lands of the Western United States. Conserv Biol 18:957–967. https://doi.org/10.1111/j.1523-1739.2004.00495.x
Bogdziewicz M, Zwolak R (2014) Responses of small mammals to clear-cutting in temperate and boreal forests of Europe: a meta-analysis and review. Eur J For Res 133:1–11. https://doi.org/10.1007/s10342-013-0726-x
Broncano MJ, Rodrigo A, Retana J (2008) Post-dispersal seed predation in Pinus halepensis and consequences on seedling establishment after fire. Int J Wildland Fire 17:407–414. https://doi.org/10.1071/WF07095
Buhk C, Meyn A, Jentsch A (2007) The challenge of plant regeneration after fire in the Mediterranean Basin: scientific gaps in our knowledge on plant strategies and evolution of traits. Plant Ecol 192:1–19. https://doi.org/10.1007/s11258-006-9224-2
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York. ISBN 978-0-387-22456-5
Castro J, Allen CD, Molina-Morales M, Maranon-Jimenez S, Sanchez-Miranda A, Zamora R (2011) Salvage logging versus the use of burnt wood as a nurse object to promote post-fire tree seedling establishment. Restor Ecol 19:537–544. https://doi.org/10.1111/j.1526-100X.2009.00619.x
Castro J, Puerta-Piñero C, Leverkus AB, Moreno-Rueda G, Sánchez-Miranda A (2012) Post-fire salvage logging alters a key plant-animal interaction for forest regeneration. Ecosphere 3:art90. https://doi.org/10.1890/es12-00089.1
Cervera T, Pino J, Marull J, Padró R, Tello E (2019) Understanding the long-term dynamics of forest transition: from deforestation to afforestation in a Mediterranean landscape (Catalonia, 1868–2005). Land Use Policy 80:318–331. https://doi.org/10.1016/j.landusepol.2016.10.006
Dellasala DA et al (2006) Post-fire logging debate ignores many issues. Science 314:51–52. https://doi.org/10.1126/science.314.5796.51b
Díaz-Ruiz F, Delibes-Mateos M, García-Moreno JL, María López-Martín J, Ferreira C, Ferreras P (2013) Biogeographical patterns in the diet of an opportunistic predator: the red fox Vulpes vulpes in the Iberian Peninsula. Mamm Rev 43:59–70. https://doi.org/10.1111/j.1365-2907.2011.00206.x
Donato DC, Fontaine JB, Campbell JL, Robinson WD, Kauffman JB, Law BE (2006) Post-wildfire logging hinders regeneration and increases fire risk. Science 311:352. https://doi.org/10.1126/science.1122855
Fernández C, Acosta FJ, Abellá G, López F, Díaz M (2002) Complex edge effect fields as additive processes in patches of ecological systems. Ecol Model 149:273–283. https://doi.org/10.1016/S0304-3800(01)00464-1
Fox J, Monette G (1992) Generalized collinearity diagnostics. J Am Stat Assoc 87:178–183. https://doi.org/10.1080/01621459.1992.10475190
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. SAGE Publications Inc, Thousand Oaks
Gómez JM (2004) Importance of microhabitat and acorn burial on Quercus ilex early recruitment: non-additive effects on multiple demographic processes. Plant Ecol 172:287–297. https://doi.org/10.1023/B:VEGE.0000026327.60991.f9
Gómez JM, Puerta-Piñero C, Schupp EW (2008) Effectiveness of rodents as local seed dispersers of Holm oaks. Oecologia 155:529–537. https://doi.org/10.1007/s00442-007-0928-3
González JR, Pukkala T (2007) Characterization of forest fires in Catalonia (North-East Spain). Eur J For Res 126:421–429. https://doi.org/10.1007/s10342-006-0164-0
Haim A, Izhaki I (1994) Changes in rodent community during recovery from fire: relevance to conservation. Biodivers Conserv 3:573–585. https://doi.org/10.1007/BF00114202
Hilbe JM (2014) Modeling count data, 1st edn. Cambridge University Press, New York. ISBN 978-1-107-61125-2
Hutto RL (2006) Toward meaningful snag-management guidelines for postfire salvage logging in North American conifer forests. Conserv Biol 20:984–993. https://doi.org/10.1111/j.1523-1739.2006.00494.x
Jędrzejewska B, Jędrzejewski W (1990) Antipredatory behaviour of bank voles and prey choice of weasels—enclosure experiments. Ann Zool Fenn 27:321–328
Keeley JE (2009) Fire intensity, fire severity and burn severity: a brief review and suggested usage. Int J Wildland Fire 18:116–126. https://doi.org/10.1071/WF07049
Kelly LT, Brotons L (2017) Using fire to promote biodiversity. Science 355:1264–1265. https://doi.org/10.1126/science.aam7672
Kelly LT, Brotons L, McCarthy MA (2017) Putting pyrodiversity to work for animal conservation. Conserv Biol 31:952–955. https://doi.org/10.1111/cobi.12861
Leverkus AB, Castro J, Puerta-Piñero C, Rey-Benayas JM (2013) Suitability of the management of habitat complexity, acorn burial depth, and a chemical repellent for post-fire reforestation of oaks. Ecol Eng 53:15–22. https://doi.org/10.1016/j.ecoleng.2013.01.003
Limousin J, Rambal S, Ourcival J, Rocheteau A, Joffre R, Rodríguez-Cortina R (2009) Long-term transpiration change with rainfall decline in a Mediterranean Quercus ilex forest. Glob Change Biol 15:2163–2175. https://doi.org/10.1111/j.1365-2486.2009.01852.x
Lindenmayer DB, Foster DR, Franklin JF, Hunter ML (2004) Salvage harvesting policies after natural disturbance. Science 303:1303. https://doi.org/10.1126/science.1093438
Longland WS, Price MV (1991) Direct observations of owls and heteromyid rodents: can predation risk explain microhabitat use? Ecology 72:2261–2273. https://doi.org/10.2307/1941576
Lüdecke D (2017) sjPlot: data visualization for statistics in social science. R package version 2.3.1. Vienna, Austria
Manning JA, Edge WD (2008) Small mammal responses to fine woody debris and forest fuel reduction in southwest Oregon. J Wildl Manag 72:625–632. https://doi.org/10.2193/2005-508
Martelletti S, Lingua E, Meloni F, Freppaz M, Motta R, Nosenzo A, Marzano R (2018) Microsite manipulation in lowland oak forest restoration results in indirect effects on acorn predation. Forest Ecol Manag 411:27–34. https://doi.org/10.1016/j.foreco.2018.01.007
Martin TG, Wintle BA, Rhodes JR, Kuhnert PM, Field SA, Low-Choy SJ, Tyre AJ, Possingham HP (2005) Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecol Lett 8(11):1235–1246. https://doi.org/10.1111/j.1461-0248.2005.00826.x
Marzano R, Garbarino M, Marcolin E, Pividori M, Lingua E (2013) Deadwood anisotropic facilitation on seedling establishment after a stand-replacing wildfire in Aosta Valley (NW Italy). Ecol Eng 51:117–122. https://doi.org/10.1016/j.ecoleng.2012.12.030
Mauri E, Pons P (2019) Handbook of good practices in post-wildfire management, 2nd edn. Anifog Project, CGL2014-54094-R, Universitat de Girona. 169pp. ISBN: 978-84-8458-564-0
Mavsar R, Varela E, Corona P, Barbati A, Marsh G (2012) Economic, legal and social aspects of post-fire management. In: Moreira F, Arianoutsou M, Corona P, De las Heras J (eds) Post-fire management and restoration of Southern European forests. Springer, Amsterdam, pp 45–78
McComb WC (2003) Ecology of coarse woody debris and its role as habitat for mammals. In: Zabel CJ, Anthony RG (eds) mammal community dynamics: management and conservation in the coniferous forests of Western North America, 1st edn. Cambridge University Press, New York, pp 374–404
Müller J, Hothorn T, Pretzsch H (2007) Long-term effects of logging intensity on structures, birds, saproxylic beetles and wood-inhabiting fungi in stands of European beech Fagus sylvatica L. Forest Ecol Manag 242:297–305. https://doi.org/10.1016/j.foreco.2007.01.046
Müller J, Noss RF, Thorn S, Bässler C, Leverkus AB, Lindenmayer D (2018) Increasing disturbance demands new policies to conserve intact forest. Conserv Lett. https://doi.org/10.1111/conl.12449
Pearson DE, Ruggiero LF (2003) Transect versus grid trapping arrangements for sampling small-mammal communities. Wildl Soc Bull 31:454–459
Perea R, San Miguel A, Gil L (2011) Acorn dispersal by rodents: the importance of re-dispersal and distance to shelter. Basic Appl Ecol 12:432–439. https://doi.org/10.1016/j.baae.2011.05.002
Peterson DW, Dodson EK, Harrod RJ (2015) Post-fire logging reduces surface woody fuels up to four decades following wildfire. Forest Ecol Manag 338:84–91
Pons P, Rost J (2017) The challenge of conserving biodiversity in harvested burned forests. Conserv Biol 31:226–228. https://doi.org/10.1111/cobi.12767
Prodon R, Lebreton J-D (1981) Breeding avifauna of a Mediterranean succession: the holm oak and cork oak series in the eastern Pyrenees, 1. Analysis and modelling of the structure gradient. Oikos 37:21–38. https://doi.org/10.2307/3544069
Puerta-Piñero C, Sanchez-Miranda A, Leverkus AB, Castro J (2010) Management of burnt wood after fire affects post-dispersal acorn predation. Forest Ecol Manag 260:345–352. https://doi.org/10.1016/j.foreco.2010.04.023
Puig-Gironès R, Brotons L, Pons P (2017) Aridity influences the recovery of Mediterranean shrubland birds after wildfire. PLoS ONE 12:e0173599. https://doi.org/10.1371/journal.pone.0173599
Puig-Gironès R, Clavero M, Pons P (2018) Importance of internal refuges and external unburnt area perimeter on the recovery of rodent populations after wildfire. Int J Wildland Fire 27:425–436. https://doi.org/10.1071/WF17102
Pulido FJ, Díaz M (2005) Regeneration of a Mediterranean oak: a whole-cycle approach. Ecoscience 12:92–102. https://doi.org/10.2980/i1195-6860-12-1-92.1
R Development Core Team (2017) R: a language and environment for statistical computing. R Development Core Team, Vienna
Revelle WR (2017) psych: procedures for personality and psychological research. Northwestern University, Evanston
Rollan À, Real J (2011) Effect of wildfires and post-fire forest treatments on rabbit abundance. Eur J Forest Res 57:201–209. https://doi.org/10.1007/s10344-010-0412-y
Rost J, Clavero M, Bas JM, Pons P (2010) Building wood debris piles benefits avian seed dispersers in burned and logged Mediterranean pine forests. For Ecol Manage 260:79–86. https://doi.org/10.1016/j.foreco.2010.04.003
Sainz-Elipe S, Saez-Duran S, Galan-Puchades MT, Fuentes MV (2012) Small mammal (Soricomorpha and Rodentia) dynamics after a wildfire in a Mediterranean ecosystem. Mammalia 76:251–259. https://doi.org/10.1515/mammalia-2011-0020
Santos X, Poquet JM (2010) Ecological succession and habitat attributes affect the postfire response of a Mediterranean reptile community. Eur J Forest Res 56:895–905. https://doi.org/10.1007/s10344-010-0387-8
Seip CR, Hodder DP, Crowley SM, Johnson CJ (2018) Use of constructed coarse woody debris corridors in a clearcut by American martens (Martes americana) and their prey. Forestry. https://doi.org/10.1093/forestry/cpy010
Sikes RS, Gannon WL, Animal Care and Use Committee of the American Society of Mammalogists (2011) Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J Mamm 92:235–253. https://doi.org/10.1644/10-MAMM-F-355.1
Slade NA, Blair SM (2000) An empirical test of using counts of individuals captured as indices of population size. J Mamm 81:1035–1045. https://doi.org/10.1644/1545-1542(2000)081%3c1035:AETOUC%3e2.0.CO;2
Smit C, Ouden J, Díaz M (2008) Facilitation of Quercus ilex recruitment by shrubs in Mediterranean open woodlands. J Veg Sci 19:193–200. https://doi.org/10.3170/2007-8-18352
Stein A, Gerstner K, Kreft H (2014) Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol Lett 17:866–880. https://doi.org/10.1111/ele.12277
Sullivan TP, Sullivan DS, Lindgren PM, Ransome DB (2012) If we build habitat, will they come? Woody debris structures and conservation of forest mammals. J Mamm 93:1456–1468. https://doi.org/10.1644/11-MAMM-A-250.1
Thorn S et al (2018) Impacts of salvage logging on biodiversity—a meta-analysis. J Appl Ecol 55:279–289. https://doi.org/10.1111/1365-2664.12945
Torre I, Arrizabalaga A (2008) Habitat preferences of the bank vole Myodes glareolus in a Mediterranean mountain range. Acta Theriol 53:241–250. https://doi.org/10.1007/BF03193120
Torre I, Díaz M (2004) Small mammal abundance in Mediterranean post-fire habitats: a role for predators? Acta Oecol 25:137–142. https://doi.org/10.1016/j.actao.2003.10.007
Torre I, Raspall A, Arrizabalaga A, Díaz M (2018) SEMICE: an unbiased and powerful monitoring protocol for small mammals in the Mediterranean Region. Mamm Biol. https://doi.org/10.1016/j.mambio.2017.10.009
Tyler CM, Kuhn B, Davis FW (2006) Demography and recruitment limitations of three oak species in California. Q Rev Biol 81:127–152. https://doi.org/10.1086/506025
Vallecillo S, Hermoso Lopez V, Possingham HP, Brotons L (2013) Conservation planning in a fire-prone Mediterranean region: threats and opportunities for bird species. Landsc Ecol 28:1517–1528
Wagenmakers E-J, Farrell S (2004) AIC model selection using Akaike weights. Psychon B Rev 11:192–196. https://doi.org/10.3758/BF03206482
Zaitsev AS, Gongalsky KB, Persson T, Bengtsson J (2014) Connectivity of litter islands remaining after a fire and unburnt forest determines the recovery of soil fauna. Appl Soil Ecol 83:101–108. https://doi.org/10.1016/j.apsoil.2014.01.007
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Statistics for biology and health. Springer, New York. ISBN 978-0-387-87457-9
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgements
We would like to thank C. Sanchez Cascante for her illustrations of Fig. 1; G. Vila, P. Eijo and the Galanthus Association for their help during this study; our thanks also to S. Herrando, X. Santos, J. Camprodon and M. Díaz for fruitful discussions and their comments on earlier drafts of this paper. This work derives from the doctoral thesis of Roger Puig-Gironès and was supported by Spanish Ministry of Economy and Competitiveness [CGL2014-54094-R].
Author information
Authors and Affiliations
Contributions
RP-G and PP conceived and designed the research; RP-G collected the data; RP-G analysed the data; RP-G, MC, LI, JR and PP wrote and reviewed the manuscript.
Corresponding author
Additional information
Communicated by Gediminas Brazaitis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Puig-Gironès, R., Imbeau, L., Clavero, M. et al. Does post-fire salvage logging affect foraging activity by rodents?. Eur J Forest Res 139, 777–790 (2020). https://doi.org/10.1007/s10342-020-01285-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-020-01285-5