Abstract
Context
Disturbances create spatial variation in environments that may influence animal foraging. Granivory by rodents can influence seed supply and thus plant establishment. However, effects of disturbance patterns on rodent seed removal in western North American conifer forests are generally unknown.
Objectives
We conducted a study in lodgepole pine (Pinus contorta var. latifolia) forests of Greater Yellowstone (Wyoming, USA) to answer: (1) How do seed removal and rodent activity vary between recently burned and adjacent unburned forests and with distance from fire perimeter? (2) Which microhabitat conditions explain variability in seed removal and rodent activity?
Methods
One or two years after wildfires, we established transects (n = 23) with four stations each: at 10 and 40 m from the fire perimeter in both burned and unburned forest. At stations, we deployed trays with lodgepole pine seeds and cameras pointed at trays for 28 days and quantified habitat structure and seed abundance.
Results
Seed removal, which averaged 85%, and diurnal rodent activity did not differ between burned and unburned forests or with distance from the fire perimeter; however, nocturnal rodent activity was lower in burned forests. Seed removal and diurnal rodent activity were not associated with any microhabitat conditions we measured. However, nocturnal rodent activity was associated with microhabitat in both burned and unburned forests.
Conclusions
High seed removal rates suggested that rodent foraging was not reduced by high-severity wildfire. If observed seed removal represents natural conditions, post-dispersal seed predation could influence post-fire recruitment of a widespread foundation tree species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disturbances create spatial patterns that can affect landscape structure for years to centuries, leading to important biological consequences for ecosystems and the organisms living within them (Turner 2010). At multiple scales, disturbances can affect vegetation succession (e.g., Turner et al. 1997; Johnstone et al. 2011) and habitat use of wildlife species (e.g., Boyce et al. 2003). Animals such as herbivores and granivores (i.e., seed predators) may also influence post-disturbance succession by affecting plants or seed supplies. While herbivore responses to changing patterns of habitat structure are well-studied (e.g., Forester et al. 2007), granivore responses remain less understood (but see Tallmon et al. 2003; Zwolak et al. 2010). Thus, understanding how granivores respond to disturbances could help elucidate potential effects of granivores on succession and how animal communities change in disturbance-prone landscapes.
Seed-eating animals, which are predominately rodents in many temperate ecosystems, can have important effects on plants (Hulme 1998; Hulme and Kollmann 2005; Crawley 2014). Granivory (i.e., seed predation) by rodents can limit plant abundance in early successional systems (Orrock et al. 2006) and affect vegetation patterns (e.g., Brown and Heske 1990; Ostfeld et al. 1997). Landscape heterogeneity at multiple scales may influence rodent granivory (Orrock and Damschen 2005; Orrock and Danielson 2005; Craig et al. 2011); however, responses of rodent granivores to disturbance-modified landscape patterns and microhabitat conditions are not often considered in the same study.
Wildfires create landscape patterns and modify microhabitat conditions, generating an ideal post-disturbance landscape within which to examine granivore behavioral responses at multiple scales. Fires affect habitat characteristics important to rodents, including food resources, canopy cover, and ground cover (Brown 1988; Manson and Stiles 1998; Orrock et al. 2004). Although rodents’ demographic responses to fires have been extensively studied (e.g., Fisher and Wilkinson 2005; Fontaine and Kennedy 2012; Griffiths and Brook 2014), behavioral responses such as foraging are less understood. The effect of granivory on post-fire plant regeneration can be substantial (e.g., Denham 2008) and even stronger than that of fire in some ecosystems (Zwolak et al. 2010). However, post-fire granivory by rodents has not been studied in lodgepole pine (Pinus contorta var. latifolia) forests, which cover millions of hectares of western North America.
Lodgepole pine, a fire-dependent foundational species (Critchfield 1980), produces serotinous cones that open and release their seeds when heated, assuring post-fire tree regeneration. Lodgepole pine often dominates subalpine conifer forests in the northern Rocky Mountains including the Greater Yellowstone Ecosystem (GYE; Wyoming, USA). The 80,000 km2 GYE, one of the world’s last and largest near-intact north-temperate ecosystems, experiences a stand-replacing fire regime that drives vegetation patterns. Broad-scale variation in post-fire lodgepole pine regeneration across the GYE depends largely on pre-fire serotiny levels and burn severity (Anderson and Romme 1991; Turner et al. 1997, 1999, 2003). Levels of serotiny vary with fire-return interval, stand age (Schoennagel et al. 2003), and the selective pressure of American red squirrels (Tamiasciurus hudsonicus) (Talluto and Benkman 2014), the dominant pre-dispersal seed predators of lodgepole pine (Smith 1970). However, the role of post-dispersal seed predation in lodgepole pine forests has not previously been studied, although researchers have suggested it could limit lodgepole pine recruitment (e.g., Lobo 2014).
Because the first few years following fire represent a critical window for lodgepole tree regeneration (Turner et al. 1997), we evaluated effects of recent stand-replacing wildfire on animal foraging on lodgepole pine seeds to determine whether granivores could drive local variation in post-fire lodgepole pine regeneration. We asked two questions: (1) How do seed removal and rodent activity vary between recently burned and adjacent unburned forests and with distance from fire perimeter? and (2) Which microhabitat conditions explain variability in seed removal and rodent activity? Because rodent activity often is equal or lower in forests after fire (Bendell 1974; Borchert et al. 2014), we hypothesized that rodent activity and seed removal would be lower in burned compared to unburned forests. We further hypothesized that, in burned forests, rodent activity and seed removal would decrease with distance from the fire perimeter and with less food resources and habitat structure, which may provide refuge from predators and/or affect rodents’ behavioral response to risk-reward tradeoffs (Holbrook and Schmitt 1988).
Methods
Study area and design
We conducted our study in lodgepole pine-mixed conifer forests in Yellowstone National Park (YNP) and Bridger-Teton National Forest (BTNF), located in the northwest corner of Wyoming (USA) within the GYE. The GYE’s climate is characterized by cold, snowy winters and cool, dry summers. Lodgepole pine dominates the subalpine forests of YNP, although subalpine fir (Abies lasiocarpa), Engelmann spruce (Picea engelmannii), and whitebark pine (Pinus albicaulis) may be locally abundant at higher elevations. Douglas-fir (Pseudotsuga menziesii) and aspen (Populus tremuloides) are more abundant in low- to mid-elevation forests, which also include limber pine (Pinus flexilis) and Rocky Mountain juniper (Juniperus scopulorum). Fire has long been a part of Yellowstone’s forested landscape, with stand-replacing fires occurring at 100–300 year intervals throughout the Holocene (e.g., Whitlock et al. 2008). A variety of granivorous rodents occur within GYE forests, including American red squirrels, northern flying squirrels (Glaucomys sabrinus), least chipmunks (Tamius minimus), yellow pine chipmunks (Tamias amoenus), deer mice (Peromyscus maniculatus), and southern red-backed voles (Myodes gapperi).
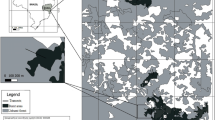
Field sampling occurred during summer 2014 in and adjacent to wildfires within the GYE that occurred in June 2012 (Fontenelle Fire), August 2012 (Cygnet Fire), and August 2013 (Alum Fire) (USDA Forest Service 2015) and burned 23,523, 1290, and 2867 hectares, respectively (USDA Forest Service/U.S. Geological Survey 2015) (Fig. 1). At each fire, we established 80-m transects (n = 23; 9 at Fontenelle, 10 at Cygnet, 4 at Alum) separated by at least 90 m to minimize the likelihood of sampling on multiple transects the same American red squirrels, as their territories average less than 1 hectare (Gurnell 1984). Transects were perpendicular to boundaries between forests affected by stand-replacing (hereafter, “burned”) and non-stand-replacing fire (hereafter, “unburned”; defined as the end of live green trees), centered on the fire’s edge, and extended 40 m into burned and unburned forest. Each transect traversed forests of the same pre-fire successional stage class and was buffered in all directions by at least 40 m of similar forest; this was the average maximum distance at which we could buffer transects due to the tortuosity of fire perimeters and variation in burn severity. The number of transects per fire and their location was largely determined by fire size and shape, burn severity pattern, and site access. We established four stations along each transect: one at 10 m and one at 40 m from the fire’s edge in both burned and unburned forests (n = 92 stations).
A map of wildfire study sites within the Greater Yellowstone Ecosystem (GYE) and wildfire perimeters (inset maps). Within each fire’s inset map, locations of transects (black lines) are overlaid on burn severity maps (USDA Forest Service/U.S. Geological Survey 2015)
Seed removal experiment with cameras
To quantify seed removal, we positioned seed removal trays at each station within 3 m of the transect in areas with clear sight lines for the subset of stations with cameras (see below). To install trays, we excavated and placed soil into a plastic container (12.5 by 18.5 cm wide and 3.5 cm deep) with a mesh windowscreen base to allow water drainage and supplied 1.0 g (equal to 259 ± 2.34 SE seeds, n = 5, i.e., a mean of 3.86 mg per seed) of lodgepole pine seeds (Sheffield’s Seed Co, Inc., collected in Wyoming). Seed removal trays were deployed for exactly 28 days (starting between July 11 and 22 and ending between August 8 and 19; Online Appendix 1) to control for variation in rodent activity during the lunar cycle (e.g., Orrock and Danielson 2004). After retrieving trays, we dried soils to prevent fungal decomposition of seeds. Data from 16 stations were excluded because trays were disturbed while deployed or soils were lost in a drying oven malfunction, leaving 76 trays for analyses. We searched material > 0.7 mm from these trays for remaining intact seeds and seed hulls (i.e., in situ seed consumption). We categorized intact seeds as those that had no visible part of the seed missing or damaged and were not empty, as determined by the apparent viability method (Ball and Miller 1990). All other seeds were assumed to have been removed and consumed.
To record activity by potential granivores at 33 stations, we secured motion-triggered cameras (22 Bushnell Trophy Cam Trophy XLTs, seven Reconyx Silent Image RM30s, three Reconyx HyperFire PC900s, and one Reconyx HyperFire PC900 Professional Covert IR) to trees < 2 m away from and aimed at trays. At each fire, cameras were deployed at stations 10 and 40 m from the fire’s edge with equal coverage in burned and unburned forests for the same 28-day period as trays. Data from two cameras were excluded due to setup errors; the remaining 31 cameras captured photos for a mean of 23.2 days per camera (23.9 in burned forests vs. 22.5 in unburned forests) because five cameras malfunctioned within the first week of deployment. After camera retrieval, we counted and identified animals (as rodents or non-rodents) in each photograph for each camera. Rodent detections were aggregated into one of the following species groups: chipmunks, mice or voles, squirrels, porcupines, and unknown rodents. Because different rodent species may be active at day and/or night, we also categorized rodent detection events as diurnal or nocturnal based on the average sunrise/sunset time for our study region during the field sampling period (6:00 am/9:00 pm MDT). As an index of rodent activity, we calculated the total number of diurnal and nocturnal detections of a taxon per total survey effort time for each camera (Kays et al. 2011), reported as detections per day (hereafter, “detection rate”).
Microhabitat conditions
At each station, we categorized burn severity class as unburned green forest (1), light-surface fire (2), severe-surface fire (3), or crown fire (4) (Turner et al. 1997, 1999), and obtained elevation with a handheld global positioning system (Garmin GPSmap 60CSx). To quantify microhabitat conditions surrounding stations, we also measured multiple habitat structure variables and estimated lodgepole pine seed abundance.
Habitat structure
We calculated total, live, and dead tree basal areas within a 3-m radius of each station from measured diameters at breast height of canopy trees. In each direction parallel and perpendicular to the transect (four records per station), we measured canopy cover at each station using a spherical densiometer and visually estimated percent ground cover within four 1-m × 1-m quadrats positioned 3 m from each station. Ground cover categories included: standing trees (not analyzed), coarse wood (> 8 cm in diameter), litter and downed wood < 8 cm in diameter, shrubs and tree seedlings, herbaceous plants, and bare soil or moss. Along four 3-m transects in each direction parallel and perpendicular to the transect, we measured coarse wood diameters (Brown 1974) at each station. At the 1-, 2-, and 3-m marks along these 3-m transects, we recorded the height of coarse wood (if present) within a 10-cm radius.
Lodgepole seed abundance
To estimate seeds available to granivores before and after fire, we calculated lodgepole pine seed abundance indices (hereafter, pre-fire SAI and post-fire SAI) from cone abundance counts at each station. In each quadrant of a 9-m radius plot centered on each station, we tallied cone abundance for the three nearest live or dead canopy trees deemed alive before the fire for a total of up to 12 trees per station. We noted whether lodgepole pines bore serotinous or non-serotinous cones (as in Tinker et al. 1994; Turner et al. 1997, 1999) and scored cone abundance in one of five categories: no cones, 1–10 cones, 11–100 cones, 101–1000 cones, or > 1000 cones. We used each category’s midpoint cone abundance (e.g., 5, 50, or 500 cones) or 1000 cones (for the “> 1000 cones” category) as the tree’s cone abundance. To represent the annual mean post-dispersal lodgepole pine seed supply (i.e., seeds released from cones, available to any granivores), we calculated SAIs for each station as:
in which S is the average number of seeds per cone that we assume to be 10 for serotinous and non-serotinous lodgepole pines (Koch 1996), i is an integer from 0 to 12 representing up to 12 sampled trees per station, and each term with the subscript i represents the per-tree cone abundance of each: non-serotinous tree alive after fire (NLi), non-serotinous tree dead after fire (NDi), serotinous tree alive after fire (SLi), and serotinous tree dead after fire (SDi). In both equations, the first term after the summation represents the total number of cones per station estimated to open in the canopy in the absence of fire, calculated as 25% of cones per tree (Mason 1915). The second term after the summation represents the total number of cones per station estimated to open on the ground, calculated as 1.44% of cones per tree under the assumptions that 3% of cones per tree are dropped (Hellum 1983), 48% of which open due to soil-surface heating (Teste et al. 2011). In the calculation of post-fire SAI, the third term after the summation represents the total number of cones per station estimated to open in and survive the presence of fire (i.e., from serotinous trees only), calculated as 100% of cones per tree.
Statistical analyses
How do seed removal and rodent activity vary between recently burned and adjacent unburned forests and with distance from fire perimeter?
To test for differences in the proportion of intact seeds remaining in trays (effectively, differences in seed removal), we used a generalized linear mixed model (GLMM) with a binomial response distribution. Our model included three fixed effects (Burned: burned or unburned forest; Distance: 10 or 40 m; and a Burned*Distance interaction term) and one random effect (Burned|Transect) to account for variation among transects within our split-plot study design of each transect. To test for differences in diurnal and nocturnal rodent detection rates among cameras at stations, we used two linear mixed models (LMMs) (one for diurnal and one for nocturnal rodent activity) with logistic transformations to improve data normality and the model structure described above, with one additional random effect to account for differences in camera models.
Prior to model fitting, we used Levene’s test to confirm equal variance among all levels of fixed effects. After fitting the GLMM using the glmer function in the lme4 package of R (R version 3.4.0, R Core Team, Vienna, Austria, 2017), we used a likelihood-ratio test of type 3 for hypotheses testing. After fitting the LMMs with maximum likelihood using the lmer function, we used the Kenward-Rogers approximation for degrees of freedom in analyses of variance (ANOVAs) of type 3 for hypotheses testing of our split-plot design (Littell et al. 2006). For all models, we performed diagnostics to check residuals for normality, linearity, and constant variance.
Which microhabitat conditions explain variability in seed removal and rodent activity?
To test for variation in seed removal and rodent activity with microhabitat conditions, we used GLMMs and LMMs. First, to scale and improve normality of our 15 microhabitat variables, we performed logit and square root transformations and a z-standardization. Because some microhabitat variables were highly correlated, we then used principal component analysis (PCA) using the princomp function in the stats package to reduce data dimensionality. We examined the scree plot to determine the number of principal component (PC) axes to use as fixed effects in models.
We constructed separate models for burned and unburned forests because some of their microhabitat conditions differed. GLMMs for seed removal included the first three principal components and all interactions as fixed effects and a random effect for Transect. LMMs for diurnal and nocturnal rodent activity included the additional random effect for camera model. We constructed candidate models containing all possible combinations of fixed effects. After fitting the models, we used Akaike’s information criterion corrected for small sample size (AICc) using the AICc function in the MuMIn package for model selection and used the hypotheses testing approaches described above. For the top models (∆ AICc < 2), we performed model diagnostics to check residuals for normality, linearity, and constant variance.
To assess whether seed removal and overall, diurnal, or nocturnal rodent activity were correlated, we used Kendall rank correlation tests. Data from an additional three cameras were excluded due to loss of the soil samples from the camera’s tray, resulting in 28 stations for these analyses. All analyses were performed in R. Because our sample size was small and we did not want to miss biologically meaningful relationships, we used α = 0.10 for hypothesis testing. When reporting variation around a mean, we report one standard error.
Results
Lodgepole pine was the dominant tree species on transects, but its relative abundance differed among fires. The Alum and Cygnet Fires were > 99% lodgepole pine, whereas the Fontenelle Fire burned a mixed conifer forest with 36% lodgepole pine. Burn severity was higher in burned than in unburned forests (mean = 3.5 ± 0.1 vs. 1.2 ± 0.1, respectively. Elevation did not differ between unburned and burned forests (mean = 2539 ± 9 vs. 2530 ± 7 m). Total (live + dead) basal area did not differ between burned and unburned forests (mean = 4.4 ± 0.8 vs. 3.4 ± 0.4 m2 ha−1). Burned forests, compared to unburned forests, had substantially more dead basal area (mean = 3.3 ± 0.6 vs. 0.8 ± 0.3 m2 ha−1), less live basal area (mean = 1.1 ± 0.7 vs. 2.6 ± 0.4 m2 ha−1), and lower canopy cover (mean = 45.3 ± 3.7 vs. 69.5 ± 2.6%), although canopy cover in burned forests was still considerable. In burned compared to unburned forests, we found no difference in coarse wood diameter (mean = 9.8 ± 1.0 vs. 8.1 ± 0.9 cm), height (mean = 3.5 ± 0.6 vs. 4.5 ± 1.0 cm) or cover (mean = 7.7 ± 0.9 vs. 9.5 ± 1.4%). Herb cover also did not differ between burned and unburned forest (mean = 11.3 ± 1.1 vs. 11.7 ± 1.1%). In burned forests compared to unburned forests, shrub cover was nearly 10x less (mean = 1.3 ± 0.2 vs. 9.4 ± 1.3%) and bare ground was nearly 3x greater (mean = 34.3 ± 3.5 vs. 12.2 ± 1.6%). Percent cover by litter was lower in burned forests than in unburned forests (mean = 44.4 ± 3.7 vs. 56.5 ± 2.5%), but still present. Pre-fire SAI did not differ between unburned and burned forests (mean = 243 ± 32 vs. 215 ± 34 seeds per 9-m radius plot), but post-fire SAI was higher in unburned than in burned forests (mean = 190 ± 29 vs. 7 ± 4 SE seeds per 9-m radius plot).
Seed removal and rodent activity between burned and unburned forests and with distance from fire perimeter
Seed removal
A total of 3016 out of approximately 19,684 supplied seeds remained in 76 trays as intact seeds (i.e., overall 85% seed removal, which includes in situ granivory of 2407 seeds remaining as hulls (12% of supplied seeds)). Among the 76 stations, seed removal ranged from 11 to 100% (mean = 85% ± 2.7% SE; Table 1a). The percentage of intact seeds remaining in trays did not differ between burned and unburned forests (86% vs. 83% seed removal, respectively; \(\chi_{7}^{2}\) = 0.76, p = 0.38) or between 40 and 10 m from the fire’s edge (83% vs. 86% seed removal, respectively; \(\chi_{7}^{2}\) = 0.12, p = 0.73), and there was no interaction between burn status and distance (\(\chi_{7}^{2}\) = 0.07, p = 0.79; Table 2a, Fig. 2a).
Rodent activity
During the total 719.8 days of survey time, the 31 cameras recorded a total of 2201 animal detection events. Rodents were detected on 90% of cameras (i.e., on 28 of 31 cameras), with an overall mean detection rate of 3.6 ± 1.9 SE detections per day. Nocturnal rodent activity was approximately twice that of diurnal rodents (mean = 2.4 ± 1.8 SE vs. mean = 1.2 ± 0.7 SE detections per day, respectively; Table 1b) and was lower in burned forests than in unburned forests (0.2 vs. 4.8 detections per day, respectively; F1,7 = 9.807, p = 0.02), but there was no difference in activity between stations at 40 and 10 m from the fire’s edge (0.7 vs. 4.5 detections per day, respectively; F1,13= 0.419, p = 0.53) and no significant interaction between burn status and distance (F1,13 = 0.032, p = 0.86; Table 2b, Fig. 2b). Diurnal rodent activity did not differ between burned and unburned forests (2.1 vs. 0.4 detections per day, respectively; F1,7 = 0.079, p = 0.79) or between 40 and 10 m from the fire’s edge (0.9 vs. 1.6 detections per day, respectively; F1,14= 0.001, p = 0.97), and there was no significant interaction between burn status and distance (F1,14 = 0.022, p = 0.89; Table 2b, Fig. 2c).
Seed removal and rodent activity with microhabitat conditions
We retained the first three PC axes, which cumulatively explained 59% of the variation in the microhabitat conditions at stations (PC1: 30%, PC2: 17%, and PC3: 12%; Table 3). The first PC axis largely separated stations in burned versus unburned forest through measurements of bare ground, canopy cover, and litter; hereafter, “burned axis”. The second PC axis was positively associated with coarse wood size and cover; hereafter, “coarse wood axis”. The third PC axis contrasted herbs and pre-fire SAI; hereafter, “pre-fire seed abundance axis”. All three PC axes contained variables with loadings whose absolute values were > 0.3 (Table 3), which is commonly interpreted to indicate significance.
Seed removal
In both burned and unburned forests, the intercept-only model was the only top model (Table 4a).
Rodent activity
In burned forests, for diurnal and nocturnal rodent activity, the intercept-only model was the best fit (Table 4b). However, for nocturnal rodents, one other supported model suggested activity in burned forests decreased with the burned axis (F1,9 = 3.52, p = 0.09, pseudo-r2 = 0.73; Table 4b). In unburned forests, for diurnal rodent activity, the intercept-only model was the best fit (Table 4b). However, the top model for nocturnal rodents suggested activity in unburned forests decreased with the coarse wood axis (F1,13 = 4.55, p = 0.05, pseudo-r2 = 0.25; Table 4b). Three other supported models included the intercept-only model and another model that suggested nocturnal rodent activity decreased with the coarse wood axis (F1,12 = 6.42, p = 0.03).
We found no significant correlations between seed removal and overall, diurnal, or nocturnal rodent activity. However, we recorded evidence of probable in situ seed consumption by rodents from the camera detections: animals eating and located on the seed removal trays of a few stations, including American red squirrels at a 40-m station in burned forest, chipmunks at 10-m station in burned forest, and mice or voles at a 10-m station in unburned forest.
Discussion
Seed removal was high, and, counter to our expectations, did not differ between recently burned and adjacent unburned subalpine forests of the GYE or vary with distance from fire perimeter. Nocturnal rodent activity was lower in burned compared to unburned forests; however, our results suggest diurnal rodents did not avoid lodgepole pine forests that burned at high severity. Although rodent activity was associated with microhabitat conditions in burned and unburned forests, seed removal was not related to any measured microhabitat conditions, and we found no direct correlations between seed removal and rodent activity. Nevertheless, high rates of seed removal suggest animal foraging could affect lodgepole pine recruitment.
Similar levels of seed removal and diurnal rodent activity between burned and unburned forests suggest some rodents exhibit behavioral resilience to stand-replacing fires as quickly as 1–2 years after fire. Rodent populations that initially decrease after North American conifer forest fires can return to pre-fire levels in as little as a year (Bond 2015). Because vegetation in the GYE is highly resilient to stand-replacing fire (e.g., Turner et al. 2007; Romme et al. 2011), diurnal rodents may perceive the disturbance-generated edge contrast between burned and unburned forests as inconsequential. For example, even in high-severity fires, little coarse wood (16%) is lost (Tinker and Knight 2000), and this likely provides cover for diurnal rodents in burned forests. While abundances of many rodent taxa are often equal in burned and unburned forests (Griffiths and Brook 2014); other diurnal species are often less abundant in burned forests (e.g., American red squirrels, Podruzny et al. 1999). Nevertheless, our results provide support for the ecological importance of post-disturbance biotic legacies in forest landscapes (Swanson et al. 2011) for diurnal rodents.
Reduced activity of nocturnal rodents in recently burned forests likely reflects differences in habitat, as stand-replacing fires in Greater Yellowstone subalpine forests kill the trees, consume fine canopy fuels and litter, and expose mineral soil. Although lower abundances of rodents in burned forests primarily have been attributed to changes in coarse wood, herb, and shrub cover that affect moisture and thermal cover (Fisher and Wilkinson 2005), our results only partially support this explanation because only shrub cover (but not coarse wood and herbs) was lower in burned forests. However, reduced canopy cover may play a role, as it likely elevates perceived predation risk for nocturnal rodents due to increased light penetration (e.g., Orrock and Danielson 2004). As habitat use of burned areas is species-specific (Zwolak and Foresman 2007; Zwolak 2009; Bond 2015), past studies have shown supporting and contrary evidence for nocturnal rodents in similar ecosystems. For example, deer mice are often found in elevated abundances in burned forests (Zwolak and Foresman 2008), likely due to increased foraging efficiency (Zwolak et al. 2012), and can contribute to high seed removal in burned forests (Zwolak et al. 2010). As deer mice consume lodgepole pine seeds (Lobo et al. 2009), they are likely also an important post-dispersal seed predator in our system. Further studies quantifying species-specific activity and granivory in lodgepole pine forests (e.g., rodent exclusion and seed addition experiments) are needed to clarify our findings.
Our results suggesting seed removal and rodent activity were not influenced by distance to fire’s edge was unexpected because, in many systems, rodent granivory varies with comparable distances from habitat edges (Bowers and Dooley 1993; Ostfeld et al. 1997). However, edge effects would likely be less apparent when considering the broad taxonomic groups we analyzed than in analyses of individual species (e.g., red squirrels; Roppe and Hein 1978), as rodent responses to habitat edges often vary among species. Nevertheless, this lack of an edge effect may reflect the substantial spatial heterogeneity present in natural disturbances (Foster et al. 1998). Shapes of burned patches were complex, and 40 m was the maximum distance from a green edge we could consistently achieve when running transects into burned areas. Even in the very large and severe 1988 wildfires in YNP, more than 75% of the severe-surface burn area was within ~ 40 m of a green forest edge (Turner et al. 1994). Complex spatial patterns of burn severity have been quantified for many large fires throughout the intermountain west (e.g., Collins et al. 2007). This spatial heterogeneity may allow for forest resilience to fire and provide adequate opportunities for some rodents to maintain their activity at various distances from habitat edges.
Multiple microhabitat conditions in burned and unburned forests were important in explaining rodent activity, but not seed removal. Interestingly, this result suggests that, although rodent activity in general was influenced by microhabitat conditions, foraging activity (i.e., resulting in seed removal) may have been unaffected by microhabitat conditions. This disconnect could stem from behavioral differences between individuals and/or species, i.e., the amount of time an animal spends in the view of a camera likely is not perfectly correlated with the amount of seeds removed. For example, we could observe the same rate of seed removal as a result of (i) one rodent quickly removing 85% of seeds to be eaten out of view of the camera (low rodent activity, high seed removal) or (ii) ten rodents on separate occasions each quickly removing 8.5% of seeds to be eaten out of view of the camera (high rodent activity, high seed removal). In burned forests, microhabitat conditions related to higher burn severity (e.g., lower canopy cover, higher bare ground cover) were associated with lower rodent activity. In unburned forests, higher coarse wood cover was associated with lower rodent activity. Although relationships between rodents and coarse wood can vary within species and across geographic areas (Bunnell et al. 2002), downed woody material and standing dead wood in forests is important to a variety of rodents (Fisher and Wilkinson 2005) for traveling, resting, nesting, refuge, and/or food resources (Carey and Johnson 1995; Bull 2002). Overall, our results support an established body of literature documenting the importance of various microhabitat conditions to rodents (e.g., Whelan et al. 1991; Bowers and Dooley 1993; Manson and Stiles 1998).
We failed to detect a direct correlation between rodent activity and seed removal. This result suggests differences in foraging behavior may be responsible this lack of a relationship (as described above) and/or granivores not considered in our analysis (e.g., birds) could also contribute to seed removal. However, rodents are considered to be major granivores in coniferous forests (Lobo 2014), and our in situ seed consumption counts and camera data (including photo evidence of probable in situ seed consumption by rodents) supported this assumption.
Although we did not study the fate of all removed seeds, we have good reasons to believe that the mean of 85% seed removal represents granivory or seed death instead of seed dispersal (Moles et al. 2003), which could enhance lodgepole pine germination. First, 99 percent of stations had evidence of in situ seed consumption in the form of seed hulls equal to 12% of supplied seeds. Second, removed seeds may be cached by animals instead of immediately eaten, but since seed-caching of lodgepole pine seems to be rare (Vander Wall 2003), lodgepole pines are unlikely to benefit from dispersal by animals (Vander Wall 2003). Studies specifically addressing the fate of removed lodgepole pine seeds are needed. However, if we assume removed seeds are consumed or relocated to unsuitable germination sites and the seed removal we observed represents natural conditions, then, at maximum, an average of 15% of seeds were potentially viable after a 28-day study period. In areas where lodgepole pine recruitment might be microsite-limited, this high rate of seed removal could have little to no impact on tree regeneration. However, where seed supply is limiting for recruitment, our results suggest that granivory could depress recruitment of a widespread foundation tree species.
Projections for increased fire activity associated with climate change in the GYE (Westerling et al. 2011) and other regions of the world suggest that future landscapes will increasingly be composed of mosaics of burned and unburned forests. Thus, it is important to improve understanding of animal community dynamics and drivers of post-fire succession within these mosaics. Changing climate conditions in the future could lead to increased rates of granivory across broad spatial extents (Orrock et al. 2015), further exacerbating potential granivore-driven reductions of lodgepole pine regeneration. The effects of pre-dispersal seed predators on lodgepole pine already are considerable (Benkman and Siepielski 2004; Talluto and Benkman 2014), and our data suggest post-dispersal seed predators may also affect seed availability for lodgepole pine recruitment. If lodgepole pine regeneration is threatened in the future [e.g., due to larger fires with shortened fire return intervals (Hansen et al. 2018)], granivores may aggravate the problem. Our research suggests, in the face of climate change, it is critical that we better understand the ecological consequences of interactions among granivore behavior, forests, and fire.
References
Anderson JE, Romme WH (1991) Initial floristics in lodgepole pine (Pinus contorta) forests following the 1988 Yellowstone fires. Int J Wildl Fire 1:119–124
Ball DA, Miller SD (1990) Weed seed population response to tillage and herbicide use in three irrigated cropping sequences. Weed Sci 38:511–517
Bendell JF (1974) Effects of fire on birds and mammals. In: Kozlowski TT, Ahlgren CE (eds) Fire and ecosystems. Academic Press Inc., New York, pp 73–138
Benkman CW, Siepielski AM (2004) A keystone selective agent? Pine squirrels and the frequency of serotiny in lodgepole pine. Ecology 85:2082–2087
Bond ML (2015) Mammals and mixed- and high-severity fire. In: DellaSala DA, Hanson CT (eds) The ecological importance of mixed-severity fires: nature’s phoenix. Elsevier Inc., New York, pp 89–117
Borchert MI, Farr DP, Rimbenieks-Negrete MA, Pawlowski MN (2014) Responses of small mammals to wildfire in a mixed conifer forest in the San Bernardino Mountains, California. Bull South Calif Acad Sci 113:81–95
Bowers MA, Dooley JL (1993) Predation hazard and seed removal by small mammals: microhabitat versus patch scale effects. Oecologia 94:247–254
Boyce MS, Mao JS, Merrill EH, Fortin D, Turner MG, Fryxell J, Turchin P (2003) Scale and heterogeneity in habitat selection by elk in Yellowstone National Park. Ecoscience 10:421–431
Brown JK (1974) Handbook for inventorying downed woody material. USFS Gen Tech Rep INT-16, Ogden, UT
Brown JS (1988) Patch use as an indicator of habitat preference, predation risk, and competition. Behav Ecol Sociobiol 22:37–47
Brown JH, Heske EJ (1990) Control of a desert-grassland transition by a keystone rodent guild. Science 250:1705–1707
Bull EL (2002) The value of coarse woody debris to vertebrates in the Pacific Northwest. In: Proceedings of the symposium on the ecology and management of dead wood in western forests. USFS Gen Tech Rep PSW-GTR-181, Albany, pp 171–178
Bunnell FL, Houde I, Johnston B, Wind E (2002) How dead trees sustain live organisms in western forests. In: Proceedings of the symposium on the ecology and management of dead wood in western forests. USFS Gen Tech Rep PSW-GTR-181, Albany, pp 291–318
Carey AB, Johnson ML (1995) Small mammals in managed, naturally young, and old-growth forests. Ecol Appl 5:336–352
Collins BM, Kelly M, van Wagtendonk JW, Stephens SL (2007) Spatial patterns of large natural fires in Sierra Nevada wilderness areas. Landscape Ecol 22:545–557
Craig MT, Orrock JL, Brudvig LA (2011) Edge-mediated patterns of seed removal in experimentally connected and fragmented landscapes. Landscape Ecol 26:1373–1381
Crawley MJ (2014) Seed predators and plant population dynamics. In: Gallagher RS (ed) Seeds: the ecology of regeneration in plant communities, 3rd edn. CAB International, Boston, pp 94–110
Critchfield WB (1980) The genetics of lodgepole pine. USFS Res Pap WO-37, Washington, DC
Denham AJ (2008) Seed predation limits post-fire recruitment in the waratah (Telopea speciosissima). Plant Ecol 199:9–19
Fisher JT, Wilkinson L (2005) The response of mammals to forest fire and timber harvest in the North American boreal forest. Mamm Rev 35:51–81
Fontaine JB, Kennedy PL (2012) Meta-analysis of avian and small-mammal response to fire severity and fire surrogate treatments in U.S. fire-prone forests. Ecol Appl 22:1547–1561
Forester JD, Ives AR, Turner MG, Anderson DP, Fortin D, Beyer HL, Smith DW, Boyce MS (2007) State-space models link elk movement patterns to landscape characteristics in Yellowstone National Park. Ecol Monogr 77:285–299
Foster DR, Knight DH, Franklin JF (1998) Landscape patterns and legacies resulting from large, infrequent forest disturbances. Ecosystems 1:497–510
Griffiths AD, Brook BW (2014) Effect of fire on small mammals: a systematic review. Int J Wildl Fire 23:1034–1043
Gurnell J (1984) Home range, territoriality, caching behavior and food supply of the red squirrel (Tamiasciurus hudsonicus fremonti) in a subalpine lodgepole pine forest. Anim Behav 32:1119–1131
Hansen WD, Braziunas KH, Rammer W, Seidl R, Turner MG (2018) It takes a few to tango: changing climate and fire regimes can cause regeneration failure of two subalpine conifers. Ecology 99:966–977
Hellum AK (1983) Seed production in serotinous cones of lodgepole pine. In: Murray M (ed) Lodgepole pine: regeneration and management. USFS Gen Tech Rep PNW-157, Portland, pp 23–27
Holbrook SJ, Schmitt RJ (1988) The combined effects of predation risk and food reward on patch selection. Ecology 69:125–134
Hulme PE (1998) Post-dispersal seed predation: consequences for plant demography and evolution. Perspect Plant Ecol Evol Syst 1:32–46
Hulme PE, Kollmann J (2005) Seed predator guilds, spatial variation in post-dispersal seed predation and potential effects on plant demography: a temperate perspective. In: Forget P-M, Lambert JE, Hulme PE, Vander Wall SB (eds) Seed fate: predation, dispersal, and seedling establishment. CAB International, Wallingford, pp 9–30
Johnstone JF, Rupp TS, Olson M, Verbyla D (2011) Modeling impacts of fire severity on successional trajectories and future fire behavior in Alaskan boreal forests. Landscape Ecol 26:487–500
Kays R, Tilak S, Kranstauber B, Jansen PA, Carbone C, Rowcliffe M, Fountain T, Eggert J, He Z (2011) Camera traps as sensor networks for monitoring animal communities. Int J Res Rev Wirel Sens Netw 1:19–29
Koch P (1996) Lodgepole pine in North America. Forest Products Society, Madison
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Press, Cary
Lobo N (2014) Conifer seed predation by terrestrial small mammals: a review of the patterns, implications, and limitations of top-down and bottom-up interactions. For Ecol Manage 328:45–54
Lobo N, Duong M, Millar JS (2009) Conifer-seed preferences of small mammals. Can J Zool 87:773–780
Manson RH, Stiles EW (1998) Links between microhabitat preferences and seed predation by small mammals in old fields. Oikos 82:37–50
Mason DT (1915) The life history of lodgepole pine in the Rocky Mountains. USFS Bull No. 154
Moles AT, Warton DI, Westoby M (2003) Do small-seeded species have higher survival through seed predation than large-seeded species? Ecology 84:3148–3161
Orrock JL, Damschen EI (2005) Corridors cause differential seed predation. Ecol Appl 15:793–798
Orrock JL, Danielson BJ (2004) Rodents balancing a variety of risks: invasive fire ants and indirect and direct indicators of predation risk. Oecologia 140:662–667
Orrock JL, Danielson BJ (2005) Patch shape, connectivity, and foraging by oldfield mice (Peromyscus polionotus). J Mamm 86:569–575
Orrock JL, Danielson BJ, Brinkerhoff RJ (2004) Rodent foraging is affected by indirect, but not by direct, cues of predation risk. Behav Ecol 15:433–437
Orrock JL, Levey DJ, Danielson BJ, Damschen EI (2006) Seed predation, not seed dispersal, explains the landscape-level abundance of an early-successional plant. J Ecol 94:838–845
Orrock JL, Borer ET, Brudvig LA, Firn J, MacDougall AS, Melbourne BA, Yang LH, Baker DV, Bar-Massada A, Crawley MJ, Damschen EI, Davies KF, Gruner DS, Kay AD, Lind E, McCulley RL, Seabloom EW (2015) A continent-wide study reveals clear relationships between regional abiotic conditions and post-dispersal seed predation. J Biogeogr 42:662–670
Ostfeld RS, Manson RH, Canham CD (1997) Effects of rodents on survival of tree seeds and seedlings invading old fields. Ecology 78:1531–1542
Podruzny SR, Reinhart DP, Mattson DJ (1999) Fire, red squirrels, whitebark pine, and Yellowstone grizzly bears. Ursus 11:131–138
Romme WH, Boyce MS, Gresswell R, Merrill EH, Minshall GW, Whitlock C, Turner MG (2011) Twenty years after the 1988 Yellowstone fires: lessons about disturbance and ecosystems. Ecosystems 14:1196–1215
Roppe JA, Hein D (1978) Effects of fire on wildlife in a lodgepole pine forest. Southwest Nat 23:279–287
Schoennagel T, Turner MG, Romme WH (2003) Influence of fire interval and serotiny on postfire lodgepole pine density in Yellowstone National Park. Ecology 84:2967–2978
Smith CC (1970) The coevolution of pine squirrels (Tamiasciurus) and conifers. Ecol Monogr 40:349–371
Swanson ME, Franklin JF, Beschta RL, Crisafulli CM, DellaSala DA, Hutto RL, Lindenmaver DB, Swanson FJ (2011) The forgotten stage of forest succession: early-successional ecosystems on forest sites. Front Ecol Environ 9:117–125
Tallmon DA, Jules ES, Radke NJ, Mills LS (2003) Of mice and men and trillium: cascading effects of forest fragmentation. Ecol Appl 13:1193–1203
Talluto MV, Benkman CW (2014) Conflicting selection from fire and seed predation drives fine-scaled phenotypic variation in a widespread North American conifer. Proc Natl Acad Sci 111:9543–9548
Teste FP, Lieffers VJ, Landhausser SM (2011) Seed release in serotinous lodgepole pine forests after mountain pine beetle outbreak. Ecol Appl 21:150–162
Tinker DB, Knight DH (2000) Coarse woody debris following fire and logging in Wyoming lodgepole pine forests. Ecosystems 3:472–483
Tinker DB, Romme WH, Hargrove WW, Gardner RH, Turner MG (1994) Landscape-scale heterogeneity in lodgepole pine serotiny. Can J For Res 24:897–903
Turner MG (2010) Disturbance and landscape dynamics in a changing world. Ecology 91:2833–2849
Turner MG, Hargrove WW, Gardner RH, Romme WH (1994) Effects of fire on landscape heterogeneity in Yellowstone National Park, Wyoming. J Veg Sci 5:731–742
Turner MG, Romme WH, Gardner RH (1999) Prefire heterogeneity, fire severity, and early postfire plant reestablishment in subalpine forests of Yellowstone National Park, Wyoming. Int J Wildl Fire 9:21–36
Turner MG, Romme WH, Gardner RH, Hargrove WW (1997) Effects of fire size and pattern on early succession in Yellowstone National Park. Ecol Monogr 67:411–433
Turner MG, Romme WH, Tinker DB (2003) Surprises and lessons from the 1988 Yellowstone fires. Front Ecol Environ 1:351–358
Turner MG, Turner DM, Romme WH, Tinker DB (2007) Cone production in young post-fire Pinus contorta stands in Greater Yellowstone (USA). For Ecol Manage 242:119–126
USDA Forest Service (2015) InciWeb: incident information system. https://inciweb.nwcg.gov. Accessed 1 Jul 2015
USDA Forest Service/U.S. Geological Survey (2015) MTBS Data Access: fire level geospatial data. In: MTBS Proj. http://mtbs.gov/direct-download. Accessed 1 Jul 2015
Vander Wall SB (2003) Effects of seed size of wind-dispersed pines (Pinus) on secondary seed dispersal and the caching behavior of rodents. Oikos 100:25–34
Westerling AL, Turner MG, Smithwick EAH, Romme WH, Ryan MG (2011) Continued warming could transform Greater Yellowstone fire regimes by mid-21st century. Proc Natl Acad Sci 108:13165–13170
Whelan CJ, Wilson MF, Tuma CA, Souza-Pinto I (1991) Spatial and temporal patterns of postdispersal seed predation. Can J Bot 69:428–436
Whitlock C, Marlon J, Briles C, Brunelle A, Long C, Bartlein P (2008) Long-term relations among fire, fuel, and climate in the north-western US based on lake-sediment studies. Int J Wildl Fire 17:72–83
Zwolak R (2009) A meta-analysis of the effects of wildfire, clearcutting, and partial harvest on the abundance of North American small mammals. For Ecol Manage 258:539–545
Zwolak R, Foresman KR (2007) Effects of a stand-replacing fire on small-mammal communities in montane forest. Can J Zool 85:815–822
Zwolak R, Foresman KR (2008) Deer mouse demography in burned and unburned forest: no evidence for source-sink dynamics. Can J Zool 86:83–91
Zwolak R, Pearson DE, Ortega YK, Crone EE (2010) Fire and mice: seed predation moderates fire’s influence on conifer recruitment. Ecology 91:1124–1131
Zwolak R, Pearson DE, Ortega YK, Crone EE (2012) Mechanisms driving postfire abundance of a generalist mammal. Can J Zool 90:51–60
Acknowledgements
We thank John Orrock and Jon Pauli for constructive comments that improved the manuscript. We thank Timothy Whitby, Winslow Hansen, and Kari Forseth for assistance with field data collection and Mike Kozuch and Claire Olsen for laboratory data collection and processing. Jon Pauli, Cristina Herren, Phil Hahn, and members of MGT’s and John Orrock’s labs provided useful feedback on study design, methodology, and/or data analyses. Field work was facilitated by Yellowstone National Park and the Bridger-Teton National Forest; we especially thank Roy Renkin and Diane Abendroth for their assistance. This work was funded by the Joint Fire Science Program (11-1-1-7), a National Science Foundation Graduate Research Fellowship (DGE-1256259) to CFF, a University of Wisconsin-Madison Department of Zoology Graduate Student Summer Research Award to CFF, and funding to MGT from the University of Wisconsin Vilas Trust.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Frock, C.F., Turner, M.G. Microhabitat conditions and landscape pattern explain nocturnal rodent activity, but not seed removal, in burned and unburned lodgepole pine forests. Landscape Ecol 33, 1895–1909 (2018). https://doi.org/10.1007/s10980-018-0717-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-018-0717-x