Abstract
Forest liming is often used as a mitigation tool to counteract soil acidification that is caused by atmospheric deposition. However, our understanding of the long-term effects of liming on forest communities is still incomplete. We examine the effects of repeated liming on the herb layer of spruce stands in the Ore Mountains in East Germany. The area is characterised by acidic bedrock and profound soil acidification caused by high levels of anthropogenic sulphur deposition in the late twentieth century. Lime has been applied as part of forest management since 1988. We created a stratified sample in which we recorded vegetation relevés covering spruce stands without liming (L0) and with one to five lime applications (L1–L5). We found a positive correlation between liming intensity and alpha diversity. Most of the indicator species were identified for spruce stands with high liming intensity (L2–L5), but no indicators were found for unlimed stands. From the third liming, the impact of lime application on species diversity and composition saturated. Several of the species promoted by liming are typical for mature stands of the natural vegetation but had declined in the twentieth century. Thus, a slight trend towards the restoration of the natural vegetation can be seen after liming. However, this is contrasted with a strong increase in species that are disturbance indicators (i.e. species of nitrogen-rich soils and open habitats). Continuation of liming as part of regular forestry management will cause these effects to establish permanently over large areas. Based on these findings, we make suggestions to adapt forest management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthropogenic acid deposition has been impacting forest ecosystems in Europe for the last 200 years. Soil acidification was particularly severe in the surroundings of industrial areas and towns due to SO2 emissions from burning coal. This caused damage, above all to conifer species, as well as the death of whole stands (Däßler 1991). The effects on forest soils were, among others, a reduction in pH, leaching of basic cations and the destruction of clay minerals together with the release of aluminium ions (Ulrich 1986). Various studies of the forest floor vegetation found a resulting decline in species requiring less acid soils and an increase in indicators of acid conditions (e.g. Wittig et al. 1985; Schmidt 1993; Økland et al. 2004).
Acid deposition in forests was widely researched and discussed in the second half of the twentieth century (Gorham 1989). The most important measure to combat soil acidification was considered to be liming (Ulrich 1987; Van Breemen 1990; Reif et al. 2014). Liming aims to ameliorate already acidified soils as well as buffer future acid inputs, but its appropriateness as a forest management measure is the subject of much debate (Lundström et al. 2003a; Reif et al. 2014). Positive effects include an increase in soil pH and base saturation (Immer et al. 1993; Leitgeb 1994; Hallbäcken and Zhang 1998), which prevents or at least slows further destruction of clay minerals (Reif et al. 2014). It also increases soil faunal activity and thus leads to the development of more favourable humus types (Anderson 1998; Kraft et al. 2003; Huber et al. 2006; Rineau and Garbaye 2009). However, the effects of liming are often limited to the organic layer and the topsoil to 30 cm depth (Guckland et al. 2012; Lorenz et al. 2001). Negative effects of liming on forest communities have also been found, for example in the almost complete change in species composition of mycorrhizal communities, which is of great importance for tree vitality and growth (Lundström et al. 2003b).
One important indicator to evaluate the effects of liming is the forest herb layer. The herb layer is relatively easy and quick to survey and is a complex systemic indicator (Beese 1996), i.e. it gives insights into the way the forest ecosystem is reacting. Numerous studies in Central Europe have investigated the effects of liming on the forest floor vegetation. They show a strong increase in species richness, mostly of nitrogen indicators (Senecio ovatus, Urtica dioica, Epilobium angustifolium, Geranium robertianum, etc.) and species of open habitats or forest fringes (Cerastium holosteoides, Cirsium vulgare, Dactylis glomerata, Ranunculus acris), but also forest species of nutrient-rich soils and natural springs, such as Cardamine flexuosa, Circaea alpina, Epilobium montanum and Lysimachia nemorum (Schlüter 1966; Wenzel 1989; Schmidt 1992, 2002; Hallbäcken and Zhang 1998; De Keersmaeker et al. 2000; Jandl et al. 2002; Kraft et al. 2003; Bergholm et al. 2015). The occurrence of nitrogen indicators and species of open habitats, however, is not directly driven by lime, but indicates high nitrogen availability. A higher microbial activity leads to a release of nitrate (Seibt and Wittich 1977; Neale et al. 1997), and higher nitrate concentrations can break dormancy in the seeds of many plant species (Hendricks and Taylorson 1972). Some nitrogen indicators and species of open habitats are already present in the seed bank of forest soils (Fischer 1987; Denner 2007), whilst others, in particular anemochorous species, are capable of travelling long distances to colonise new areas (Maas and Pfadenhauer 1994). This seed potential is activated by liming. The species present before liming do not necessarily disappear, but species adapted to nutrient-poor, acid soils decline (Becker et al. 1992; De Keersmaeker et al. 2000; Jandl et al. 2002; Höcke 2006).
Almost all previous investigations of the long-term effects of liming were conducted in study areas treated with a single, large dose of lime, usually in combination with other nutrients, as used to be practiced historically (e.g. Hartmann 1959; Becker et al. 1992; Hallbäcken and Zhang 1998; Jandl et al. 2002). Schornick (1990) and Höcke (2006) state that the effects of a single lime application decline over time, but are still measurable even decades later. However, current forestry best practice now recommends periodic applications at lower concentrations instead of a single application of lime. There are so far very few studies that investigated the effects of this means of liming: one exception is Schmidt (2002), who documented the development of the vegetation over two liming events over the space of 10 years, each with a dose of 3 t/ha. This study showed an increase in nitrophilic species and in Ellenberg indicator values (EIV) for soil reaction (R) and nitrogen (N) after the first liming but this was not continued after the second lime application. Seibt and Wittich (1977) found similar results, whereby initial changes in the forest herb layer after first liming did not continue when the treatment was repeated, although the observed changes were faster. Furthermore, the diversity of the vegetation played only a small role in the analyses. Only the average species richness was used as a measure of alpha diversity (e.g. De Keersmaeker et al. 2000; Schmidt 2002; Reif et al. 2014), which provides only a limited insight into the ecological processes involved. Further studies are therefore necessary to determine to what extent the results based on single liming events are relevant for areas that have been treated with multiple applications over longer periods. This is essential to derive recommendations for future forestry management.
Our study area is a large forested area in which liming began around 30 years ago. The area contains parcels of spruce plantation that have never been limed, as well as those that have been limed one up to five times. We analyse the effects of liming intensity on the species composition and the diversity of the herb layer of spruce stands. The main aim is to answer the following questions: (1) Does periodic lime application in spruce stands lead to a steadily advancing change of the herb layer? Or are the herb layer composition and diversity in stands with multiple additions similar to those in stands with only one or two lime applications? (2) Does periodic lime application cause the loss of certain plant species or does it solely impact the cover? (3) Are the plant species showing a hump-shaped response to liming intensity? (4) Based on the presumption that lime applications contribute to restore soil conditions, are plant species of the typical forest herb layer vegetation promoted by lime additions?
Methods
Study area
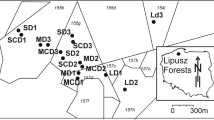
The study was carried out in the Ore Mountains, a low mountain range in the east of Germany. We identified four areas of 50–100 ha that had never been limed in a large, continuously forested area north-west of the “Fichtelberg” mountain. We defined the study area as a window of ca. 8 × 8 km around these unlimed areas. The forest stands belong to the forest district Neudorf. The study area covers elevations between 600 and 1025 m a.s.l., has an average annual temperature of 3.8–6.3 °C, and total precipitation of 900–1070 mm/year. The area is in the transition zone between suboceanic and subcontinental climates (Mannsfeld and Syrbe 2008). The bedrock is dominated by gneiss and phyllite with relatively low nutrient content. Weathering of this rock leads to the formation of coarse Cambisols and cambic Podzols. With increasing elevation, the cambic Podzols become pure Podzols (Bierögel et al. 1957). Chemical analyses of weathered gneiss and phyllite soils from the study area show little variation in nutrient content. This is supported by the nutrient classification produced by the forest site mapping (VEB Forstprojektierung 1985).
The potential natural vegetation is dominated by the Calamagrostio villosae-Fagetum. This forest community constitutes the transition between beech and spruce forests in the Ore Mountains. It is characterised by the dominance of Calamagrostis villosa in the herb layer, further important species are Trientalis europaea, Prenanthes purpurea and Polygonatum verticillatum (Schmidt et al. 2002). The natural tree species composition, however, was changed already several centuries ago, in particular by the medieval mining (Mannsfeld and Syrbe 2008). At present, Norway spruce (Picea abies (L.) H. Karst) dominates the tree layer of forests in the study area. According to data from the local forest inventory, spruce stands currently make up 91.3% of the forest area belonging to the forest district Neudorf.
With only a few exceptions, the stands in the study area are regularly managed, with interventions at approximately 10-year intervals. Due to the high elevation and the large proportion of spruce, storm and snow damage is frequent.
The SO2 concentrations in the Ore Mountains rose to very high levels in the middle of the twentieth century (SMUL 1996). With average annual values of over 200 μg/m3, the area received around ten times the critical levels defined as safe for forest ecosystems (UN/ECE 1992). As a result, thousands of hectares of spruce stands died, particularly at higher elevations and on ridges (Dittrich 2006). In the herb layer, SO2-tolerant grasses (Calamagrostis villosa, Deschampsia flexuosa) spread, and in the deciduous mixed forests the decline of species sensitive to low pH values led to a severe impoverishment of the forest communities (Schmidt 1993). Due to technical measures, the atmospheric pollution was steadily reduced during the 1990s, and currently the average annual SO2 concentration only amounts to 5 μg/m3 (LfULG 2018). The first liming treatment was carried out in the Ore Mountains in 1986 and in our study area in 1988. The initial applications were 2.5 t/ha of dolomite lime, applied aerially. Lime applications from 1995 to 2012 followed guidelines (Leube 2000), which specified 4.5 t/ha for higher elevations in the Ore Mountains in cycles of 6 years. Areas such as small stream valleys and wetlands were not limed. These guidelines were modified in practice, as the annual applications of lime were affected by changes in local responsibility and changes in stand boundaries. This led to patches of forest with very different histories of lime application. Since 2013, the applications have been set at 3.0 t/ha in a cycle of 10 years.
Vegetation analysis
We analysed pre-existing spruce stands with varying liming intensities. Measuring the intensity as number of lime applications over the last 30 years produces a gradient from zero (L0) over one (L1) to five (L5) in the study area. The patches of different liming intensities are of different sizes and are not regularly distributed. The study area can therefore be divided into six management classes (L0, L1–L5), which formed the basis for our stratified sampling (see Usher 1991; Krebs 1999).
Within each liming class, we selected spruce stands on condition that the other environmental factors were kept as constant as possible (see Greene 1979). We therefore restricted the stand selection to sites of the Calamagrostio villosae-Fagetum and did not consider sites of other forest communities. Only stands with well-developed forest floor vegetation were selected. This meant that we excluded young, dense stands and only used stands with a minimum age of 60 years. To avoid spatial autocorrelation between the plots, we selected several stands (at least five) for each class distributed across the study area and limited the number of plots to three per stand (see Hurlbert 1984).
We selected plots for vegetation surveys in areas of homogeneous vegetation (Dierschke 1994), avoiding vehicle tracks, disturbed areas (e.g. large gaps, upended root plates) and piles of brushwood from thinning. Plots were located at least 20 m away from the nearest forest road and at least 40 m from the border to the next liming class. We avoided areas with mixed tree species composition. The herb layer covered minimum 30% of the plot and the shrub layer maximum 30%.
We surveyed in total 81 vegetation plots in June–September 2016, i.e. 14 plots in each class, apart from L1 and L5, in which less suitable stands were found. Each plot was 400 m2 in the form of a square. In cases, where this meant that the distance between the skid trails was 20 m, we used a rectangle shape instead. We recorded each herbaceous vascular plant species (including woody species < 0.5 m in height) in the plot following the method of Braun-Blanquet. We quantified the cover of each species in the plot using the scale of Pfadenhauer et al. (1986): r = one individual; + ≤ 1% cover; 1 = 1–5%; 2a = 5–15%; 2b = 15–25%; 3 = 25–50%; 4 = 50–75%; 5 = 75–100%. Nomenclature of the vascular plant species follows Buttler et al. (2017) and of the vegetation communities Rennwald (2000).
As lime was not uniformly applied within the classes over the last 30 years, we furthermore determined the exact cumulative quantity of lime applied (t/ha) for each plot by checking the forestry records. Furthermore, we determined how many years passed since the last liming.
As a local reference for the natural forest vegetation, we included vegetation relevés of semi-natural beech (Fagus sylvatica L.) stands (SB). They were sampled in the nature reserve Zweibach simultaneously with those in the spruce stands. Zweibach is located within the study area in the transition zone between the Luzulo-Fagetum and the Calamagrostio villosae-Fagetum. The beech stands sampled are both managed and unmanaged, but the whole nature reserve has never been limed.
Data analysis
All statistical analysis was performed using the R 3.0.3 software (R Core Team 2014). For quantitative analysis, the cover value classes of the relevés were transformed to mean average per cent values (r = 0.1%, + = 0.5%, 1 = 3%, etc.). We produced tables for each class (L0, L1–L5, and also for SB) with the constancy and average cover value for each species. We then calculated the unweighted EIV for each plot following Ellenberg et al. (2001). To investigate differences in herb layer composition between the classes L0 and L5, we carried out a correspondence analysis (CA) using log-transformed cover values (Legendre and Legendre 2012). The environmental variables “liming” and the average EIV of the plots were then overlaid for the ecological interpretation of the axes. We used package “vegan”, version 2.2-1 (Oksanen et al. 2015) for the analysis with the following functions: decorana; ira = 1: perform a CA; iweigh = 1: reduce the weighting of rare species; envfit: overlay the environmental variables and the EIV.
To identify indicator species for the various liming intensities, we used the Indicator Value Index (IndVal) (Dufrêne and Legendre 1997; De Cáceres et al. 2010). This method defines indicator species as the species characterising the group in question, i.e. they occur exclusively or predominantly in that group, as well as being found in the majority of records from that group. Rare species are thus largely excluded from being indicator species. We tested significance using permutation tests. The analysis was carried out using the package “indicspecies”, version 1.7.4 (De Cáceres and Legendre 2009).
We further examined the influence of liming intensity on different measures of diversity. As a measure of alpha diversity, we used the average species richness per plot as well as the species pool for each class using the Chao estimator (Chiu et al. 2014). We considered beta diversity measured as Sørensen dissimilarity (Koleff et al. 2003). The dominance structure was calculated using the Evenness Index E1:0, which is derived from the diversity numbers N0 and N1 (Hill 1973). The analyses were carried out in the package “vegan”, to calculate beta diversity we used the package “betapart”, version 1.3 (Baselga et al. 2013).
We used the analytical indicators (constancy and coverage of the species) to derive normative indicators by differentiating into typical for the habitat and disturbance indicators. Typical forest herb layer vegetation is defined here as the characteristic species combination (“Charakteristische Artenverbindung”—CAV) for the natural vegetation in mature stands without disturbances. Dierschke (1994) defines the CAV as containing all species that regularly occur in a community. This includes, alongside the character species themselves, also the accompanying species with high levels of constancy. We define the term “disturbance indicator” in a broad sense and also include forest species with higher nutrient demand, which are typically not part of the Calamagrostio villosae-Fagetum community.
We first assigned each species to a species group according to their EIV and their phytosociology (Table 1). This assignment is mainly based on the ecological species groups of Ewald (2007), the indicators of disturbance of Grabherr et al. (1998), the strength of their association to forest habitats according to Schmidt et al. (2011) and the indicator values and phytosociological classification according to Ellenberg et al. (2001) and Oberdorfer et al. (2001). We adapted these values to the study region following Reinhold (1939), Schubert (1972), Schmidt et al. (2002) and Chytrý (2013). We then calculated the relative coverage values of the species groups for each plot.
We computed the percentage similarity of the classes following Czekanowsky (cited in Goodall 1973). This parameter describes the relationship between the cover of the species that occur in the compared classes to the total sum cover in the classes. The percentage similarity was calculated for the classes L0–L5 compared to the semi-natural beech stands (SB).
The cover values in the vegetation surveys are not exact measurements, but rather estimations. This is also the case for derived values such as total and proportional coverage. In addition, the sample size per class (8–17) is relatively small. We therefore used conservative tests to compare averages that do not require normal distribution of data or homogeneity of variance, namely the Kruskal–Wallis test and the Dunn test with Bonferroni correction for pairwise comparisons. We did not analyse the EIV statistically as these are neither measured nor estimated values, but rather based on expert opinion.
Results
Multivariate analysis
The plots are aligned in the CA along a gradient corresponding to the first ordination axis (Fig. 1). The control (L0) and single application (L1) plots are clearly separated from the other classes on the first axis. Only the class L1 shows same overlap with plots of L0. The plots in class L2 are mostly in the middle of the axis, whilst plots in L3–L5 are concentrated on the right-hand side of the axis. The EIV show that this axis represents a gradient in nitrogen/nutrient availability and pH. The average EIV for soil reaction (pH) increases from the control (2.8) to L3 (4.3), as does the EIV for nitrogen (3.9–5.4) (Table 2). Liming intensity is strongly correlated with the first ordination axis. Note that the eigenvalue of the first axis is more than three times higher than the eigenvalue of the second axis.
Indicator species analysis
The indicator species analysis failed to find indicator species for the control plots (L0). Apart from Luzula sylvatica, this was also the case for spruce stands with low liming intensities. We therefore found no evidence of species that completely disappear with liming, although Vaccinium myrtillus and particularly Deschampsia flexuosa show a clear decrease in cover (Table 3).
In contrast, we found several indicator species differentiating the limed spruce stands (L1–L5) from the control (L0). Apart from Urtica dioica and Hieracium lachenalii, these species also occur at very low densities in unlimed spruce stands. They increase considerably with increasing liming intensity but, with the exception of Oxalis acetosella, do not exceed an average coverage of 1% in the plot. We also found multiple further indicators for spruce stands with high liming intensity (L2–L5). The majority of these indicator species occur only in limed spruce stands in our dataset; only Athyrium filix-femina, Luzula luzuloides, Senecio ovatus, Taraxacum sect. Ruderalia, Rubus idaeus and Mycelis muralis occur in the control plots. The average cover of these species is generally very low, apart from Athyrium filix-femina, Senecio ovatus, Rubus idaeus, Mycelis muralis and Circaea alpina, which have cover values of > 1%. Further indicators of the most intensively limed plots are Stellaria media (L4) and Ranunculus acris (L5). Whilst seedlings of Picea abies, Sorbus aucuparia and Fagus sylvatica occur in all classes with high constancy, Acer pseudoplatanus and Prunus avium seedlings only occur in limed spruce stands. There are some indicator species for spruce stands with high liming intensity which also show a higher frequency in the semi-natural beech stands (Luzula luzuloides, Rubus idaeus and Lysimachia nemorum).
Diversity indices
Alpha diversity is lowest in the unlimed stands (L0, see Table 2; Fig. 2a). With increasing liming intensity, the mean species richness increases from 12.6 in L0 to 34.9 in L3 (significantly different to the control in L2–L5). The class L3 also contained the plots with the highest species richness (53) and the largest species pool (117). Alpha diversity remains at this level with further increases in liming intensity.
Beta diversity is highest in the control (Table 2), and, in contrast to alpha diversity, there is a tendency towards decreasing values from the unlimed to the limed stands. The lowest values are found in the classes L4 and L5. Evenness shows the same tendency (Fig. 2b). The values of beta diversity and evenness in the semi-natural beech stands are somewhat higher than those of the unlimed spruce stands (Table 2).
Normative indicators
The herb layer in the unlimed spruce stands (L0) is comprised to almost 90% of species of the group CAV1 (Table 2; Fig. 3a). With increasing liming intensity, the cover of CAV1 species declines to 35% in L3 (with significant differences to the control in L2–L5). In contrast, more nutrient-demanding species of CAV2 increase in cover with liming (significant to control from L3), from an average of 12% in the unlimed spruce stands to 44% in L5 (Fig. 3b).
Relative cover of the species groups CAV1 (a), CAV 2 (b), BC1 (c), BC2 (d), disturbance indicators (e) and number of disturbance indicators (f) (see text for explanation of species groups) in the control (L0), and in stands of spruce with different liming intensities (L1–L5). Letters indicate significant differences (Dunn’s post hoc test for multiple comparisons after positive test result with the Kruskal–Wallis test, p ≤ 0.05)
The cover of species typically associated with acid beech forests (group BC1) also increases with liming intensity. However, the difference to L0 is only significant for L2, L4 and L5 (Fig. 3c). The level of BC1 species at these highest liming intensities is close to that of the semi-natural beech stands, but does not quite reach them (Table 2). Species associated with mesophilic beech forests increase slightly with liming intensity (Fig. 3d), but average cover in all classes of spruce stand remains < 1% (Table 2). These species only occur at high densities in the semi-natural beech stands.
Liming had a particularly strong effect on the group of species that are indicators of disturbance. Unlimed spruce plots only supported on average one species from this group with a cover of 1%. Increasing liming intensity corresponded with an increase of both the cover and the species richness (both significant to control from L2) of disturbance indicators up to L3 (Table 2; Fig. 3e, f). Plots in L3 showed an average of 16 (max. 37) species from this group with a cover of c. 28%. Species of non-forest habitats and numerous species of nutrient-demanding forest communities were only found in limed spruce stands (Table 3). The similarity of the spruce stands to the reference of semi-natural beech stands decreases steadily with increasing liming until L3, after which the decrease is only very slight (Table 2).
Discussion
Influence of liming intensity on the herb layer
Our results showed a strong influence of liming intensity on the species composition of the herb layer. Changes are visible after just one lime application, although with the exception of some indicator species they are not significant. This contrasts with the findings of other studies: Schlüter (1966); Becker et al. (1992); Schmidt (1992) and Jandl et al. (2002) found major changes in the herb layer after only a single lime application. In some of these studies the impact of various lime quantities was compared, but with mixed results. Schlüter (1966) did not find an influence of the lime dose on the species composition of the herb layer. By contrast, in the long-term studies of Schornick (1990) and Höcke (2006) the herb layer in plots with low lime quantities partly converged to those in unlimed plots after 30 and 40 years, respectively, whereby at higher lime doses large differences to the control remained. In our study, we found significant changes in the occurrences of the indicator species only after the second liming. However, after the third lime application a saturation effect was observed. From the class L3 only very few additional indicator species occurred, and also the EIV remained at a certain level. This is an important finding of our study. There are so far no studies that investigate the effects of three or more lime applications, and also the cumulative quantity of lime applied of up to 20.4 t/ha in the class L5 is far above those quantities analysed elsewhere. However, a saturation effect was reached in the investigations of Seibt and Wittich (1977) and Schmidt (2002) probably after the first liming. After the second liming, no amplification of the effects was found. One reason why these earlier results differ from our findings could be environmental differences between the study areas, i.e. some forest stands require only relatively small quantities of lime for strong changes in the herb layer to occur, whilst others require large quantities. Priehäußer (1958), for example, found no effect of liming on the herb layers of experimental plots in the Bavarian Forest at elevations of 1200–1250 m a.s.l. Effects were only seen in stands that had also been thinned. It seems that, due to the low temperatures at this elevation, liming alone was not sufficient in the quantities used to increase microbial activity enough to cause observable changes. Our study areas are also at relatively high elevations, which could be the cause of the delayed reaction of the herb layer to the liming. This is in accordance with the findings of Krause (1989). He carried out vegetation relevés in the western part of the Ore Mountains in elevations from 800 to 950 m a.s.l. in the years 1989–1993. In some plots the first liming was conducted some years earlier. With the exception of an increase in cover of Epilobium angustifolium there was no change in the herb layer.
We observed also a strong influence of liming intensity on the diversity of the herb layer of spruce stands. We found a significant increase in the average species richness in limed stands, which is in agreement with the results of numerous other studies (e.g. Schlüter 1966; Schornick 1990; Schmidt 1992, 2002; Rodenkirchen 1993; Nohrstedt 2001; Höcke 2006). The above-mentioned saturation effect also emerges in alpha diversity. Our results for beta diversity could indicate that existing differences in environmental factors between stands had been moderated by the effect of liming. The homogenising effect on species composition in spite of increasing alpha diversity caused by atmospheric nitrogen deposition have long been known (e.g. Ellenberg 1991; Jenssen and Hofmann 2005), and liming intensifies this effect (see Reif et al. 2014). Further research is necessary in order to fully understand herb layer dynamics after liming, in particular regarding the changes in beta diversity in permanent plots which have not been limed at the time of the establishment.
Does liming restore natural forest communities?
In contrast to previous studies that only used qualitative analyses (e.g. Schmidt 2002; Kraft et al. 2003), our study quantifies the occurrence of disturbance indicators and compares this to the cover of species typical for mature stands of the natural vegetation. This allows us to conclude that liming shows some signs of restoring the herb layer of spruce stands to a more natural species composition. In stands in Calamagrostio villosae-Fagetum habitat, liming leads to a significant increase in cover of nutrient demanding species of the characteristic species combination. This is, however, mainly due to large changes in the occurrence of one species (Oxalis acetosella). This species declines as a result of acidification (Rodenkirchen 1993; Økland et al. 2004), and similar effects of liming on Oxalis acetosella were found in the Höglwald project (Rodenkirchen 1993). The cover of the character species of beech forest communities (BC1/BC2) also increases significantly, although the absolute value is much lower. Historical vegetation records in spruce plantations on areas of natural Calamagrostio villosae-Fagetum in the Ore Mountains (Reinhold 1939) show numerous species of the groups CAV2, BC1 and BC2 (Luzula luzuloides, Prenanthes purpurea, Phegopteris connectilis, Oxalis acetosella and Athyrium filix-femina), in some cases with high constancy. Oxalis acetosella also shows high cover values here, suggesting that liming has reversed earlier degradation of the habitat (see Schmidt 1992).
The decline after liming of species promoted by acidification can be similarly interpreted (Schmidt 1992). In our study, this is particularly the case for Deschampsia flexuosa, which experienced a well-documented increase in the second half of the twentieth century in forests of the Ore Mountains affected by acid deposition (Schmidt 1993). This species was recorded by Reinhold (1939) with cover values always < 5%, whilst our study found Deschampsia flexuosa to be an important species in the classes L0 and L1, with the highest cover values after Calamagrostis villosa. In the classes L3–L5, however, Deschampsia flexuosa occurs with low cover values.
Even clearer than these tendencies towards community restoration is the promotion by liming of species that are disturbance indicators. Almost all the indicator species we found for limed stands were not typical for mature phases of the natural forest community. The promotion of species of high nitrogen and non-forest habitats we observed agrees with the findings of numerous earlier studies (e.g. Schlüter 1966; Wenzel 1989; Schmidt 1992; Hallbäcken and Zhang 1998; De Keersmaeker et al. 2000; Kraft et al. 2003; Bergholm et al. 2015). Many of these species occur in the natural development cycle of forests under these site conditions, but are limited to phases with disturbances. Grabherr et al. (1998) classify for example Cirsium vulgare, Dactylis glomerata, Epilobium angustifolium, Galeopsis bifida, Geranium robertianum, Ranunculus repens, Tussilago farfara, Urtica dioica and Veronica chamaedrys as characteristic species for disturbances in spruce forests. On skid trails in spruce stands Ebrecht and Schmidt (2005) found, e.g. Cardamine flexuosa, Mycelis muralis, Prunella vulgaris and Urtica dioica. Some of these species are occasionally present in the soil seed bank of forests, which can be interpreted as a remnant of earlier disturbances (Fischer 1987). As such, their spread as a result of liming is seen critically (Schmidt 2002). This puts the clear increase in alpha diversity with increasing liming intensity into perspective, as it is mainly due to the appearance of these indicators of disturbance. These species of habitats with high nitrogen availability and of non-forest habitats are largely absent in the historical records of Reinhold (1939), with only isolated occurrences of Rubus fruticosus agg., Senecio ovatus and Urtica dioica mostly in spruce forest communities with more nutrient-rich soils.
Several studies have shown the benefits of liming for species of nutrient-rich forest communities or natural spring habitats (Hartmann 1959; Schlüter 1966; Immer et al. 1993; Leitgeb 1994; Jandl et al. 2002). Schmidt (2002) considers this to be reversal of earlier degradation of the habitat. However, this cannot be generalised, and the specific environmental conditions in the stand have to be considered. We thus categorised these species as disturbance indicators, as they are not elements of the potential natural vegetation for this area.
Overall, despite the observed tendencies towards restoration of the natural herb layer, increasing liming intensity caused a continuous decrease in the similarity of the vegetation to that of the reference beech stands. The gradual decline of the disturbance indicators after many years, as documented by Schornick (1990); Immer et al. (1993); Jandl et al. (2002) and Kraft et al. (2003), is suppressed by the 10-year cycle of lime application. Our results show that by periodic liming the non-equilibrium state, i.e. the common occurrence of species that are typical for the habitat and disturbance indicators, is stabilized. This is in agreement with the intermediate disturbance hypothesis (Connell 1978).
Our indicator approach enables to evaluate the effect of liming, in particular with regard to the reaction of a part of the ecosystem, and it allows drawing conclusions about the future liming management. It should be noticed, however, that our analysis only concerns the rooting space of the herb layer. Oxalis acetosella, a species which is promoted by liming, for example, mainly roots in the humus (Rodenkirchen 1998). Generally, the need for lime applications in forest ecosystems is determined using chemical parameters of the mineral soil, and an important intention of liming is to meliorate also deeper soil horizons. Decisions about the necessity of liming are, thus, a complex task and should be based on multiple factors (Huettl and Zoettl 1993).
Conclusions
When evaluating liming in forest habitats, it is important to recognise that this is a measure applied to systems that are already highly disturbed through anthropogenic acid deposition. Lime applications can to some extent ameliorate the effects of chemical imbalances in the system caused by acid deposition, but can never fully restore it to its original condition. The complexity of this issue is demonstrated in our results. We observed a trend towards habitat restoration in terms of increasing cover of some nutrient-demanding species of the characteristic species combination and of beech forest communities. On the other hand, we found a considerable expansion of disturbance indicators. In this context, liming does not act to restore earlier conditions but act as a disturbance itself by causing the sudden release of nitrogen that was bound in the humus. With regard to the development cycle of spruce forests, the periodic liming leads to the extensive and permanent establishment of a herb layer which is typical for disturbance phases. Of particular concern is the impact on diversity. The homogenisation of environmental conditions and the vegetation caused by anthropogenic nitrogen deposition is compounded by liming, and these developments should be carefully monitored. Our results do not rule out using liming as a measure to counteract soil acidification, but support a more differentiated approach. Given that the herb layer is representative for the site conditions, the liming intervals should be raised to 15–20 years in order to avoid saturation effects. Above all, large, contiguous areas distributed throughout the treatment area should be kept as controls to be able to monitor progress.
Change history
18 May 2019
In the original publication of the article, the Table 2 column heading that reads “L6” should read “SB” and the ORCID iD of the fourth author was missed in the original publication and included in this correction.
References
Anderson TH (1998) The influence of acid irrigation and liming on the soil microbial biomass in a Norway spruce (Picea abies [L.] Karst.) stand. Plant Soil 199:117–122
Baselga A, Orme D, Villeger S, De Bortoli J, Leprieur F (2013) betapart: partitioning beta diversity into turnover and nestedness components. R package version 1.3. http://CRAN.R-project.org/package=betapart
Becker M, Bonneau M, Le Tacon F (1992) Long-term vegetation changes in an Abies alba forest: natural development compared with response to fertilization. J Veg Sci 3(4):467–474
Beese F (1996) Indikatoren für eine multifunktionelle Waldnutzung. Forstw Cbl 115:65–79
Bergholm J, Olsson BA, Vegerfors B, Persson T (2015) Nitrogen fluxes after clear-cutting. Ground vegetation uptake and stump/root immobilization reduce N leaching after experimental liming, acidification and N fertilization. For Ecol Manag 342:64–75
Bierögel L, Bitterlich G, Brandhoff H, Dexelberger F, Hänel H, Kegel H, Lux H, Mauersberger G, Neuhof G, Ranft H, Weise A, Wünsche M (1957) Erläuterungen zur Standortserkundung und -Kartierung der Hochlagen des Erzgebirges im Staatlichen Forstwirtschaftsbetrieb Annaberg
Buttler KP, Thieme M, Mitarbeiter (2017) Florenliste von Deutschland - Gefäßpflanzen. Version 9. http://www.kp-buttler.de/florenliste/index.htm
Chiu CH, Wang YT, Walther BA, Chao A (2014) An improved nonparametric lower bound of species richness via a modified Good-Turing frequency formula. Biometrics 70:671–682
Chytrý M (2013) Vegetace České republiky. 4. Lesní a křovinná vegetace. Academia, Prag
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199:1302–1310
Däßler HG (1991) Einfluß von Luftverunreinigungen auf die Vegetation. Gustav Fischer, Jena, 4. Aufl
De Cáceres M, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574
De Cáceres M, Legendre P, Moretti M (2010) Improving indicator species analysis by combining groups of sites. Oikos 119:1674–1684
De Keersmaeker L, Neirynck J, Maddelein D, De Schrijver A, Lust N (2000) Soil water chemistry and revegetation of a limed clearcut in a nitrogen saturated forest. Water Air Soil Pollut 122:49–62
Denner M (2007) Auswirkungen des ökologischen Waldumbaus in der Dübener Heide und im Erzgebirge auf die Bodenvegetation. Forstwissenschaftliche Beiträge Tharandt 29
Dierschke H (1994) Pflanzensoziologie: Grundlagen und Methoden. Ulmer, Stuttgart
Dittrich K (2006) Neue Chancen für den Wald im Mittleren Erzgebirge. FuH 3:89–94
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Ebrecht L, Schmidt W (2005) Einfluss von Rückegassen auf die Vegetation. Forstarchiv 76:83–101
Ellenberg H (1991) Ökologische Veränderungen in Biozönosen durch Stickstoffeintrag. Ber Ökol Forsch 4:74–90
Ellenberg H, Werner HE, Düll R, Wirth V, Werner W (2001) Zeigerwerte von Pflanzen in Mitteleuropa. 3. Aufl. Scr Geobot 18
Ewald J (2007) Beurteilung von Waldstandorten und Waldgesellschaften mit Zeigerarten-Ökogrammen. Tuexenia 27:7–18
Fischer A (1987) Untersuchungen zur Populationsdynamik am Beginn von Sekundärsukzessionen. Die Bedeutung von Samenbank und Samenniederschlag für die Wiederbesiedlung vegetationsfreier Flächen in Wald- und Grünlandgesellschaften. Diss Bot 110, J. Cramer, Berlin, Stuttgart
Goodall DW (1973) Sample similarity and species correlation. In: Whittaker RH (ed) Ordination and classification of communities. Handbook of vegetation science, vol 5, pp 105–156
Gorham E (1989) Scientific understanding of ecosystem acidification: a historical review. Ambio 18:150–154
Grabherr G, Koch G, Kirchmeir H, Reiter K (1998) Hemerobie österreichischer Waldökosysteme. Veröffentlichungen des österreichischen MaB-Programmes Bd. 17, Wagner, Innsbruck
Greene RH (1979) Sampling design and statistical methods for environmental biologists. Wiley, New York
Guckland A, Ahrends B, Paar U, Damman I, Evers J, Meiwes KJ, Schönfelder E, Ullrich T, Mindrup M, König N, Eichhorn J (2012) Predicting depth translocation of base cations after forest liming—results from long-term experiments. Eur J For Res 131:1869–1887
Hallbäcken L, Zhang LQ (1998) Effects of experimental acidification, nitrogen addition and liming on ground vegetation in a mature stand of Norway spruce (Picea abies (L.) Karst.) in SE Sweden. For Ecol Manag 108:201–213
Hartmann FK (1959) Über die Wirkung von Kalkungen auf Waldböden verschiedenen Nährstoff- und Basengehaltes in soziologisch-ökologischer Betrachtung. In: Kalkdienst. Der Wald braucht Kalk (3):30–44
Hendricks SB, Taylorson RB (1972) Promotion of seed germination by nitrates and cyanides. Nature 237:169–170
Hill MO (1973) Diversity and evenness: a unifying notation ant its consequences. Ecology 54:427–432
Höcke CE (2006) Langfristige Veränderungen der Bodenvegetation und der Bodeneigenschaften durch Walddüngungen im Nordschwarzwald und auf der Baar. Dissertation, TU Freiburg
Huber C, Weis W, Göttlein A (2006) Tree nutrition of Norway spruce as modified by liming and experimental acidification at the Höglwald site, Germany, from 1982 to 2004. Ann For Sci 63:861–869
Huettl RF, Zoettl HW (1993) Liming as a mitigation tool in Germany`s declining forests—reviewing results from former and recent trials. For Ecol Manag 61:325–338
Hurlbert S (1984) Pseudoreplication and the design of ecological experiments. Ecol Monogr 54:187–211
Immer A, Schmidt W, Meiwes KJ, Beese F (1993) Langzeitwirkungen von Kalkung und Düngung auf den chemischen Zustand im Oberboden, die Humusform und die Bodenvegetation in einem Fichtenforst. Forstw Cbl 112:334–346
Jandl R, Starlinger F, Englisch M, Herzberger E, Johann E (2002) Long-term effects of a forest amelioration experiment. Can J For Res 32:120–128
Jenssen M, Hofmann G (2005) Einfluss atmogener Stickstoffeinträge auf die Vielfalt der Vegetation in Wäldern Nordostdeutschlands. Beitr Forstwirtsch u Landschökol 39(3):132–141
Koleff P, Gaston KJ, Lennon JJ (2003) Measuring beta diversity for presence-absence data. J Anim Ecol 72:367–382
Kraft M, Reif A, Schreiner M, Aldinger E (2003) Veränderungen der Bodenvegetation und der Humusauflage im Nordschwarzwald in den letzten 40 Jahren. Forstarchiv 74:3–15
Krause S (1989) Waldvegetationskundliche Untersuchungen in ausgewählten Naturschutzgebieten der Hoch- und Kammlagen des Erzgebirges sowie Ableitung eines Behandlungskonzeptes. Dissertation, TU Dresden
Krebs C (1999) Ecological methodology, 2nd edn. Addison Wesley Longman, Menlo Park
Legendre P, Legendre L (2012) Numerical ecology: 3rd English edition. Elsevier, Amsterdam
Leitgeb E (1994) Reaktion der Bodenvegetation auf Düngung und Kalkung in einem Fichtenbestand. Cbl gesamte Forstwes 111:229–241
Leube F (2000) Leitfaden Forstliche Bodenschutzkalkung in Sachsen. Schriftenreihe der Sächsischen Landesanstalt für Forsten 21
LfULG (ed) (2018) Luftqualität in Sachsen. Jahresbericht 2017. Dresden
Lorenz K, Feger KH, Kandeler E (2001) The response of soil microbial biomass and activity of a Norway spruce forest on liming and drought. J Plant Nutr Soil Sci 164:9–19
Lundström US, Bain DC, Taylor AFS, van Hees PAW (2003a) Effects of acidification and its mitigation with lime and wood ash on forest soil processes: a review. Water Air Soil Pollut Focus 3:5–28
Lundström US, Bain DC, Taylor AFS, van Hees PAW, Geibe CA, Holmström SJM, Melkerud PA, Finlay R, Jones DL, Nyberg L, Gustafsson JP, Riise G, Tau Strand L (2003b) Effects of acidification and its mitigation with lime and wood ash on forest soil processes in Southern Sweden. A joint multidisciplinary study. Water Air Soil Pollut Focus 3:167–188
Maas D, Pfadenhauer J (1994) Effizienzkontrollen von Naturschutzmaßnahmen – fachliche Anforderungen im vegetationskundlichen Bereich. Schr-R f Landschaftspflege u. Naturschutz 40:25–50
Mannsfeld K, Syrbe RU (2008) Naturräume in Sachsen. Forschungen zur deutschen Landeskunde 257:1–268
Neale SP, Shah Z, Adams WA (1997) Changes in microbial biomass and nitrogen turnover in acidic organic soils following liming. Soil Biol Biochem 29:1463–1474
Nohrstedt HÖ (2001) Response of coniferous forest ecosystems on mineral soils to nutrient additions: a review of Swedish experiences. Scan J For Res 16:555–573
Oberdorfer E, Schwabe A, Müller T (2001) Pflanzensoziologische Exkursionsflora. Für Deutschland und angrenzende Gebiete. 8. Aufl., Stuttgart, Ulmer
Økland T, Bakkestuen V, Økland RH, Eilertsen O (2004) Changes in forest understorey vegetation in Norway related to long-term soil acidification and climatic change. J Veg Sci 15:437–448
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2015) Vegan: Community Ecology Package. R package version 2.2-1. http://CRAN.R-project.org/package=vegan
Pfadenhauer J, Poschlod P, Buchwald R (1986) Überlegungen zu einem Konzept geobotanischer Dauerbeobachtungsflächen für Bayern. Teil I. Berichte der Akademie für Naturschutz und Landschaftspflege 10:41–60
Priehäußer G (1958) Änderungen im Artenbestand der Flora auf den Rohhumusböden der Hochlagen des Bayerischen Waldes bei natürlicher Auflichtung und bei künstlicher Auflichtung mit Kalkung. Ber Bayer Bot Ges 32:108–117
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Reif A, Schulze ED, Ewald J, Rothe A (2014) Waldkalkung – Bodenschutz contra Naturschutz? Waldökologie, Landschaftsforschung und Naturschutz 14:5–29
Reinhold F (1939) Versuch einer Einteilung und Übersicht der natürlichen Fichtenwälder (Piceion excelsae) Sachsens. Thar Forstl Jb 90:229–271
Rennwald E (2000) (Bearb.). Verzeichnis und Rote Liste der Pflanzengesellschaften Deutschlands. Schriftenreihe für Vegetationskunde 35. Bonn—Bad Godesberg
Rineau F, Garbaye J (2009) Does forest liming impact the enzymatic profiles of endomycorrhizal communities through specialized fungal symbionts? Mycorrhiza 19:493–500
Rodenkirchen H (1993) Wirkungen von Luftverunreinigung und künstlichem sauren Regen auf die Bodenvegetation in Koniferenwäldern. Forstw Cbl 112:70–75
Rodenkirchen H (1998) Evidence for a nutritional disorder of Oxalis acetosella L. on acid forest soils. I. Control Situation and effects of dolomitic liming and acid irrigation. Plant Soil 199:141–152
Schlüter H (1966) Untersuchungen über die Auswirkung von Bestandskalkungen auf die Bodenvegetation in Fichtenforsten. Die Kulturpflanze 14:47–60
Schmidt W (1992) Der Einfluß von Kalkungsmaßnahmen auf die Waldbodenvegetation. Z Ökol Natursch 1:79–88
Schmidt PA (1993) Veränderung der Flora und Vegetation von Wäldern unter Immissionseinfluß. Forstw Cbl 112:213–224
Schmidt W (2002) Einfluss der Bodenschutzkalkung auf die Waldvegetation. Forstarchiv 73:43–45
Schmidt PA, Hempel W, Denner M, Döring N, Gnüchtel A, Walter B, Wendel D (2002) Potentielle Natürliche Vegetation mit Karte 1:200000. In: LfUG (ed) Materialien zu Naturschutz und Landschaftspflege. Dresden
Schmidt M, Kriebitzsch WU, Ewald J (2011) Waldartenlisten der Farn- und Blütenpflanzen, Moose und Flechten Deutschlands. BfN-Skripten 299
Schornick OK (1990) Änderung der Bodenvegetation in Waldbeständen als Folge einer künstlichen Düngung. KfK-PEF 63
Schubert R (1972) Übersicht über die Pflanzengesellschaften im südlichen Teil der DDR. III. Wälder Teil 3. Hercynia NF 9 (1972) 3: 197–228
Seibt G, Wittich W (1977) Ertragskundliche und bodenkundliche Ergebnisse langfristiger Kalkungsversuche im nord- und westdeutschen Bergland. Der älteste Kalkungsversuch in Neuenheerse und bodenkundliche Grundlagen. Schr-R forstl Fak Univ Göttingen 50:5–88
SMUL (ed) (1996) Waldschadensbericht 1996. Vetters, Radeburg
Ulrich B (1986) Natural and anthropogenic components of soil acidification. Z Pflanzenernaehr Bodenk 149:702–717
Ulrich B (1987) Stability, elasticity and resilienc of terrestrical ecosystems with respect to matter balance. Ecol Stud 61:11–49
UN/ECE (1992) Critical levels of air pollutants for Europe. Background Papers prepared for the UN/ECE Workshop on Critical Levels in Engham, U.K. 23.-26-March 1992. Air Quality Division, Department of the Environment, London
Usher MB (1991) Scientific requirements of a monitoring programme. In: Goldsmith FB (ed) Monitoring for conservation and ecology. Chapman & Hall, London, pp 15–32
Van Breemen N (1990) Deterioration of forest land as a result of atmospheric deposition in Europe: a review. In: Gessel SP, Lacate DS, Weetman GF, Powers RF (eds) Sustained productivity of forest soils. Proceedings of the 7th North America Forest Soils Conference on University of British Columbia, Faculty of Forest Publication, Vancouver, pp 1–525
VEB Forstprojektierung (Ed.) (1985) Erläuterungen zur Standortskarte des Staatlichen Forstwirtschaftsbetriebes Schwarzenberg. Potsdam
Wenzel B (1989) Kalkungs- und Meliorationsexperimente im Solling: Initialeffekte auf Boden, Sickerwasser und Vegetation. Ber Forschungszentrums Waldökosysteme A 51
Wittig R, Ballach HJ, Brandt CJ (1985) Increase of number of acid indicators in the herb layer of the millet grass-beech forest of the Westphalian Bight. Angewandte Botanik 59:219–232
Acknowledgements
Thanks to Dr. R. Petzold for many fruitful discussions and useful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Rüdiger Grote.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baumann, M., Dittrich, S., Körner, M. et al. Liming in spruce stands: What effect does the number of lime applications have on the herb layer?. Eur J Forest Res 138, 723–735 (2019). https://doi.org/10.1007/s10342-019-01191-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-019-01191-5