Abstract
Large samples of citrus genotypes need to be evaluated to find and improve the genetic resources for producing better hybrid rootstocks. Two well-known tolerant (‘Cleopatra’ mandarin) and sensitive (‘Troyer’ citrange) cultivars, and 10 genetically diverse citrus genotypes from Iran were examined under four sodium chloride (NaCl) levels, including 0, 2, 4, and 6 dS m−1, to screen and discover salt-tolerant genotypes. Salinity (especially at 6 dS m−1) had a detrimental effect on plants by reducing relative water content (RWC; −27.34%), water potential (−220%), total chlorophyll content (−61.97%), and enhancing Na+ (500%), Cl− (136%) concentration, as well as cell oxidative level (electrolyte leakage [EL; 61.92%], malondialdehyde [MDA; 64.05%]). In reaction to salinity, osmoprotectant content (soluble sugars [163%] and proline [101%]) and antioxidant enzymes activity (superoxide dismutase [SOD; 336%], catalase [CAT; 53.54%], peroxidase [POD; 77.06%], and ascorbate peroxidase [APX; 421%]) increased dramatically especially at 6 dS m−1. In addition, under different salinity levels, genotypes exhibited different responses, but ‘Cleopatra’ mandarin and G5 exhibited the highest RWC, water potential, chlorophylls, soluble sugars, proline, and antioxidant enzymes activity, as well as the lowest Na+, Cl− concentrations, EL, and MDA. Overall, G5 was identified as the genotype with the highest salt tolerance and can be used in gardens that have salt stress problems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, there have been significant climatic changes. Climate change has raised the likelihood of abiotic stressors (flooding, drought, salt, etc.) and has a negative impact on agricultural growth and development. One of the most significant effects of global climate change is salinity. It is also a significant negative abiotic factor in worldwide agricultural crop production. More than 6% of the Earth’s total land area and about 20% of its arable land area are under salinity stress (Aparicio-Durán et al. 2021; Ullah et al. 2021).
Citrus is a subtropical crop with little tolerance for less-than-ideal circumstances. Its cultivation has been expanded to varied climatic locations between 40° north and south latitudes, where citrus output is exceptionally high in dry and semiarid regions. In key citrus-producing areas, salinity limits its productivity, and since citrus is a “salinity-sensitive” crop, excessive salt concentrations have a negative effect on its yield (Colmenero-Flores et al. 2020).
Conversely, Iran is one of the leading citrus-producing nations (FAO 2020). According to reports, 20% of Iran’s overall landmass is saline or alkaline. Due to too much evaporation and transpiration, not enough rain, and poor irrigation water quality in drylands, the salinity of inland water is steadily getting worse (Raoufi et al. 2021). It has been found that a high concentration of sodium (Na+) and chloride (Cl−) causes salinity stress. For every one dS m−1 rise in salinity over 1.3 dS m−1 in the saturated soil extract, citrus output decreases by about 13%; salinity levels above three dS m−1 are crucial for citrus production (Vincent et al. 2020; Colmenero-Flores et al. 2020).
In abiotically damaging circumstances, plants react with a variety of measures to meet the anticipated impact of stress, ensuring a new stage of development. Plants mitigate the detrimental effects of abiotic stress by undergoing morphophysiological, biochemical, and metabolic changes, as well as by achieving an adapted state. The key characteristics associated with abiotic stress tolerance in plants include osmoprotectants and compatible solutes (proline and soluble sugars), water potential, antioxidant enzyme activity, and changes in plant pigment content (Latef 2021).
Citrus genotypes with a high tolerance capacity may escape saltwater because they can exclude Na+ and Cl−, exhibiting different responses than salt-sensitive genotypes (dos Santos et al. 2021). In this instance, Etehadpour et al. (2019) showed that salt-tolerant genotypes had lower leaf Na+ and Cl− content and increased antioxidant enzyme activity, protein, and chlorophyll content compared to salt-sensitive genotypes.
Physicochemical methods are used to evaluate plants under salinity stress because these methods provide a comprehensive understanding of the physiological and biochemical characteristics of the plants, which are crucial for assessing their response to stress conditions. These analyses help in identifying the specific physiological and biochemical traits that contribute to stress tolerance in citrus cultivars, enabling a more targeted approach towards breeding and selecting plants with enhanced resilience to salinity stress (Vives-Peris et al. 2023).
Considering the possibility of interspecific hybridization in citrus and the mutations that might lead to desirable features, salinity-tolerant accessions are likely to be found among the genetically diverse germplasm. Consequently, the purpose of this study was to evaluate the salinity responses of many citrus genotypes from Iran based on their physicochemical properties.
Materials and Methods
Plant Material and Treatments
At a commercial greenhouse in Tonekabon province, Mazandaran, Iran, seeds of 10 Persian citrus cultivars, ‘Cleopatra’ mandarin (tolerant) and ‘Troyer’ citrange (sensitive), were planted. Half-strength Hoagland’s solution was used to irrigate the plants twice each week. For 12 weeks, 8‑month-old well-grown seedlings were subjected to salinity treatment. Seedlings treated with salinity solution contained 0, 2, 4, and 6 dS m−1 NaCl (Merk). The soil of each plant was flushed with 3 L of tap water 2 days prior to treatment. Using the following formula (Etehadpour et al. 2019), the quantity of water or salt solution was determined with three replications based on sandy loam soil and field capacity:
where d is the soil depth (pot), FC is the field capacity, PWP is the permanent wilting threshold, 0.5 is the permitted shortfall for management, and A is the soil area. The quantity of irrigation solution equals B minus 30% leaching.
To avoid osmotic shock from high concentrations, treated plants began with lower salt concentrations, which were gradually raised until each group achieved the treatment-specific concentration. Following salt treatments, the electrical conductivity (EC) of the solution in the pots was tested, and if the EC increased, the plants were watered with non-saline water.
The leaf Na+ and Cl− concentration was measured 3 weeks after salinity treatments. Just ripe leaves from the stem’s center were harvested.
Measurements
All measurements were repeated in three replications. After salinity treatment for 12 weeks, specific parameters were assessed. Five leaves were similarly removed from each tree to determine the relative water content (RWC). The petiole was immediately placed in distilled water in a sealed glass tube after being sliced. The increased weight of the tubes was then used to calculate leaf fresh weight (FW) in the laboratory. After 48 h in dim light, the leaves were weighed to determine their turgidity. After oven drying at 80 °C for 48 h, the dry weight was measured, and the relative water content was determined according to the Wahbi et al. (2005) method.
Using a pressure chamber (Scholander pressure bomb, Soil Moisture Equipment Corp., USA), the leaf water potential of outer canopy leaves at midday was determined as described by Turner (1981). Chlorophylls content of fresh leaf tissue was measured at 470, 645, and 663 nm using a spectrophotometer (NanoDrop® ND-1000 UV-Vis, USA) (Arnon 1967). Using the anthrone reagent, soluble sugars were quantified at 625 nm (Irigoyen et al. 1992). The proline content was determined using the Bates et al. (1973) technique at 520 nm. Electrolyte leakage (EL) was assessed in accordance with Shi et al. (2006). Using a thiobarbituric acid reaction, the concentration of malondialdehyde (MDA) was measured (Tajvar et al. 2011).
For enzyme extractions, 0.5 g leaf samples were homogenized with 50 mM potassium phosphate buffer (pH = 7) including 0.5 mM EDTA and 2% (w/v) polyvinylpolypyrrolidone (PVPP). Samples were centrifuged at 14,000 rpm for 15 min, and supernatants were used for measurement of enzyme activity (Tajvar et al. 2011). Superoxide dismutase (SOD) activity was measured by its ability to inhibit the photochemical reduction of nitro blue tetrazolium (NBT) at 560 nm. Catalase (CAT) activity was determined by the initial rate of disappearance of H2O2 at 240 nm. Peroxidase (POD) activity was determined by formation of tetraguaiacol at 470 nm. One unit of enzyme was defined as the amount of enzyme to decompose 1 µM of H2O2 per min at 25 °C. Ascorbate peroxidase (APX) activity was estimated according to Tajvar et al. (2011). The measure depends on the decrease in absorption at 290 nm as ascorbate is oxidised. The Na+ and Cl− content were evaluated based on the Waling et al. (1989) method.
Experimental Design and Statistical Analysis
Our investigation was conducted as a factorial experiment with three replications using a random design. Using SAS software, the PROC ANOVA approach was used to analyze the data (ver. 9.1 2002–2003, SAS Institute, Cary, NC). The data were examined for normality and homoscedasticity using the Kolmogorov–Smirnov and Cochran tests prior to variance analysis. After a significant ANOVA effect, Duncan’s multiple range test was computed to assess the differences between means.
Results and Discussion
The main impact and interaction effect of genotype and salinity were found to influence all examined characteristics substantially (P ≤ 0.01) (Table 1).
Na+ concentration enhanced considerably from 15.76% under non-salinity circumstances (0 dS m−1) to 31.68% at the maximum salinity level (6 dS m−1) (Table 1). At various salinity settings, ‘Cleopatra’ mandarin and G5 exhibited the lowest Na+ concentration (Table 2). According to Table 1, the Cl− content increased considerably (P ≤ 0.01) from 0.79 to 1.89% under non-saline circumstances at the maximum salinity level. ‘Cleopatra’ mandarin and G5 had the lowest Cl− concentrations among examined genotypes at almost all salinity conditions (Table 2). ‘Cleopatra’ mandarin and G5 accumulated less Na+ and Cl− than other genotypes, indicating their suitability for regions with salt-rich soils and water.
All citrus genotypes had distinct Na+ and Cl− concentrations, which may be attributed to their unique absorption capabilities and root structures. Na+ and Cl− ions may generate lethal circumstances for plants, although Cl− is more hazardous. Instead, nutritional imbalance during salt exposure seems to be caused by membrane selectivity and competitive antagonist interactions (Shelke et al. 2019; van Zelm et al. 2020; Hasanuzzaman et al. 2021). Due to the capacity to exclude or prevent absorption or transmit salt ions from the roots to the shoots, citrus is tolerant to salinity. Many studies demonstrate that a high Cl− concentration is connected with susceptibility to salt stress, and the lower presence of this ion in the leaves implies an exclusion mechanism, which is linked to a tolerant phenotype (Wu 2018; Aparicio-Durán et al. 2021). Similar to our results, El Yacoubi et al. (2022) reported that salt stress adversely influenced several citrus genotypes’ Na+ and Cl− content.
The leaf RWC content reduced considerably (P ≤ 0.01) from 90.27% in innon-salinity conditions to 65.59% in 6 dS m−1 of salinity (Table 1). Several genotypes responded differently to various salt levels; however, ‘Cleopatra’ mandarin and G5 often had the greatest leaf RWC concentration (Table 2). According to Table 1, plant water potential reduced considerably (−0.40 to −1.28 MPa) in response to salinity stress (−0.40 to −1.28 MPa; Table 1). Compared to other genotypes, ‘Cleopatra’ mandarin, and G5 exhibited the maximum plant water potential under varying salt concentrations (Table 2).
These findings are consistent with those of Shafieizargar et al. (2015) and Etehadpour et al. (2019), who discovered that salinity considerably decreased the RWC and water potential content of several citrus genotypes. When plants are exposed to high salinity levels, the salt concentration in the soil increases, leading to a lower water potential than in the plant’s root cells. This osmotic imbalance causes water to move out of the plant cells into the soil, decreasing the plant’s water content and potential. As a result, the plant experiences water stress, affecting its physiological processes and overall growth. The decrease in RWC and water potential is a typical response to salinity stress as plants struggle to maintain water balance and cope with the adverse effects of high salt concentrations in the soil (Etehadpour et al. 2019; Vives-Peris et al. 2023).
According to Table 1, the MDA concentration increased considerably (P ≤ 0.01) from 17.16 g g−1 FW in non-salinity circumstances to 28.15 g g−1 FW at a salinity of 6 dS m−1. Several genotypes responded differently to various salinity levels; however, ‘Cleopatra’ mandarin and G5 typically had the lowest MDA concentrations (Table 3). The leaf EL content increased considerably (P ≤ 0.01) from 29.02% in non-salinity circumstances to 46.99% in the most salinity condition (Table 1). ‘Cleopatra’ mandarin and G5 had the lowest leaf EL content among investigated genotypes at almost all salt concentrations (Table 3).
Due to the quick rise under abiotic stress, the EL and MDA have been deemed the most sensitive indicators under stressed circumstances. Additionally, EL is used as a marker to assess cell membrane damage. In our investigation, EL in the leaves was associated with cell damage. The rise of reactive oxygen species (ROS) generation during cell damage causes oxidative damage to numerous cell components, such as the cell membrane, and abnormalities in cell metabolic processes, leading to an increase in EL level (Hernández 2019). Less EL content is connected with maintaining cell membrane integrity under stressful situations (Hniličková et al. 2019; Maryum et al. 2022). Similar to our results, Madani et al. (2022) and El Yacoubi et al. (2022) reported that under salt stress, oxidative cell markers (EL and MDA) increased in citrus genotypes.
Chl‑a, Chl‑b, and T‑Chl levels decreased considerably (P ≤ 0.01) in response to varying salt concentrations (Table 1). ‘Cleopatra’ mandarin and G5 exhibited the maximum chlorophyll concentration at almost all salinity levels, according to Table 3. Moreover, the ratio of Chl‑a to Chl‑b (Chla/b) as a stress indicator increased considerably (P ≤ 0.01) from 0.393 in non-salinity conditions to 0.577 in 4 dS m−1 of salinity and subsequently declined somewhat to 0.416 in 6 dS m−1 of salinity (Table 3). Under varying salinity conditions, genotypes exhibited various Chla/b ratio responses (Table 3).
These results are in agreement with Othman et al. (2023). During abiotic stress, structural damage to chloroplasts due to the generation of ROS or photodegradation of chlorophylls causes a decrease in chlorophylls (Yang et al. 2020). The reduction in chlorophyll concentration may be attributable to the cytotoxic effects of Na+ and Cl− ions, which inhibit pigment synthesis (Yang et al. 2011; Madani et al. 2022). Destruction of chloroplast membranes, severe swelling, destruction of lamellae vesiculation, and the formation of lipid droplets have also been linked to the salinity-induced decrease in chlorophyll content (Angon et al. 2022). On the other hand, maintaining a low chlorophyll content under harsh salinity conditions may assist plants in decreasing photo-oxidative damage, which occurs when photosynthesis is inhibited, and light excitation energy is in excess (van Zelm et al. 2020; Maryum et al. 2022). Extra excitation energy acquired by chlorophylls will disrupt photosynthetic capability, increasing ROS generation and oxidative stress (van Zelm et al. 2020; Pintó-Marijuan and Munné-Bosch 2014).
At varying salinity conditions, the soluble sugar content increased considerably from 25.48 to 67.14 mg g−1 FW (Table 1). In addition, the soluble sugar content of ‘Cleopatra’ mandarins and G5 was determined (Table 4). Proline concentration rose considerably from 15.76 mg g−1 FW in non-salinity settings to 31.68 mg g−1 FW at the highest salinity level. Several genotypes responded differently to various salt levels; however, ‘Cleopatra’ mandarin and G5 had the greatest proline concentration (Table 4).
In stressful situations, plants modify their morphological, biochemical, physiological, molecular, and signaling levels, among others (van Zelm et al. 2020; Maryum et al. 2022). In stressful situations, the plant increases osmoprotectants or osmolytes synthesis and regulates nutritional homeostasis at the cellular level as part of its defense mechanisms. These organic chemicals include categories such as ammonium compounds, carbohydrates, and amino acids. Osmoprotectants are ubiquitous and regulate cellular osmotic adjustment, mitigate ROS-induced deleterious effects, minimize membrane damage, and protect proteins and enzymes. Osmoprotectants protect cellular organelles from dehydration-induced damage and do not interfere with normal cellular metabolic activities (Singh et al. 2015; Omari Alzahrani et al. 2021).
In line with the results of the present study, Balal et al. (2011) mentioned that sugars content increased during salinity stress in some citrus rootstocks. Sugar molecules provide carbon and energy for the normal functioning of cellular activities, and sugars regulate plant growth and development. Sugars are often assumed to operate as osmoprotectants, which regulate osmotic regulation, provide membrane integrity, and detoxify ROS under various stressful situations (Koyro et al. 2012; Singh et al. 2022). Sugars (as osmoprotectants) were shown to raise salinity levels considerably. In addition, higher sugar content under salinity conditions might aid cellular processes such as energy storage for stress recovery, signal transduction, and osmoprotectant production (Ghosh et al. 2021; Omari Alzahrani et al. 2021). Ziogas et al. (2021) and Snoussi et al. (2022) validated the variations in sugar content under varied salinity levels in different citrus varieties.
Proline is among the essential active amino acid molecules. It acts as a primary osmolyte and has a molecular signaling function that is typically located in the cytosol. It is also involved in stabilizing and preserving membranes of various organelles, scavenging the harmful effects of ROS, and buffering the cellular redox capacity under different abiotic stresses (Kavi Kishor and Sreenivasulu 2013; Singh et al. 2022). It may alleviate cytoplasmic acidosis in quantity essential for regulating homeostasis between NADP+ and NADPH under circumstances of normal metabolism (Singh et al. 2015; Ghosh et al. 2021). The build-up of proline inside stressed plants is caused by an increase in proline synthesis (due to a decrease in glutamate oxidation) and a decrease in proline consumption for protein synthesis (due to a halt in plant development) (Omari Alzahrani et al. 2021; Singh et al. 2022). In accordance with our findings, Snoussi et al. (2022) reported that the proline content of citrus rootstocks rose dramatically under various salinity conditions.
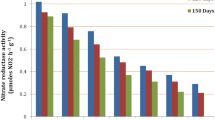
As indicated in Table 1, the activity of all antioxidant enzymes (SOD, CAT, POD, and APX) increased considerably (P ≤ 0.01) under varied salinity levels compared to non-salinity conditions (Table 4). In addition, among the genotypes studied, ‘Cleopatra’ mandarin and G5 had the maximum antioxidant enzyme activity at almost all salinity levels (Table 4).
These findings are in agreement with Etehadpour et al. (2019), who previously reported an increase in the activity of antioxidant enzymes in several citrus species grown under salt. Salinity stress causes ionic and osmotic imbalances in plant cells, leading to the excessive production of ROS like superoxide radicals, hydrogen peroxide, and hydroxyl radicals. These ROS can damage cellular components such as proteins, lipids, and nucleic acids. To protect against ROS-induced damage, plants activate their antioxidant defense systems. ROS also act as signaling molecules that trigger the activation of stress-responsive genes, including those encoding antioxidant enzymes. This signaling leads to an increase in the synthesis and activity of these enzymes as part of the plant’s adaptive response to salinity stress. By enhancing the activities of these antioxidant enzymes, citrus plants maintain cellular redox homeostasis, protecting cells from oxidative stress, and ensuring normal cellular functions (Ahmad et al. 2019; Sachdev et al. 2021). SODs are the earliest step of cellular defense against ROSs in a succession of detoxifying processes; they convert O2¯ and water (H2O) to H2O2 and molecular oxygen O2. The catalase activity swiftly converts the generated H2O2 to H2O and 1/2 O2. Principally active in the chloroplast organ, the APX enzyme plays a crucial role in the conversion of H2O2 to water, using ascorbate as an electron donor. Peroxidase is the primary protein responsible for oxidizing aromatic electron donors, such as guaiacol and pyrogallol, at the cost of H2O2 (Hasanuzzaman et al. 2020; Hasanuzzaman et al. 2021). Moreover, it is assumed that GPX found in the cytosol, vacuole, cell wall, and apoplast reduces lipid hydroperoxides to their respective alcohols and frees H2O2 to water (Gupta et al. 2018; Ahmad et al. 2019).
Conclusion
Based on the findings, integrated selection for physicochemical features is more accurate. RWC, water potential, and chlorophyll content were dramatically decreased by salinity, but Na+, Cl− concentration, EL, MDA, soluble sugars, proline, and antioxidant enzyme activity were significantly increased. G5 was superior to the other genotypes based on several characteristics, including the lowest Na+, Cl− concentration, and cell oxidative level, as well as the highest plant water relations, osmoprotectants content, and antioxidant enzyme activity. Based on physicochemical data, G5 was the most resistant genotype and may be a highly promising genotype in Iran and other salinity-challenged citrus-growing locations. Using some of the more tolerant genotypes identified in this research, it may be possible to cultivate citrus in salty water and soil for commercial purposes.
References
Ahmad R, Hussain S, Anjum MA, Khalid MF, Saqib M, Zakir I, Ahmad S (2019) Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In: Hasanuzzaman M, Hakeem KR, Nahar K, Alharby HF (eds) Plant abiotic stress tolerance. Springer, Cham
Alzahrani OF (2021) Metabolic engineering of osmoprotectants to elucidate the mechanism(s) of salt stress tolerance in crop plants. Planta 253:24. https://doi.org/10.1007/s00425-020-03550-8
Angon PB, Tahjib-Ul-Arif M, Samin SI, Habiba U, Hossain MA, Brestic M (2022) How do plants respond to combined drought and salinity stress?—A systematic review. Plant 11:2884. https://doi.org/10.3390/plants11212884
Aparicio-Durán L, Hervalejo A, Calero-Velázquez R, Arjona-López JM, Arenas-Arenas FJ (2021) Salinity effect on plant physiological and nutritional parameters of new huanglongbing disease-tolerant citrus rootstocks. Agron 11:653. https://doi.org/10.3390/agronomy11040653
Arnon AN (1967) Method of extraction of chlorophyll in the plants. Agron J 23:112–121
Balal RM, Ashraf MY, Khan MM, Jaskani MJ, Ashfaq M (2011) Influence of salt stress on growth and biochemical parameters of citrus rootstocks. Pak J Bot 43:2135–2141
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Colmenero-Flores JM, Arbona V, Morillon R, Gómez-Cadenas A (2020) Salinity and water deficit. In: Talon M, Caruso M, Gmitter FG (eds) The genus citrus, pp 291–309 https://doi.org/10.1016/B978-0-12-812163-4.00014-0
El Yacoubi H, Mouhssine F, Imtara H, Ouallal I, Ech-cheddadi S, Koutoua A, Lagzouli M, Alotaibi BS, Al kamaly O, Parvez MK, Rochdi A (2022) Insight into membrane stability and physiological responses of selected salt-tolerant and salt-sensitive cell lines of Troyer Citrange (Citrus sinensis [L.] x Citrus trifoliata [L.] Raf.) under salt stress. Sustain 14:9583. https://doi.org/10.3390/su14159583
Etehadpour M, Fatahi R, Zamani Z, Golein B, Naghavi MR, Gmitter F (2019) Evaluation of the salinity tolerance of Iranian citrus rootstocks using morph-physiological and molecular methods. Sci Hortic 261:109012. https://doi.org/10.1016/j.scienta.2019.109012
FAO (2022) https://www.fao.org/faostat/en/#data/QC
Ghosh UK, Islam MN, Siddiqui MN, Khan MAR (2021) Understanding the roles of osmolytes for acclimatizing plants to changing environment: a review of potential mechanism. Plant Signal Behav 16(8):1913306. https://doi.org/10.1080/15592324.2021.1913306
Gupta DK, Palma JM, Corpas FJ (2018) Antioxidants and antioxidant enzymes in higher plants. Springer, Berlin Leipzig https://doi.org/10.1007/978-3-319-75088-0
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681. https://doi.org/10.3390/antiox9080681
Hasanuzzaman M, Raihan MRH, Masud AAC, Rahman K, Nowroz F, Rahman M, Nahar K, Fujita M (2021) Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int J Mol Sci 22:9326. https://doi.org/10.3390/ijms22179326
Hernández JA (2019) Salinity tolerance in plants: trends and perspectives. Int J Mol Sci 20:2408
Hniličková H, Hnilička F, Orsák M, Hejnák V (2019) Effect of salt stress on growth, electrolyte leakage, Na+ and K+ content in selected plant species. Plant Soil Environ 65:90–96. https://doi.org/10.17221/620/2018-pse
Irigoyen JJ, Emerich DW, Sanchez-Diaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa L.) plants. Physiol Plant 84:55–60. https://doi.org/10.1111/j.1399-3054.1992.tb08764.x
Kavi Kishor PB, Sreenivasulu N (2013) Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ 37:300–311. https://doi.org/10.1111/pce.12157
Koyro HW, Ahmad P, Geissler N (2012) Abiotic stress responses in plants: an overview. In: Ahmad P, Prasad MNV (eds) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, New York, pp 1–28 https://doi.org/10.1007/978-1-4614-0815-4_1
Latef AAHA (2021) Organic solutes, oxidative stress, and antioxidant enzymes under abiotic stressors, 1st edn. CRC Press https://doi.org/10.1201/9781003022879
Madani SM, Piri S, Sedaghathoor S (2022) The response of three Mandarin cultivars grafted on sour orange rootstock to salinity stress. Int J Fruit Sci 22(1):264–274. https://doi.org/10.1080/15538362.2022.2036669
Maryum Z, Luqman T, Nadeem S, Khan SM, Wang B, Ditta A, Khan MK (2022) An overview of salinity stress, mechanism of salinity tolerance and strategies for its management in cotton. Front Plant Sci 13:907937. https://doi.org/10.3389/fpls.2022.907937
Othman YA, Hani MB, Ayad JY, Hilaire RS (2023) Salinity level influenced morpho-physiology and nutrient uptake of young citrus rootstocks. Heliyon 9(2):e13336. https://doi.org/10.1016/j.heliyon.2023.e13336
Pintó-Marijuan M, Munné-Bosch S (2014) Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: advantages and limitations. J Exp Bot 65:3845–3857. https://doi.org/10.1093/jxb/eru086
Raoufi A, Salehi H, Rahemi M, Shekafandeh A, Khalili S (2021) In vitro screening: The best method for salt tolerance selection among pistachio rootstocks. J Saudi Soc Agric Sci 20(3):146–154. https://doi.org/10.1016/j.jssas.2020.12.010
Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M (2021) Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antioxid 10(2):277. https://doi.org/10.3390/antiox10020277
dos Santos MÂCM, Filho MAC, Modesto FJN, Patt JM, Fancelli M (2021) Behavioral responses of Asian citrus psyllid (Hemiptera: Liviidae) to salinity-stressed citrus. Environ Entomol 50(3):719–731. https://doi.org/10.1093/ee/nvab028
Shafieizargar A, Awang Y, Ajamgard F, Juraimi AS, Othman R, Kalantar Ahmadi A (2015) Assessing five citrus rootstocks for NaCl salinity tolerance using mineral concentrations, proline and relative water contents as indicators. Asian J Plant Sci 14:20–26. https://doi.org/10.3923/ajps.2015.20.26
Shelke DB, Nikalje GC, Nikam TD, Maheshwari P, Punita DL, Rao KRSS, Suprasanna P (2019) Chloride (Cl−) uptake, transport, and regulation in plant salt. In: Roychoudhury A, Tripathi D (eds) Molecular plant abiotic stress: biology and biotechnology, pp 241–268 https://doi.org/10.1002/9781119463665.ch13
Shi L, Chen B, Wang Z, Elias DA, Mayer MU, Gorby YA, Ni S, Lower BH, Kennedy DW, Wunschel DS (2006) Isolation of a high-affinity functional protein complex between OmcA and MtrC: two outer membrane decaheme c‑type cytochromes of Shewanella oneidensis MR‑1. J Bacteriol 188:4705–4714. https://doi.org/10.1128/jb.01966-05
Singh M, Kumar J, Singh S, Singh VP, Prasad SM (2015) Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev Environ Sci Biotechnol 14:407–426. https://doi.org/10.1007/s11157-015-9372-8
Singh P, Choudhary KK, Chaudhary N, Gupta S, Sahu M, Tejaswini B, Sarkar S (2022) Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Front Plant Sci 13:1006617. https://doi.org/10.3389/fpls.2022.1006617
Snoussi H, Askri H, Nacouzi D, Ouerghui I, Ananga A, Najar A, El Kayal W (2022) Comparative transcriptome profiling of salinity-induced genes in citrus rootstocks with contrasted salt tolerance. Agric 12:350. https://doi.org/10.3390/agriculture12030350
Tajvar Y, Fotouhi Ghazvini R, Hamidoghli Y, Hassan Sajedi R (2011) Antioxidant changes of Thomson Navel orange (Citrus sinensis) on three rootstocks under low temperature stress. Hort Environ Biotechnol 52(6):576–580. https://doi.org/10.1007/s13580-011-0052-5
Turner NC (1981) Techniques and experimental approaches for the measurement of plant water status. Plant Soil 58:339–366. https://doi.org/10.1007/BF02180062
Ullah A, Bano A, Khan N (2021) Climate change and salinity effects on crops and chemical communication between plants and plant growth-promoting microorganisms under stress. Front Sustain Food Syst 5:618092. https://doi.org/10.3389/fsufs.2021.618092
Vincent C, Morillon R, Arbona V, Gómez-Cadenas A (2020) Citrus in changing environments. In: Talon M, Caruso M, Gmitter FG (eds) The genus citrus, pp 271–289 https://doi.org/10.1016/B978-0-12-812163-4.00013-9
Vives-Peris V, López-Climent MF, Moliner-Sabater M, Gómez-Cadenas A, Pérez-Clemente RM (2023) Morphological, physiological, and molecular scion traits are determinant for salt-stress tolerance of grafted citrus plants. Front Plant Sci 14:1145625. https://doi.org/10.3389/fpls.2023.1145625
Wahbi S, Wakrim R, Aganchich B, Tahi H, Serraj R (2005) Effects of partial rootzone drying (PRD) on adult olive tree (Olea europaea) in field conditions under arid climate; I. Physiological and agronomic responses. Agric Ecosyst Environ 106:289–301. https://doi.org/10.1016/j.agee.2004.10.015
Waling I, Van Vark W, Houba VJG, Van der lee JJ (1989) Soil and plant analysis, a series of syllabi. Part 7. Plant analysis procedures. Agriculture University, Wageningen
Wu H (2018) Plant salt tolerance and Na+ sensing and transport. Crop J 6(3):215–225. https://doi.org/10.1016/j.cj.2018.01.003
Yang JY, Zheng W, Tian Y, Wu Y, Zhou DW (2011) Effects of various mixed salt-alkaline stresses on growth, photosynthesis, and photosynthetic pigment concentrations of Medicago ruthenica seedlings. Photosynt 49:275284
Yang Z, Li JL, Liu LN, Xie Q, Sui N (2020) Photosynthetic regulation under salt stress and salt tolerance mechanism of sweet sorghum. Front Plant Sci 10:487808. https://doi.org/10.3389/fpls.2019.01722
van Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Annu Rev Plant Biol 71:403–433. https://doi.org/10.1146/annurev-arplant-050718-100005
Ziogas V, Tanou G, Morianou G, Kourgialas N (2021) Drought and salinity in citriculture: optimal practices to alleviate salinity and water stress. Agron 11:1283. https://doi.org/10.3390/agronomy11071283
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. Naghashi, B. Babakhani, M. Asadi, P. Rahdari and M.A. Shiri declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Naghashi, Y., Babakhani, B., Asadi, M. et al. Screening of Some Citrus Genotypes for Salinity Tolerance Using Physiochemical Methods. Applied Fruit Science (2024). https://doi.org/10.1007/s10341-024-01132-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10341-024-01132-6