Abstract
The productivity of important crops, particularly sugarcane, is greatly impacted by increased crop vulnerability to changing climatic conditions, including various abiotic stress factors like temperature, waterlogging, drought, etc. Salinity tolerance potential of five high yielding sugarcane genotypes was determined based on morpho-physiological, biochemical, and yield traits. The findings showed that morphological features (plant height, leaf area, stem diameter, number of internodes, and internodal length) were suppressed under salinity stress (ECiw 4, 8 and 10 dS/m). The relative water content (RWC) decreased by 4.4–12.5% as salinity level increased. Solute potential (Ψs) ranged from − 1.11 to − 2.27 MPa, whereas the water potential (Ψw) dropped from − 0.86 to − 1.99 MPa (from control to ECiw ~ 10 dS/m). Genotypes Co 13035 and Co 0118 maintained higher plant water status. There was a reduction in pigments and gas exchange traits due to increase in salinity in comparison to their respective control. Proline concentration increased up to seven times under salinity stress, with greatest accumulation in Co 0238 and Co 13035. The ionic (Na+/K+) ratio increased by 4, 6, and 8 times respectively under ECiw 4, 8 and 10 dS/m as compared to the control. The genotypes that were most resistant to salinity stress were Co 13035, Co 0238, and Co 0118, which had low Na+/K+ ratio. The results concluded that genotype Co 13035 had highest survival rate, low Na+/K+, maintained higher water content and osmolyte accumulation, better chlorophyll content, and single cane weight under salinity stress, thereby could be considered as tolerant to salinity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global food supply faces a serious environmental threat from soil salinity. It has a disproportionate impact on the world’s arid and semiarid regions, which rely heavily on irrigation to sustain their agricultural economics. It is projected that unrestrained soil salinization, climate change, an increase in irrigated agricultural output, and an exponential rise in human population will exacerbate future soil salinization, rendering near about 1.5 million hectares of arable land annually (Kumar et al., 2023a, 2023b, 2023c; Sheoran et al., 2023). Numerous factors like genotype, plant developmental stage, stress progression rate, severity, and duration affect plant responses to salinity stressors. The crops productivity on saline soil may be sustained by growing salt-tolerant plants, comparing to remediation methods like leaching, drainage, and the application of additives, which are frequently expensive and ad hoc fixes (Kumar et al., 2019; Mann et al., 2023). Salinization of soil because of regular irrigation practices is a serious constraint in the environment that rigorously affects agricultural sustainability, particularly in high water guzzling crop like sugarcane. Like most other crop species, sugarcane is usually a glycophyte that exhibits high sensitivity to salinity during various growth and developmental phases (Dhansu et al., 2022a, 2022b; Kumar et al., 2022).
Earlier studies showed that the water having salinity level > 8 dS/m and soil EC of > 1.7 dS/m severely affect the physiological characteristics during critical growth stages in sugarcane and caused reduction in cane yield up to 50% (Dhansu et al., 2022a, 2022b; Plaut et al., 2000; Santana et al., 2007) and every unit increase beyond ECe—1.7 dS/m reduced crop yield by 5.9% (Rao et al., 2015). Sugarcane (a C4 plant) is mainly a cash crop on global prospective, and has four major growth stages (germination, formative/tillering, grand growth and maturity). Though all growth stages of sugarcane are susceptible towards salinity stress, but germination and tillering phases are most vulnerable (Sengar et al., 2013). Soil salinity impairs crop physiology and associated metabolic processes that are attributed to reduced osmotic potential of the soil solution, oxidative stress, ionic toxicity (Na+ and Cl− ions), and nutritional imbalances (Pooja et al., 2012; Verma et al., 2023). Besides inhibiting leaf emergence and extension, the excess salt ions can induce leaf yellowing, burning, abscission and depletion of photosynthetic pigments that impeded photosynthetic absorption (Pooja and Sharma, 2016). Oxidative stress develops in response to salinity stress because of the buildup of reactive oxygen species (ROS). Plants induce their antioxidant defense system by activation of various enzymes to combat negative impacts of oxidative stress (Kumar et al., 2021; Pooja et al., 2020). Capacity of sugarcane to tolerate salinity stress is complex and greatly influenced by a variety of traits like high number of roots, high leaf area and numbers of tillers, wax-coated stem and strong ratooning ability (Mahadevaiah et al., 2023). Availability of salt tolerant sugarcane clones is still inadequate in all cropping regions. Hence, the present study was designed to explore salinity tolerance potential of high yielding available sugarcane genotypes and characterise the bio-chemical and physiological parameters contributing salinity tolerance and to identify salinity tolerant sugarcane clones for the use of farmer community and as donor in future breeding programme.
Material and methods
Plant material and experimental site
Single-budded setts of five Genotypes namely Co 0118, Co 13035, Co 15023, Co 15025, and Co 0238 were planted in 50 kg capacity cemented pots having soil of ECe—0.55 dS/m, pH—7.9, field capacity of 28% v/v (bulk density ~ 1.45 g/cc), and a porosity of ~ 40%. The whole experiment was performed under CRBD (completely randomized block design) design during second fortnight of March 2022, at ICAR—SBIRC (Sugarcane Breeding Institute, Regional Centre), Karnal in five replications. The study region has a semi-arid environment where hot summers exist during April to October and cold/winters exist from November to March along with an annual rainfall of 700–800 mm.
Experiments design and salinity treatments
Chloride dominant salinity was created using 4 salts i.e. calcium chloride (CaCl2), sodium chloride (NaCl), Magnesium chloride (MgCl2), and Magnesium sulphate (MgSO4) as (1:1:1:1) to prepare four levels of irrigation i.e. Control (best available water), ECiw ~ 4 dS/m (moderate saline irrigation), ECiw ~ 8 dS/m (severe saline irrigation) and ECiw ~ 10 dS/m (extreme saline irrigation) for this study. Prior to applying the various salt treatments, the plants of the selected genotypes were given 45 days to germinate and acclimatise. We filled the pots to an initial capacity of 13.2 L (based on the size of the field). Then evaporation was noted through the pan. Thereafter, 7.5 L of water was provided every week during the summer and every two weeks during the winter. The drainage hole at the bottom of the pots was plugged with glass wool to prevent the potting mix from flowing out. This experiment was conducted in a naturally ventilated net house, open on all sides but covered on the top with a polyethylene sheet to prevent the influx of rainwater. The experimental pots were reshuffled fortnightly to avoid any potential pot position effect.
Observations recorded
The TVD (top visible dewlap) leaves on the plant in each replication both in control and stressed conditions were tagged to record the functional traits of leaves, gas exchange attributes, chlorophyll fluorescence, and relative leaf chlorophyll. The same leaves were subsequently used for ion analyses. Plant height, leaf area, number of internodes, and stem diameter were measured after 60–75 days of exposure to salinity treatments using randomly selected samples of five plants from each treatment. A metre stick was used to calculate the plant's stature. Vernier measuring instruments were used to ascertain the diameter of the stem. The CI-203 Handheld Laser Leaf Area Metre was used to measure the leaf area. Three plants from each treatment were chosen at random, and between 9:00 and 10:00 a.m., the first TVD leaf was removed from each plant. Traditional techniques were used to calculate the leaf osmotic potential (s) and leaf water potential (w), as well as the relative water content (RWC%). RWC (%) in leaf was determined using the methodology proposed by Weatherley (1950). Using KCl standards for calibration, a Vapour Pressure Osmometer (WescorInc, Logan) was used to psychrometrically determine Ψs (Kaur et al., 2022). A pressure chamber (Model WP4C; Dew Point Potentiometer, WA) with a cooled mirror dew point technique was used to determine the Ψw in MPa (Soni et al., 2021).
Estimation of total leaf chlorophyll (Gu et al., 2016) and carotenoids (Lichtenthaler & Buschmann, 2001) was done in acetone and absorbance was taken at 663, 645 and 440.5 nm for the estimation of photosynthetic pigments. During the morning hours (9:00 AM to 11:30 AM), the LI-COR 6800 (LI-COR, Lincoln, NE, USA) portable photosynthesis system was used to record various parameters associated with gas exchange, including photosynthetic rate (Pn), transpiration rate (E), and stomatal conductance (gS) that was connected to a standard cuvette of 6 cm2. (WUEins) and intrinsic (WUEint) water usage efficiency, the ratios of Pn to E (Pn/E) and Pn to gs (Pn/gs) were recorded.
Fresh, separated TVD leaf samples of 100 mg were used to calculate membrane damage (Dionisio-Sese & Tobita, 1998). The percentage of membrane damage was then determined using the established methodology:
Homogenising 200 mg of leaf tissue in 10 ml of 3% sulphosalicyclic acid allowed quantifying the proline concentration of the extract (Bates et al., 1973). Using toluene as a standard, the absorbance of the extract combination was measured at 520 nm. As a marker of lipid peroxidation, the levels of malondialdehyde (MDA) were calculated (Heath & Packer, 1968). On a UV spectrophotometer, the absorbance was measured in comparison to a standard at 532 nm and 600 nm.
Statistical analysis
DUNCAN's Test was used to compare the means of the observed parameters at the 5% confidence level. SAS software (Version 9.3, SAS Institute Inc., USA) was used to conduct statistical analysis on the collected data.
Results
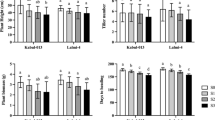
The Table 1 depicts the impact of salinity environment on growth and development of sugarcane plants along with the variability in these traits among different genotypes. The table shows the mean values of morphological characters (plant height, leaf area, stem diameter, intermodal length and number of internodes) for sugarcane plants and it was observed that as the salinity level (ECiw) increases from 4 dS/m to 10 dS/m, all of the morphological traits showed decreasing trend. Plant height and stem girth, are the important morphological attributes for cane development showed reduction of 19.87% and 8.66% under ECiw ~ 4dS/m, 30.21% and 19.52% under ECiw ~ 4dS/m and 43.75% and 30.06% under ECiw ~ 10 dS/m, respectively. Among the studied Genotypes, Co 13035 was found statistically superior or at par to the studied checks. Other important morphological attributes include TVD leaf area, showed reduction of 4.22%, 10.83% and 17.91% at ECiw ~ 4, 8 and 10 dS/m, respectively (Table 1). Genotype, Co 13035 also showed superiority in terms of leaf area followed by Co 0118 and Co 0238 (Table 1). Whereas, number of internodes also reduced alongwith increasing salinity and noted maximum reduction of 27.68% under higher salinity of ECiw ~ 10 dS/m (Table 1). Genotypic differences for number of internodes were also found significant and Co 15025 had the highest number of internodes. Similar results were also revealed by intermodal length and noted 5.82, 7.24 and 7.45% reduction with increasing salinity from ECiw ~ 4dS/m to ECiw ~ 10dS/m (Table 1). The intermodal length is highest in Co 13035 (10.50) and lowest in Co 15025 (7.2).
Under the control condition, Genotypes have a high relative water content (80.16%) and a slightly negative water potential and osmotic potential (− 0.86 and − 1.11 MPa), indicating good hydration with the turgor potential of 0.25 MPa. It was noted from the results that RWC decreased by 4.40–12.46% with increasing salinity (Table 2) and Genotypes Co 15025 (76.33%) and Co 15023 (74.83%) maintained higher mean RWC under salinity stress. The water potential (Ψw) is a measure of potential energy of water of a system in comparison to pure water at atmospheric pressure. With increase in salinity, Ψw decreased from − 0.86 MPa (control) to − 1.11 MPa (ECiw ~ 10 dS/m), and genotype Co 13035 (− 1.24 MPa) and Co 0118 (− 1.3 MPa) retained lowest Ψw (Table 2). Osmotic potential (Ψs) an important attribute represents the effect of dissolved solutes on water movement. Results showed decreased in mean values of Ψs with increasing salinity (Table 2) i.e., − 1.11 MPa, − 1.41 MPa, − 1.86 MPa, − 2.27 MPa under control, ECiw of 4 dS/m, 8 dS/m and 10 dS/m respectively. Similar to Ψw, lowest Ψs values were also noted in genotype Co 13035 (− 1.48 MPa) and Co 0118 (− 1.55 MPa).
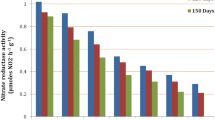
Turgor potential is another component of water potential that helps in maintaining the shape and rigidity of plant cells. Significant effects of salinity as well as genotypes were noted for turgor potential (Table 2). Results showed increased in mean values of turgor pressure with increasing salinity (Table 2) i.e., 0.25 under control whereas 0.28, 0.29, 0.31 at salinity level of ECiw ~ 4 dS/m, 8 dS/m and 10 dS/m. It was also observed from the results that genotype Co 0238 shows a lower relative water content (73.52%) and 0.33 MPa turgor potential, whereas genotype Co 15025 shows more negative osmotic and water potential (− 1.93 and − 1.59 MPa). Results revealed statistically (p < 0.001) significant decline in the studied gas exchange attributes. Chlorophyll, photosynthetic pigment showed decline of 6.25, 20.14 and 25.69% under variable salinity regimes of ECiw ~ 4, 8 and 10 dS/m, respectively. Similarly, SPAD reading (measure of greenness index) also declined by 8.78, 18.16 and 27.31% under salinity stress of ECiw ~ 4, 8 and 10 dS/m, respectively. Genotypes also depicted significant variability and noted that Genotype Co 0118 and Co 13035 displayed higher mean chlorophyll content (1.62 mg/g) and SPAD reading (40.79) respectively over salinity regimes, statistically followed by Co 13035 and Co 0238 (Fig. 1A, B). Such genotypic variations might be attributed towards photo-inhibition followed by ROS formation. Carotenoids content was also showed similar decreasing trend with increasing salinity stress and genotype Co 0238 had higher carotenoid content followed by Co 13035 (Fig. 1C).
For negating the injurious effects of salinity, the crop genotypes slow down their metabolism by reducing the photosynthetic rate (Pn). Present study found a reduction of 15.16, 29.34 and 57.7% at ECiw of 4, 8 and 10 dS/m, respectively. Among different sugarcane genotypes, the Co 0238 substantially showed higher Pn values (13.21 µmol CO2 m−2 s−1) that was followed by Co 13035 (12.48 µmol CO2 m−2 s−1) in comparison to others (Fig. 2A). Stomatal conductance (gS) and the rate of transpiration also found decreased in the range 10.03–28.09% and 9.79–28.93%, respectively over salinity levels. Stomatal conductance was higher in Co 0238 while transpiration was maximum in Co 0118, whereas Co 15025 and Co 15023 displayed lowest Pn, gS and E (Fig. 2A–B).
Water use efficiency was also calculated as instantaneous water use efficiency (rate of photosynthesis/rate of transpiration; Pn/E) and the intrinsic water use efficiency (Rate of Photosynthesis/stomatal conductance; Pn/gS). Similarly, both WUE decreased with increasing stress and Genotypes displayed statistically significant differences in WUE (Fig. 3A, B).
Results revealed that all these biochemical attributes enhanced with stress intensification and noted an increase of 3.21-fold in membrane injury, 3.69-fold in lipid peroxidation in term of MDA content and 7.09-fold in proline content under high salinity of 10 dS/m with respect to their control as shown in Table 3. MI refers to cell membrane damage resulting in cellular contents leakage as well as disruption of their structural integrity and function. Genotypes Co 13035 (17.8%) and Co 0118 (19.47%) depicted lower mean MI in comparison to others. Similar to MI, LP (measure in terms of malondialdehyde content) was minimum in Co 0118 (0.503 nmol/g) followed by Co 13035 (0.510 nmol/g). Genotypes differentially depicted in their ability to grow and yield satisfactorily, and noted with intensified stress condition the per cent survival in terms of plant population and single cane weight decreased (Fig. 4A, B). Genotype Co 15025 displayed significant reduction in survival rate (52%) under low (ECiw of 4 dS/m) and high (ECiw of 10 dS/m) salinity treatments, only 5.5% were survived (Fig. 4A). Other studied Genotypes showed nearly 40–50% survival up to ECiw ~ 8 dS/m which further reduced with stress intensification and Genotype Co 13,035 showed maximum survival of 33% under ECiw ~ 10 dS/m (Fig. 4A). All investigated clones exhibited significant gradual decrease in weight of single cane (SCW) with increasing salinity level (Fig. 4B).
Correlation analysis
To identify the trait significance, Pearson`s correlation analysis was done among traits both at control condition and ECiw ~ 8 dS/m. Under control conditions, Genotypes showed highly significant correlation among SCW and plant height (r = 0.86), Ψs and Ci (r = 0.8) while Proline, MI, MDA and Na+/K+ revealed negative association (Fig. 5A).
Whereas as, TCC also showed strong positive correlation with biochemical attributes (r > 0.8). Under salinity stress, SCW revealed strong negative relation with stem girth, leaf area, Ψw, Ψs, gS, Ci, proline (Fig. 5B). Overall, it was noted from the morphological, physiological, biochemical traits and yield, Genotype Co 13035 performed better than studied check Co 0118, while Co 15025 was found sensitive towards salinity stress.
Discussion
Plant height and stem girth, are the important morphological attributes for cane development and reductions in these traits suggest that salinity stress hinders growth of plants and foliage development that might be because of lowering of soil water potential which further restricts the absorption of water by plant roots (Dhansu et al., 2022a; Simões et al., 2023; Soni et al., 2021). In addition to this, salinity stress disrupts their water and nutrient uptake mechanisms, leading to diverse physiological and biochemical alterations that affect their growth and development (Sharma et al., 2021). Salt stress also interferes with cell expansion and the uptake of essential nutrients by the plant roots, as well as also creates an osmotic imbalance between the soil and plant cells, causing water to move out of the plant cells. This lead to cellular dehydration, wilting, and overall water stressed conditions that severely affect growth and development of plants (Kumar et al., 2023a, 2023b, 2023c). The reductions in number of internodes and intermodal length might be because of increase in concentration of toxic ions (Na+, Cl−) that interfere with growth of shoot and root systems (Vasantha et. al. 2010). Hence the plants allocate less energy to the growth of above-ground parts (shoots) and below-ground parts (roots), resulting in smaller and less vigorous plants (Pooja et al., 2012; Pooja and Sharma, 2016). Plant water relations under salt stress are significantly affected because of the existence of high concentrations of salt (Na+ and Cl−) either in the soil or irrigation water. Salt stress imposes multiple challenges on plants that disrupts uptake of water, water transport and overall water balance (Kumar et al., 2023a, 2023b, 2023c; Pooja et al., 2019). Relative Water Content (%) represents the percentage of water content in plant tissues compared to the maximum water content possible (Dhansu et al., 2022b). High accumulation of salts inside the soil particularly, in the root zone decreases the water availability that will be absorbed by the plant roots. Because of this, the plants may undergo water loss through transpiration at a higher rate than water uptake resulting into physiological drought, leading to a decrease in RWC and water potential (Soni et al., 2021). Overall, this data indicates that increasing salinity leads to a reduction in both content and potential of water, while different genotypes also exhibit variations in water-related traits. The turgor potential remains relatively stable across treatments and genotypes (Table 2). Such reduction in Ψw and Ψs generally rely upon the inherent potential of the crop genotypes capable to absorb water from the rhizosphere. Additionally to meet their evaporative demands under salinity stress, plants lowered Ψw and Ψs which regulates conductance of stomata and also alter the photosynthesis and transpiration phenomenon. Moreover the water uptake by roots is mediated by the potential difference exist between the soil water and leaf.
Salinity provoked osmotic and ionic stresses are responsible for severe reduction in leaf expansion area and the pigments involved in photosynthesis. This further cause undeniably damage and reduces the CO2 assimilation rate during photosynthesis that regulate various growth stages of the plant and development (Kumar et al., 2017; 2019). Results revealed statistically (p < 0.001) significant decline in the studied gas exchange attributes and photosynthetic pigments. Reduction in chlorophyll content and SPAD reading may be due to suppression in the activity of ALA synthase enzyme/increase in the chlorophyllase activity or might be due to the lack of uptake of the minerals such as magnesium which is required for the biosynthesis of the chlorophyll pigments (Chakraborty et al., 2016). Photosynthesis is an essential phenomenon to be performed by the plants under stressed environment for adequate plant growth and development. However, the opened stomata under saline stress shows uptake of salts or toxic ions and excessive water loss. Increase in ion concentration under saline environment might be another factor that produces variable stomatal and non-stomatal restrictions that affect the physiological processes of the plants under stress (Thiem et al., 2020). Decline in the stomatal conductance also suggested an adaptive approach to resist the loss of water via transpiration under salt stress. These alterations in various studied gas exchange parameters (Fig. 2A–B) may be related to the osmotic and ionic stress induced structural modifications in the photosynthetic machinery at multiple levels. These includes decreased efficiency of Rubisco, feedback inhibition as a result of reduced sink activity, closure of stomata, decrease in photochemical efficiency of chlorophyll and the electron transport activity (Mittal et al., 2012). Accumulation of salts in plant leaves via transpiration stream causes inhibition of growth. Therefore, the association between stomatal conductance or water-use efficiency, transpiration and photosynthesis is most considerable aspect under saline environment (Ran et al., 2021).
The interplay between, membrane injury (MI), lipid peroxidation (LP) and proline is a critical aspect of how plants cope with salinity stress and their mechanistic understanding is crucial for developing strategies to improve salinity tolerance in crop plants. Alteration in cell membrane structure due to membrane injury and modification of membrane fluidity because of oxidative stress might compel more distraction of plant resources to repair membrane. These adjustments play significant role in developing salinity tolerance (Gomathi & Rakkiyapan, 2011; Kumar et al., 2021). Similar to MI, LP was minimum in Co 0118 and Co 13035 which might have an efficient protection mechanism to suppress the cellular damage either predominantly higher capacity of the antioxidant system to scavenge ROS or inducing the accumulation of osmo-protectant proline (Azeem et al., 2023). Proline, a well-known osmo-protectant that helps in maintaining water balance, stabilizing membrane proteins and cellular structures as well as directly and indirectly scavenging ROS. Co 0238, Co 13035 and Co 0118 had higher accumulation of proline, which acts as balancer of cellular redox via maintaining osmotic balance of the cytosol, vacuole and within the external environment (Azeem et al., 2023; Kumar et al., 2023a, 2023b, 2023c). Crosstalk between MI, LP and proline helps in attenuation of adverse effects of ionic, osmotic and oxidative stress in the studied genotypes.
Maintaining an adequate equilibrium between sodium (Na+) and potassium (K+) ions is required by the plants to survive under saline conditions. Ionic stress mediated nutrient deficiency hampered the plant growth due to alterations in availability of nutrients, their absorption and further transport to the entire plant parts (Sharma et al., 2021). Increase in Na+/K+ under salinity was due to high levels of sodium ions competes with uptake of potassium ions and might obstruct the specific transporters for K+ ions through Na+/K+ co-transporters which can exacerbate the injurious effects of salinity (Lata et al., 2022). It was also noted that genotypes Co 13035, Co 0238 and Co 0118 maintained low Na+/K+ over salinity regimes which showed these genotypes maintained low Na+ in their leaves with adequate potassium levels, that were beneficial in minimizing the damage caused by salinity and promoting growth and survival of the plants (Dhansu et al., 2022a; Shabala & Cuin, 2008). For developing salinity tolerant crop varieties, the varieties exhibiting low Na+/K+ ratios and efficient ion management under saline environment would be on priority basis. All investigated clones exhibited significant gradual decrease in weight of single cane (SCW) with increasing salinity level (Fig. 4B). These reductions in SCW revealed that salinity stress reduced the cane’s ability to utilize and assimilate the resources which interacts with several components to contribute final harvest of the plants. The extent of reduction in survival and SCW could vary, but generally, under severe and prolonged salinity stress, these might be due to reduction in availability of water and cell expansion, impaired nutrient uptake and limited availability of essential elements, reduced photosynthetic activity and energy production, decreased cell division and elongation in the growing cane and increased metabolic costs to manage salinity stress generated ionic, oxidative and osmotic stresses (Apon et al., 2023; Kumar et al., 2023a, 2023b, 2023c). Similarly, Vasantha et al. (2010), Vasantha and Gomathi (2012), Gomathi and Thandapani (2014), Dhansu et al. (2022a) suggested that Genotypes with better ion exclusion capacity, adequate water and osmotic balancer, along with better ROS scavenger could ameliorate the damaging effects of salinity.
Data availability
The authors state that the data will be made accessible upon reasonable request.
References
Apon, T. A., Ahmed, S. F., Bony, Z. F., Chowdhury, M. R., Asha, J. F., & Biswas, A. (2023). Sett priming with salicylic acid improves salinity tolerance of sugarcane (Saccharum officinarum L.) during early stages of crop development. Heliyon, 9(5), e16030.
Azeem, M., Pirjan, K., Qasim, M., Mahmood, A., Javed, T., Muhammad, H., Yang, S., Dong, R., Ali, B., & Rahimi, M. (2023). Salinity stress improves antioxidant potential by modulating physio-biochemical responses in Moringa oleifera Lam. Scientific Reports, 13, 2895.
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39, 205–207.
Brindha, C., Vasantha, S., & Arunkumar, R. (2019). The response of sugarcane genotypes subjected to salinity stress at different growth phases. Journal of Plant Stress Physiology, 5, 28–33.
Chakraborty, K., Bhaduri, D., Meena, H. N., & Kalariya, K. (2016). External potassium (K+) application improves salinity tolerance by promoting Na+-exclusion, K+-accumulation and osmotic adjustment in contrasting peanut cultivars. Plant Physiology and Biochemistry, 103, 143–153.
Dhansu, P., Kulshreshtha, N., Kumar, R., Raja, A. K., Pandey, S. K., Goel, V., & Ram, B. (2021). Identification of drought-tolerant genotypes based on physiological traits, yield attributes and drought tolerance indices. Sugar Tech, 23, 741–767.
Dhansu, P., Kumar, R., Kumar, A., Vengavasi, K., Raja, A. K., Vasantha, S., Meena, M. R., Kulshreshtha, N., & Pandey, S. K. (2022a). Differential physiological traits, ion homeostasis and cane yield of sub-tropical sugarcane varieties in response to long-term salinity stress. Sustainability, 14(20), 13246.
Dhansu, P., Nandwal, A. S., Kumar, S., Chand, M., Rani, B., & Kulshreshtha, N. (2022b). Comparative evaluation of growth, yield and yield attributing traits in sugarcane (Saccharum officinarum) under different soil moisture regimes. Indian Journal of Agriculture Science, 92(8), 942–946.
Dionisio-Sese, M. L., & Tobita, S. (1998). Antioxidant responses of rice seedlings to salinity stress. Plant Science, 135(1), 1–9.
Gomathi, R., & Rakkiyapan, P. (2011). Comparative lipid peroxidation, leaf membrane thermostability, and antioxidant system in four sugarcane genotypes differing in salt tolerance. International Journal of Plant Physiology and Biochemistry, 3(4), 67–74.
Gomathi, R., & Thandapani, P. (2014). Influence of salinity stress on growth parameters and yield of sugarcane. IOSR Journal of Pharmacy and Biological Sciences, 9(3), 28–32.
Gu, D. D., Wang, W. Z., Hu, J. D., Zhang, X. M., Wang, J. B., & Wang, B. S. (2016). Non-destructive determination of total chlorophyll content in maize using three-wavelength diffuse reflectance. Journal of Applied Spectroscopy, 83, 541–547.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125, 189–198.
Kaur, G., Sanwal, S. K., Sehrawat, N., Kumar, A., Kumar, N., & Mann, A. (2022). Getting to the roots of Cicer arietinum L. (chickpea) to study the effect of salinity on morpho-physiological, biochemical and molecular traits. Saudi Journal of Biological Sciences, 29(12), 103464.
Kumar, A., Mann, A., Lata, C., Kumar, N., & Sharma, P. C. (2019). Salinity-induced physiological and molecular responses of halophytes. Research developments in saline agriculture, 331–356.
Kumar, A., Mann, A., Kumar, A., Kumar, N., & Meena, B. L. (2021). Physiological response of diverse halophytes to high salinity through ionic accumulation and ROS scavenging. International Journal of Phytoremediation, 23(10), 1041–1051.
Kumar, R., Meena, M. R., Dhansu, P., Karuppaiyan, R., Appunu, C., Kulshreshtha, N., Kaushik, P., & Ram, B. (2022). Winter tolerance potential of genetically diverse sugarcane clones under subtropical climate of Northern India. Sustainability, 14(18), 11757.
Kumar, A., Sheoran, P., Mann, A., Yadav, D., Kumar, A., Devi, S., Kumar, N., Dhansu, P., & Sharma, D. K. (2023a). Deciphering trait associated morpho-physiological responses in pearlmillet hybrids and inbred lines under salt stress. Frontiers in Plant Science, 14, 1121805.
Kumar, R., Dhansu, P., Kulshreshtha, N., Meena, M. R., Kumaraswamy, M. H., Appunu, C., Chhabra, M. L., & Pandey, S. K. (2023b). Identification of salinity tolerant stable sugarcane cultivars using AMMI, GGE and some other stability parameters under multi environments of salinity stress. Sustainability, 15(2), 1119.
Kumar, R., Meena, M. R., Kulshreshtha, N., Kumar, A., & Ram, B. (2017). Genotypic response of recently evolved sugarcane Co-clones under different levels of saline irrigation water. Journal of Sugarcane Research, 7(2), 159–168.
Kumar, R., Sagar, V., Verma, V. C., Kumari, M., Gujjar, R. S., Goswami, S. K., Kumar, S., Pandey, H., Dubey, A. K., Srivastava, S., Singh, S. P., Mall, A. K., Pathak, A. D., Singh, H., Jha, P. K., & Prasad, P. V. V. (2023c). Drought and salinity stress induced physio-biochemical changes in sugarcane: An overview of tolerance mechanism and mitigating approaches. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2023.1225234
Lata, C., Kumar, A., Mann, A., Soni, S., Meena, B. L., & Rani, S. (2022). Mineral nutrient analysis of three halophytic grasses under sodic and saline stress conditions. Indian Journal of Agriculture Science, 92(9), 1051–1055.
Lichtenthaler, H.K., & Buschmann, C. (2001). Chlorophylls and Carotenoids: Measurement UNIT F4.3 and Characterization by UV-VIS Spectroscopy. Current Protocols in Food Analytical Chemistry, F4.3.1-F4.3.8.
Mahadevaiah, C., Vignesh, P., Appunu, C., Valarmathi, R., Dhansu, P., Kumar, A., Dharshini, S., Padmanabhan, T. S. S., Narayan, J. A., & Selvamuthu, K. (2023). Physiological characterization of tripidium arundinaceum and sugarcane (Saccharum spp.) germplasm for salinity stress tolerance at the formative stage. Sustainability, 15, 6962.
Mann, A., Bishi, S.K., Mahatma, M.K., & Kumar, A. (2015). Metabolomics and salt stress tolerance in plants. Managing Salt Tolerance in Plants: Molecular and Genomic Perspectives, Taylor and Francis Group LLC. (2015), pp. 251–266 1.
Mann, A., Lata, C., Kumar, N., Kumar, A., Kumar, A., & Sheoran, P. (2023). Halophytes as new model plant species for salt tolerance strategies. Frontiers in Plant Science, 14, 1137211.
Mittal, S., Kumari, N., & Sharma, V. (2012). Differential response of salt stress on Brassica juncea: Photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiology and Biochemistry, 54, 17–26.
Plaut, Z., Meinzer, F. C., & Federman, E. (2000). Leaf development, transpiration and ion uptake and distribution in Sugarcane cultivars- grown under salinity. Plant and Soil, 218, 59–69.
Pooja, A., & Sharma, K. D. (2016). Salicylic acid induced amelioration of salinity stress in mungbean. KG, Germany: Scholars Press Omni Scriptum GmbH & Co.
Pooja, Nandwal, A. S., Chand, M., Pal, A., Kumari, A., Rani, B., Goel, V., & Kulshreshtha, N. (2020). Soil moisture deficit induced changes in antioxidative defense mechanism of sugarcane (Saccharum officinarum) varieties differing in maturity. Indian Journal of Agriculture Science, 90, 507–512.
Pooja, Nandwal, A. S., Chand, M., Singh, K., Mishra, A. K., Kumar, A., Kumari, A., & Rani, B. (2019). Varietal variation in physiological and biochemical attributes of sugarcane varieties under different soil moisture regimes. Indian Journal of Experimental Biology, 57, 721–732.
Pooja, Sharma, K. D., & Kumar, A. (2012). Improvement in plant water relation and photosynthetic activity of mungbean (Vigna radiata L.) in response to salicylic acid under salinity stress. Indian Journal of Plant Physiology, 17(3), 268–274.
Ran, X., Wang, X., Gao, X., Liang, H., Liu, B., & Huang, X. (2021). Effects of salt stress on the photosynthetic physiology and mineral ion absorption and distribution in white willow (Salix alba L.). PLoS ONE, 16(11), e0260086.
Rao, V. P., Sengar, R. S., Singh, S., & Sharma, V. (2015). Molecular and metabolic perspectives of sugarcane under salinity stress pressure. Progressive Agriculture, 15, 77–84.
Santana, M. J., Carvalho, J. A., Souza, K. J., Sousa, A. M. G., Vasconcelos, C. L., & Andrade, L. A. B. (2007). Effects of irrigation water salinity on sprouting and initial development of sugarcane (Saccharum sp.) and in soils with different textural levels. Ciência e Agrotecnologia, 31, 1470–1476.
Sengar, K., Sengar, R. S., & Singh, A. (2013). Biotechnological and genomic analysis for salinity tolerance in sugarcane. International Journal of Biotechnology and Bioengineering Research, 4, 407–414.
Shabala, S., & Cuin, T. A. (2008). Potassium transport and plant salt tolerance. Physiologia Plantarum, 133(4), 651–669.
Sharma, P.C., Kumar, A., & Mann, A. (2021). Physiology of salt tolerance in crops. Sustainable Agriculture, 199.
Sheoran, P., Kamboj, P., Kumar, A., Kumar, A., Singh, R. K., Barman, A., Prajapat, K., Mandal, S., Yousuf, D. J., Narjary, B., & Kumar, S. (2023). Matching N supply for yield maximization in salt–affected wheat agri–food systems: On-farm participatory assessment and validation. Science of the Total Environment, 875, 162573.
Simões, W. L., de Oliveira, A. R., Tardin, F. D., de Oliveira, C. P. M., de Morais, L. K., Teodoro, L. P. R., & Teodoro, P. E. (2023). Saline stress affects the growth of Saccharum complex genotypes. Journal of Agronomy and Crop Science. https://doi.org/10.1111/jac.12647
Soni, S., Kumar, A., Sehrawat, N., Kumar, A., Kumar, N., Lata, C., & Mann, A. (2021). Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi Journal of Biological Sciences, 28, 2510–2517.
Thiem, D., Tyburski, J., Golebiewski, M., & Hrynkiewicz, K. (2020). Halotolerant fungi stimulate growth and mitigate salt stress in Alnus glutinosa Gaertn. Dendrobiology, 83, 30.
Vasantha, S., & Gomathi, R. (2012). Growth and development of sugarcane under salinity. Journal of Sugarcane Research, 2(1), 1–10.
Vasantha, S., Venkataramana, S., Rao, P. N. G., & Gomathi, R. (2010). Long term salinity effect on growth, photosynthesis and osmotic characteristics in sugarcane. Sugar Tech, 12, 5–8.
Verma, K. K., Song, X. P., Verma, C. L., Malviya, M. K., Guo, D. J., Rajput, V. D., Sharma, A., Wei, K. J., Chen, G. L., & Solomon, S. (2021). Prediction of photosynthetic leaf gas exchange of sugarcane (Saccharum spp) leaves in response to leaf positions to foliar spray of potassium salt of active phosphorus under limited water irrigation. ACS Omega, 6, 2396–2409.
Verma, K. K., Song, X. P., Zeng, Y., Li, D. M., Guo, D. J., Rajput, V. D., Chen, G. L., Barakhov, A., Minkina, T. M., & Li, Y. R. (2020). Characteristics of leaf stomata and their relationship with photosynthesis in Saccharum officinarum under drought and silicon application. ACS Omega, 5, 24145–24153.
Verma, K., Kumar, R., Kumar, A., Bhardwaj, A. K., & Verma, R. C. (2023). Host plant regulates growth processes, ion homeostasis, and salinity tolerance of sandalwood (Santalum album L.). J Plant Growth Regul, 1–13. https://doi.org/10.1007/s00344-023-10906-3.
Weatherley, P. E. (1950). Studies in the water relations of the cotton plant I.The field measurements of water deficit in leaves. New Phytologist, 49, 81–97.
Acknowledgements
We appreciate ICAR-SBI, RC, Karnal and MM (DU), Mullana, Ambala for the help with logistics that allowed us to finish this study.
Funding
This research received no grant in the form external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Preet, K., Dhansu, P., Sehrawat, N. et al. Morpho-physiological analysis of salinity tolerance in sugarcane genotypes. Plant Physiol. Rep. 29, 356–366 (2024). https://doi.org/10.1007/s40502-024-00782-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-024-00782-8