Abstract
Leaf area and some physical leaf properties play an important role in the vital events of the plant, such as photosynthesis, respiration, growth, yield, and quality. Therefore, knowing the leaf area and models that estimate the leaf area is crucial in the development of a fruit tree. Determination of leaf area, especially by non-destructive methods, is very important in terms of examining leaf development in different periods. Therefore, a leaf area estimation model and some physical leaf properties were produced using ‘Jeromine’, ‘Fuji Zehn Astec’, ‘McIntosh’, ‘Granny Smith Challenger’, ‘Buckeye Gala’, ‘Honeycrisp’, ‘Rosy Glow’, ’Super Chief’, ‘Golden Reinders’, ‘Ginger Gold’ and ‘Amasya’ apple cultivars. This study was conducted in the Bafra Plain in Northern Anatolia in 2023. Leaf width, length and leaf area were measured to develop the model. The leaf area of the cultivars were measured by PLACOM Digital planimeter, and multiple regression analysis with Microsoft Office XP Excel 2016 program was performed for the cultivars separately. The developed leaf area estimation model in the present study was: LA = [0.887–0.224*(W + L) + 0.786*W*L] (r2 = 0.975). In addition to the model generation procedure, the model was validated using the residual values between predicted and measured leaf areas from new leaf samples collected from different apple trees. R2 values for the relationships between actual and predicted leaf areas of the tested apple cultivars were found to be 0.971. In this study, the brightest colored leaves were obtained from the ‘Golden Reinders’ cultivar. The highest chlorophyll content was obtained from ‘Honeycrisp’ and ‘Ginger Gold’ cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Determining the leaf area in horticultural cultivation is important for photosynthesis, respiration, transpiration, light interception, flowering, fruit set, quality and yield. All of these processes occur directly or indirectly in the leaf. Photosynthesis, the main source of growth and development, especially in green plants, occurs in the leaf. As a matter of fact, although photosynthesis occurs in all green parts of a tree, including green fruits, the main production site of photosynthesis is the leaf. Many cultural practices, especially training and pruning, are performed on fruit trees for better leaf development and for these leaves to benefit from the sun better. Leaf area fruit ratio is also very important, especially in obtaining high-quality products and high yields. For example, it is known that a leaf area of 200 cm2 per fruit is needed for high quality fruit in cherries (Long et al., 2021). All information is presented to emphasize the importance of leaf and leaf area. Therefore, determining the leaf area accurately, practically and cheaply is very important both in terms of yield and quality and in terms of providing scientific data. In addition, leaf area models provide great convenience for researchers in orchard plant experiments.
Leaf area measurements can be determined by using some instruments (such as a planimeter) and developing a leaf area prediction model. When measuring leaf area with instruments such as planimeters, the time and labor required for measurements are increased, as well as the possibility of damaging the tree while removing the leaves from the tree. For these reasons, studies have been conducted on non-destructive methods of leaf area estimation models (Robbins and Pharr 1987; Bindi et al. 1997; Uzun and Çelik 1999; Demirsoy et al. 2005; Serdar and Demirsoy, 2006). The most important advantage of these models is that they help determine the leaf area without damaging the plant or removing the leaves from the plant. Leaf area prediction models have been developed by researchers (Uzun and Çelik 1999; Demirsoy and Demirsoy 2003; Demirsoy et al. 2005; Demirsoy et al. 2004; Serdar and Demirsoy 2006; Demirsoy 2009; Demirsoy and Lang 2010; Öztürk et al. 2019) in many fruit species (avocado, kiwifruit, pear, sweet cherry, strawberry, peach, chestnut, kiwifruit, pistachio). In recent years, studies have been conducted using artificial neural networks (ANNs) to determine the leaf area index (Shabani et al. 2017; Ercanlı et al. 2018; Öztürk et al. 2019).
Leaf area is a shape that emerges from the physical change of the leaf, such as size and color, starting from spring. It comes in different shapes and sizes in different periods. Therefore, when determining the leaf area or developing a formula or model on the leaf area, it is necessary to examine the physical changes in the leaf. This study aimed to determine the leaf area estimation model and some physical leaf properties in apples, which are grown very intensively worldwide.
Materials and Methods
The study was carried out in 2023 to develop a leaf area prediction model and to determine change in some physical properties of leaves such as color and size in apples in the 8‑year-old trial orchard of Ondokuz Mayıs University, Faculty of Agriculture. In the trial, ‘Jeromine’, ‘Fuji Zehn Astec’, ‘McIntosh’, ‘Granny Smith Challenger’, ‘Buckeye Gala’, ‘Honeycrisp’, ‘Rosy Glow’, ‘Super Chief’, ‘Golden Reinders’, ‘Ginger Gold’ and ‘Amasya’ apple cultivars on M9 rootstock were used. These cultivars are important cultivars traded both in Türkiye and worldwide.
In the experiment, leaves were taken from three trees of each and three different periods (26 April, 6 June, and 14 July) so that depending on the leaf size at the time the developed model was taken, its borders were as wide as possible and it could respond to leaves of all sizes. Later, the developed model was validated with different leaves. In addition, some physical properties such as leaf size, color and chlorophyll content (CCI) were also determined in the study.
Development of the Model
Leaf samples were selected randomly from apple trees from different levels of the canopy during spring–summer growth period. A total of 640 leaves samples were used in the experiment, 448 (70%) for developing a model and 192 (30%) for validating. Initially, each leaf was glued on A4 paper and then measurements were taken with the help of a Placom Digital Planimeter (Sokkisha Planimeter Inc., Model KP-90, Japan). The leaf width (cm) and length (cm) of the leaf samples taken were measured to be used in model construction. Leaf width (W) was measured from tip to tip at the widest part of the lamina and leaf length was measured from lamina tip to the point of petiole intersection along the midrib. All values were recorded to the nearest 0.1 cm.
Multiple regression analysis of the data was performed. For this reason, analysis was conducted with different subsets of the independent variables viz., length (L), length square (L2), width (W), leaf width*leaf length square (W*L2) to develop the best model for predicting leaf area (LA) by using the Microsoft Office XP Excel 2016 package program. Multiple regression analysis was carried out till the deviation sum of squares was minimized.
In this research, to validate the developed leaf area estimation model, 192 leaves collected at the three different periods like model were used. Leaf width, length, and actual leaf area of these leaf samples were measured as mentioned in the model construction section. For the validation procedure, leaf area values obtained by using the model were plotted against actual leaf areas measured using a planimeter. The Microsoft Office XP Excel 2016 package program was used for this procedure.
Some Physical Properties of Leaves
Leaf color values of the cultivars examined in the experiment were determined by reading the L (brightness), C (color intensity) and ‘H◦’ (hue) values of the digital color measuring device (CE Minolta CR300). Chlorophyll content was recorded by using a chlorophyll meter (CCM-200, Opti-Sciences, USA) in fully expanded young leaves of 10 marked plants in the early hours of the day (9:00–10:00). SPSS 20.0 statistical package program was used to evaluate leaf color values (L, a, b) and chlorophyll content characteristics of the cultivars and Duncan multiple comparison test was applied according to the importance level of p ≤ 0.01 and p ≤ 0.05 by using the same package program to determine the difference between the averages.
Results and Discussion
Development of the Model
Multiple regression analysis was used to determination of the best-fitting equation for leaf area prediction. Regression analysis in the studied apple cultivars showed that most of the variation in the leaf area values was explained by the selected parameters (length and width). The overall variation explained by the parameters was 97.53% for apple cultivars (Table 1). There was a highly reliable relationship between actual and predicted leaf areas for the apple cultivars (Fig. 1).
In their study, Boyacı and Küçükönder (2022) developed a leaf area prediction model for four different apple cultivars. In the model they developed separately for each cultivar, the researchers determined r2 = 0.9792 for the ‘Summer Red’ cultivar, r2 = 0.9914 for the ‘Mondial Gala’ cultivar, r2 = 0.9858 for the ‘Golden Delicious’ cultivar, and r2 = 0.9508 for the ‘Braeburn’ cultivar.
In accordance with the present study, many studies carried out to establish reliable relationships between leaf area and leaf dimensions of different plant species such as kiwifruit, avocado, pepper, lotus plum, aubergine, red currant species (Uzun and Çelik 1999), sweet cherry, (Demirsoy and Demirsoy 2003), strawberry (Demirsoy et al. 2005), peach (Demirsoy et al. 2004), chestnut (Serdar and Demirsoy 2006), and pear (Öztürk et al. 2019) showed that there was a close relationship between leaf width, leaf length, and leaf area (r2 = 0.983 for avocado, lotus plum, kiwifruit, aubergine, and pepper; r2 = 0.986 for red currant; r2 = 0.9809 for sweet cherry; r2 = 0.989 for pear; r2 = 0.993 for strawberry; r2 = 0.9975 for peach; r2 = 0.988 for chestnut).
Plotting processes were carried out between actual leaf area values measured by using a Placom digital planimeter and predicted leaf areas of the tried cultivars calculated by the developed model in this research to determine the degree of accuracy of the model (Fig. 2). It was found that the relationship (r2 values) was 0.971.
Validation of a leaf area model is an important step to overcome the implications of produced equations for prediction of leaf area. After determining the level of usability of these kinds of models, a trustable way would be given to the researchers to lead studies on plant growth phenomena such as respiration, photosynthesis, and transpiration without destructive leaf harvesting. In regression analysis, the proportion of the variation accounted for by a relationship is equivalent to the coefficient of determination (r2) (Bindi et al. 1997). The objective of regression analysis and modeling is to maximize the proportion of the variation accounted for by the model, while minimizing the unattributable variation. Many researchers validated their own developed leaf area prediction model. For example, Çelik and Uzun (2002) found that the relationship (r2 values) between actual and predicted leaf areas varied from 0.918 in lotus plum to 0.988 in pepper (from the lowest to the highest value). In another study, Demirsoy and Lang (2010) reported that the actual and predicted leaf area values of r2 = 0.9886 in ‘Regina’/Gisela 6 combinations and r2 = 0.9849 in ‘Regina’/Gisela 5 combinations. In the present study, it was found that the relationship (r2 values) was 0.9757.
Some Physical Properties of Leaves
There was no statistical difference among the cultivars in terms of leaf color values. However, among the cultivars, the brightest (L) leaves were obtained from the ‘Golden Reinders’ cultivar (39.48), followed by ‘Buckeye Gala’ and ‘Fuji Zehn Astec’ cultivars (36.65 and 36.15, respectively) (Fig. 3). The cultivar with the least leaf brightness (L) was ‘Super Chief’ (30.82). In the study, a values were the highest ‘Super Chief’ (−3.70) and the lowest ‘Golden Reinders’ (−10.43) cultivar. With regard to b values, these were highest in ‘Golden Reinders’ cultivar (10.24) and lowest in ‘Super Chief’ (3.60) cultivar. The −a value indicates that the leaf color is green and the +a value indicates that the leaf color is red. The −b value indicates that the color is blue, the +b value indicates that the color is yellow.
There was a significant difference between cultivars in terms of chlorophyll content. The highest chlorophyll content was obtained from ‘Honeycrisp’ and ‘Ginger Gold’ cultivars. The lowest chlorophyll content was obtained from the ‘Golden Reinders’ cultivar (Fig. 4).
Keeping plants’ green leaves for a long time helps them perform photosynthesis for a longer period of time (Ellis et al. 1990). The photosynthetic activities of leaves are directly proportional to the concentration of chlorophyll, the green color pigment. Chlorophyll content in apple trees may be an indicator of the photosynthetic efficiency and, therefore, leaf area. The change in chlorophyll density of leaves can provide information about leaf area and other physiological states of plants (Chen et al. 2007). Cultivars with larger leaf areas, such as ‘Granny Smith Challenger’ in this study, have higher chlorophyll density of leaves as they will be exposed to more sunlight.
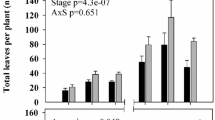
The leaf area changes of the cultivars in April, June and July are given in Figs. 5, 6 and 7. When the total leaf areas of the cultivars were examined at the end of three different periods, it was determined that the most was obtained from the ‘Granny Smith Challenger’ cultivar and the least was obtained from the ‘McIntosh’ and ‘Amasya’ cultivars (Fig. 8).
Conclusion
As mentioned above, as in many fruit species, there is a model consisting of different formulas for each cultivar based on a previous study on apples. That model cannot be used to determine the areas of cultivars not included in that study. However, in this study, 11 cultivars were used to create the model and the aim was to estimate the leaf area of apples with a single model, regardless of the cultivar. As a result of the study, r2 values between actual and predicted leaf areas were found to be relatively high (0.971) with non-destructive modeling. Considering the accuracy of the model, it can be seen that this formula can be used safely in studies on leaf area. The study also examined some physical leaf properties, such as leaf color and chlorophyll contents. In this context, this study has achieved its goal.
References
Bindi M, Miglietta F, Gozzini B, Orlandini S, Seghi L (1997) A simple model for simulation of growth in grapevine (Vitis vinifera L.). 2. Model validation. Vitis 36:73–76
Boyacı S, Küçükönder H (2022) A research on non-destructive leaf area estimation modeling for some apple cultivars. Erwerbs-Obstbau 64(1):1–7. https://doi.org/10.1007/s10341-021-00619-w
Çelik H, Uzun S (2002) Validation of leaf area estimation models (UZCELIK-I) evaluated for some horticultural plants. Pak J Bot 34(1)
Chen W, Yang X, He Z, Feng Y, Hu F (2007) Differential changes in photosyn the ticcapacity, 77K chlorophyll fluorescence and chloroplas tultra structure between Zn-efficienct and Zn in efficient rice genotypes (Oryza sativa L.) underlow Zn stress. Plant Physiol 132:89–101. https://doi.org/10.1111/j.1399-3054.2007.00992.x
Demirsoy H (2009) Leaf area estimation in some species of fruit tree by using models as a non-destructive method. Fruits 64(1):45–51. https://doi.org/10.1051/fruits/2008049
Demirsoy H, Lang GA (2010) Validation of a leaf area estimation model for sweet cherry. Span J Agric Res 8(3):830–832. https://doi.org/10.5424/sjar/2010083-1285
Demirsoy L, Demirsoy H (2003) Leaf area estimation model for some local cherry genotypes in Turkey. Pak Biol Sci 6(2):153–156
Demirsoy H, Demirsoy L, Uzun S, Ersoy B (2004) Non-destructive leaf area estimation in peach. Eur J Hortic Sci 69(4):144–146
Demirsoy H, Demirsoy L, Öztürk A (2005) Improved model for the non-destructive estimation of strawberry leaf area. Fruits 60(1):69–73. https://doi.org/10.1051/fruits:2005014
Ellis RH, Hadley P, Roberts EH, Summerfield RJ (1990) Quantitative relations between temperature and crop development and growth. Climatic change and plant genetic resources. Belhaven Press, London, New York
Ercanlı İ, Günlü A, Şenyurt M, Keleş S (2018) Artificial neural network models predicting the leaf area index: a case study in pure even-aged Crimean pine forests from Turkey. For Ecosyst 5:1–12. https://doi.org/10.1186/s40663-018-0149-8
Long LE, Lang GA, Kaiser C (2021) Sweet cherries. CABI, p 391
Öztürk A, Cemek B, Demirsoy H, Küçüktopcu E (2019) Modelling of the leaf area for various pear cultivars using neuro computing approaches. Span J Agric Res 17(4):e206. https://doi.org/10.5424/sjar/2019174-14675
Robbins NS, Pharr DM (1987) Leaf area prediction models for cucumber from linear measurements. HortScience 22(6):1264–1266. https://doi.org/10.21273/HORTSCI.22.6.1264
Serdar Ü, Demirsoy H (2006) Non-destructive leaf area estimation in chestnut. Sci Hortic 108(2):227–230. https://doi.org/10.1016/j.scienta.2006.01.025
Shabani A, Ghaffary KA, Sepaskhah AR, Kamgar-Haghighi AA (2017) Using the artificial neural network to estimate leaf area. Sci Hortic 216:103–110. https://doi.org/10.1016/j.scienta.2016.12.032
Uzun S, Çelik H (1999) Leaf area prediction models (Uzcelik-I) for different horticultural plants. Turk J Agric For 23(6):645–650
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. Soysal declares that she has no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soysal, D. A Non-destructive Leaf Area Prediction Model and Some Physical Leaf Properties in Apples. Applied Fruit Science 66, 1181–1186 (2024). https://doi.org/10.1007/s10341-024-01103-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-024-01103-x