Abstract
Low grape quality in early maturing table grape cultivars is a common problem, which reduces production efficiency. Despite the fact that there are numerous measures to be taken, foliar applications of different substances can be commonly used to overcome this low-quality problem in early maturing table grape cultivars. In this research, it was utilized from various doses of biostimulant (BSt) and plant growth regulator (s-abscisic acid, S‑ABA) applications, including Control, BSt 3000 ppm, BSt 4000 ppm, BSt 5000 ppm, S‑ABA 300 ppm, S‑ABA 400 ppm and S‑ABA 500 ppm for improving table grape quality of early maturing cv. Trakya Ilkeren. In the study, various doses of both foliar applications had considerable roles on physical and quality characteristics of cultivar, S‑ABA applications particularly triggered significant increases in total soluble solids content, total phenolic compounds content and total anthocyanin content. As a consequence, it has been highlighted in present study that S‑ABA 400 ppm application provides table grape growers with a tool to increase table grape quality characteristics such as anthocyanins and phenolic compounds in early maturing cv. Trakya Ilkeren.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
‘Trakya Ilkeren’ is an early season fine table grape cultivar grown in various regions of Turkey where the ecological conditions are appropriate. It was derived from crossing of ‘Alphonse Lavallée’ × ‘Perlette’ and produces berries with blue-black color (Kok 2018a).
In growing of early season table grape varieties, there can be occasional problems in grape skin coloration and soluble solids content (İşçi and Gökbayrak 2014; Kamiloğlu 2013) caused by different reasons such as excessive crop load, the lack of sunlight on clusters (Kishino and Roberto 2007) and excessive warm regions (Peppi et al. 2006).
Traditionally, table grape quality evaluation is achieved by various methods, including visual and taste assessments, physical and biochemical attributes of grapes (Maoz et al. 2016; Kok 19,20,a, b; Kristic et al. 2003).
Grape growth and quality are influenced by several pre-harvest factors such as genetic, viticultural practices and ecological conditions (Tyagi et al. 2017).
Among the different viticultural practices, there has been growing interest in using various foliar spray applications, including agrochemicals, biostimulants and plant growth regulators for enhancing table grape quality (Cantin et al. 2007; Peppi and Fidelibus 2008; Kok 19,20,a, b).
Biostimulant applications affect quality and yield of fruits by encouraging and stimulating plant metabolism and reducing stress (Parrado et al. 2008; Chehade et al. 2017) and are also known to increase fruit size, appearance and quality by having direct impacts on fruit growth and development or indirectly by adjusting crop load, plant vigor and canopy design (Looney 1993).
Plant growth regulators (PGRs), either produced naturally by the plant or synthetically, are small organic molecules, behaving inside the plant cells and change the growth and development of plants (Giannakoula et al. 2012).
Abscisic acid (ABA) has already been used to increase skin anthocyanin content and flavonol content in table grapes (Peppi et al. 2006; Sandhu et al. 2011).
The objective of current study was to analyze the effects of various doses of biostimulant and abscisic acid applications on quality characteristics of early maturing cv. Trakya Ilkeren table grape.
Material and Methods
Plant Material and Research Area Characteristics
This study was performed in a commercial vineyard located in territory of Tekirdağ, Turkey (40°58′23.95″N; 27°27′15.54″E; 105 m a.s. l.) during the growing season of 2015 year.
In the research, it was benefited from 11-year old cv. Trakya Ilkeren grapevines trained to guyot trained system and grafted on Kober 5BB rootstock (‘Berlandieri’ × ‘Riparia Teleki’ 8B, Selection Kober 5BB). The grapevines were planted at a spacing of 3.0 m between rows and 1.5 m between grapevines within a row in the vineyard. All vineyard operations were conducted according to standard viticultural practices for grape cultivar and the region.
In terms of region climate, the research area is under the control of a Mediterranean Climate. Annual mean temperature, sunshine duration per day, relative humidity and total precipitation were in order of 15.5 °C, 5.0 h, 80.9% and 850.8 mm for 2015 year. Common soil characteristics of research area were clay-loam with pH of 7.51 and organic matter of 1.20%.
Applications of Biostimulant and Plant Growth Regulator
In this research, two different foliar application sources, including biostimulant and plant growth regulator were used to increase grape quality characteristics. One of these foliar substances was a biostimulant (BSt), consisting of 26.6 g/L of organic nitrogen, 13.3 g/L of mineral nitrogen, 93.1 g/L of K2O and 186.2 g/L of organic carbon. However, other substance used in the study was a plant growth regulator as s‑abscisic acid (S-ABA, 10%), which is natural isomer of abscisic acid.
In the study, it was made use of various doses of foliar biostimulant (BSt) and s‑abscisic acid (S-ABA) applications, consisting Control, BSt 3000 ppm, BSt 4000 ppm, BSt 5000 ppm, S‑ABA 300 ppm, S‑ABA 400 ppm and S‑ABA 500 ppm. Foliar applications were performed thrice with 10-day intervals, containing 10-day before vérasion period (first application time), at vérasion period (second application time) and 10-day after vérasion period (third application time) by using a back pump. Prior to all foliar applications, both biostimulant and s‑abscisic acid were firstly dispersed in water with 0.01% (v/v) of Tween 20 and were subsequently applied to grapevines until run-off.
Physical and Quality Parameters
After the grapes were harvested at ideal maturity values of grape cultivar, physical variables such as grape length (mm), grape width (mm), grape weight (g), grape firmness (N), bunch length (cm), bunch width (cm), bunch weight (g) and bunch shape were measured. On the other hand, total soluble solids content (%), titratable acidity (g/L), must pH, p-value of must (µW), total phenolic compounds content (mg GAE/kg fw) and total anthocyanin content (mg GAE/kg fw) were also found out as quality variables.
Description of Bunch Shape
Bunch shape of present grape cultivar were determined according to Code 208 of OIV (International Organization of Vine and Wine) descriptor list. Based on this descriptor list, there are three different bunch shapes, defining for grape cultivars and codes of 1, 2 and 3 indicate grape bunch shapes of cylindrical, conical and funnel, respectively (Anonymous 2009).
Making Decision for Harvest Time and Grape Saplings for Quality Analysis
During the 2015 growing season, ripening grapes on the grapevines of cv. Trakya Ilkeren were continuously observed and were harvested when the control grapes achieved 16% total soluble solids content. After grapes were picked at harvest time, samples of 250-grapes were collected from each foliar application and eventually employed to determine total soluble solids content, titratable acidity, must pH, p-value of must. Besides this, it was utilized from 300-grape samples for determining total phenolic compounds content and total anthocyanin content. All grape samples were stored at −25 °C until the analyzes of total phenolic compounds and total anthocyanin were performed. Previous to these analyzes, grape samples were moved away from −25 °C, permitted to thaw overnight at 4 °C and were subsequently homogenized in a commercial laboratory blender for 20 s.
Determination of Grape Firmness
Grape firmness was gauged with an analog penetrometer (FT 02, Wagner Instruments, Riverside, USA) and measurement values were read as g/mm. After this, values were converted from g/mm to N with the conversion factor given below:
Calculation of P-value
P-value, including redox potential (mV), pH, p-value (μW) and resistivity (Ώ) were formulated with an equation informed by Hoffmann (1991). In current study, p-values of grape must sample from various doses of preharvest foliar applications were calculated according to equation mentioned above and were expressed as μW.
Analyzes for Total Phenolic Compounds Content and Total Anthocyanin Content
Determination of total phenolic compounds content and total anthocyanin content of grapes were successively conducted by employing spectrophotometric methods declared by Singleton et al. (1978) and Di Stefano and Cravero (1991). Results of both analyzes were explained as milligrams of gallic acid equivalent per kilogram of fresh weight (mg GAE/kg fw).
Statistical Analysis
The study was carried out by using a completely randomized blocks design with four replicates. Data from research findings were submitted to analysis of variance (ANOVA) by means of TARIST statistical software. The means were separated by using Fisher’s Least Significant Difference (LSD) multiple range test at 5%.
Results and Discussion
Physical Characteristics
Findings of physical variables remarked in Table 1 reveal that some of the physical characteristics are significantly influenced by foliar biostimulant and s‑abscisic acid applications except for grape width, grape weight bunch width, bunch weight and bunch shape (P ≤ 0.05).
Grape and bunch sizes in grape cultivars are influenced by grape cultivars, ecological conditions and viticultural practices (Gougolias and Masheva 2010; Kok et al. 2013). In present study, it is observed in Table 1 that foliar biostimulant and s‑abscisic acid applications have significant effects on grape length (P ≤ 0.05) and means of grape length ranged from 18.43 (Control) to 20.00 mm (S-ABA 400 ppm).
As mentioned in Table 1, that applications of foliar biostimulant and s‑abscisic acid give rise to non-significant differences in grape width (P ≤ 0.05). In the study, while the highest grape width mean was 20.52 mm for S‑ABA 400 ppm application, the lowest grape width mean was recorded for Control application (19.51 mm).
As far as grape weight means are concerned in Table 1, there are no significant differences among the foliar biostimulant and s‑abscisic acid applications (P ≤ 0.05) and S‑ABA 400 ppm application had the highest grape weight (5.98 g) than Control application (5.44 g).
Grape firmness of table grapes is one of the major factors, determining their eating quality (Plessis 2008). As shown in Table 1, grape firmness is significantly affected by foliar biostimulant and s‑abscisic acid applications (P ≤ 0.05) and the highest grape firmness means were obtained from applications of S‑ABA 400 ppm (6.07 N) and S‑ABA 500 ppm (5.85 N) when the compared with Control application (4.15 N).
Research findings of bunch length indicated in Table 1 show that foliar biostimulant and s‑abscisic acid applications are found to be statistically significant (P ≤ 0.05) and rising means of bunch length were successively 19.11 cm (Control), 19.90 cm (BSt 3000 ppm), 20.53 cm (BSt 5000 ppm), 20.62 cm (BSt 4000 ppm), 20.96 cm (S-ABA 300 ppm), 21.36 cm (S-ABA 500 ppm) and 21.78 cm (S-ABA 400 ppm).
As pointed out in Table 1, foliar biostimulant and s‑abscisic acid applications have no significant effects on bunch width (P ≤ 0.05) and while the lowest mean was 18.53 cm for Control application, the highest bunch width was 21.00 cm 21.00 cm for S‑ABA 400 ppm.
Based on bunch weight means presented in Table 1, it seems that foliar biostimulant and s‑abscisic acid applications have non-significant differences on bunch weight means (P ≤ 0.05). In the study, S‑ABA 400 ppm application caused the highest bunch weight (647.13 g) whereas the lowest mean was obtained from Control application (543.05 g).
Bunch shapes on the grapevines are influenced by cultivar characteristic of grape and viticultural practices employed in vineyard (Çelik 2011). Bunch shapes indicated in Table 1 are not significantly influenced by foliar biostimulant and s‑abscisic acid applications (P ≤ 0.05). In the study, while all doses of S‑ABA applications were leading to bunch shape no 2.25, meaning a shape between the conical and funnel, Control and foliar biostimulant (BSt) applied grapevines had bunches shaped no 2.00, meaning conical bunch shape according to Code 208 of OIV descriptor list (Anonymous 2009).
Quality Characteristics
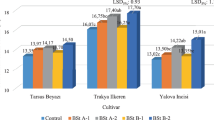
Figs. 1 and 5 and 6 show that total soluble solids content, total phenolic compounds content and total anthocyanin content of grapes are significantly influenced by foliar biostimulant and s‑abscisic acid applications (P ≤ 0.05).
Sugar and organic acid compositions, which are measured through total soluble solids and titratable acidity are the most frequently associated with the taste of table grapes (Ferguson and Boyd 2002; Shiraishi et al. 2010). As illustrated in Fig. 1, foliar biostimulant and s‑abscisic acid applications significantly affect total soluble solids content (P ≤ 0.05) and means of total soluble solids content varied from 16.07 (Control) to 17.90% (S-ABA 400 ppm).
Although organic acids are existing in small quantities in grapes when they compared with sugars, these compounds contribute significantly to the overall taste (Nelson 1985). Regarding titratable acidity indicated in Fig. 2, foliar biostimulant and s‑abscisic acid applications have no significant effects on titratable acidity (P ≤ 0.05) and means of titratable acidity changed from 2.67 (S-ABA 400 ppm) to 3.07 g/L (Control).
pH values of grape must be generally between 3.0 and 3.5 (Keller 2015). In terms of must pH represented in Fig. 3, there are no significant differences among the foliar biostimulant and s‑abscisic acid (P ≤ 0.05) and while the highest pH mean was 3.52 for S‑ABA 400 ppm, the lowest pH mean was recorded for Control application (3.38).
Determining of p-value is a recently developed method for assessing product quality (Keppel 2001; Velimirov 2004) and lower p-values signify better product quality (Hoffmann 1991). In this study, it is clearly seen in Fig. 4 that foliar biostimulant and s‑abscisic acid applications have no significant effects on p-value of must (P ≤ 0.05) and S‑ABA 400 ppm application resulted in the lowest p-value of must (103.11 µW) when the compared with Control application (115.98 µW).
Grape skins are the main sources of grape phenolic compounds and total phenolic compounds content of grapes varies from 400 to 3000 mg/L depending on grape cultivar, soil composition, climate, geographic origin, viticultural practices or exposure to fungal diseases and reactions occurring during the storage (Cosme et al. 2018; Kok 2017; Kok and Bal 2017). As indicated in Fig. 5, total phenolic compounds content is significantly influenced by foliar biostimulant and s‑abscisic acid applications (P ≤ 0.05) and while the grapes of Control grapevines exhibited the lowest total phenolic compounds content (2401.99 mg GAE/kg fw), S‑ABA 400 ppm applied grapes tented to have the highest total phenolic compounds content (2897.57 mg GAE/kg fw).
Skin colors of table grapes are crucial characteristics demanded by the consumers and anthocyanin pigment plays important roles for red coloration of grape skin (Wei et al. 2011). As demonstrated in Fig. 6, foliar biostimulant and s‑abscisic acid applications significantly affect total anthocyanin content (P ≤ 0.05). In current study, S‑ABA 400 ppm application resulted in the highest total anthocyanin content (334.29 mg/GAE kg fw) compared to Control application (159.38 mg GAE/kg fw).
Conclusions
Foliar application of different substances is a remarkable tool for increasing quality parameters of table grapes. Findings of present study revealed that increasing doses of both foliar applications had potent impacts on quality characteristics of table grape and s‑abscisic acid applications were particularly found to be more effective on quality parameters to a greater extent than the biostimulant applications. In conclusion, S‑ABA 400 ppm application especially caused the highest improvements in quality characteristics of early maturing ‘Trakya Ilkeren’ table grape.
References
Anonymous (2009) 2nd edition of the OIV descriptor list for grape varieties and Vitis species. OIV technical standards and documents. http://www.oiv.int/public/medias/2274/code-2e-edition-finale.pdf. Accessed 4 Oct 2018
Cantin MC, Fidelibus MW, Crisosto CH (2007) Application of abscisic acid (ABA) at version advanced red color development and maintained postharvest quality of ‘Crimson Seedless’ grapes. Postharvest Biol Tec 46(3):237–241
Çelik S (2011) Viticulture (ampelology), 3rd edn. Avcı Press, Istanbul
Chehade LA, Chami ZA, Pascali SA, Cavoski I (2017) Biostimulants from food processing by-products: agronomic, quality and metabolic impacts on organic tomato (Solanum lycopersicum L.). J Sci Food Agric 98:1426–1436
Cosme F, Pinto T, Vilela A (2018) Phenolic compounds and antioxidant activity in grape juices: a chemical and sensory view. Beverages 4(1):4–22
Di Stefano R, Cravero MC (1991) Metodi per lo studio deipolifenolidell’uva. Riv Vitic Enol 2:37–45
Ferguson IB, Boyd LM (2002) Inorganic nutrients and fruit quality. In: Knee M (ed) Fruit quality and its biological basis. Sheffield Academic Press, Sheffield, pp 14–45
Giannakoula AE, IIias IF, Maksimovic JJD, Maksimovic VM, Zivanovi BD (2012) The effects of plant regulators on growth, yield and phenolic profile of lentil plants. J Food Compos Anal 28:46–53
Gougolias N, Masheva L (2010) Effect of gibberellic acid (GA3) on polyphenols content and antioxidative activity of some table grape varieties. Oxid Commun 33(3):652–660
Hoffmann M (1991) Elektrochemische Merkmale zur Differenzierung von Lebensmitteln. In: Meier-Ploeger A, Vogtmann H (eds) Lebensmittelqualität – Ganzheitliche Methoden und Konzepte. Alternative Konzepte, vol 66. CF Müller, Karlsruhe, pp 67–86
İşçi B, Gökbayrak Z (2014) Effect of brassinosteroids on yield and quality of table grape cultivar ‘Trakya Ilkeren’. J Agric Fac Uludag Univ 28(1):13–17
Kamiloğlu Ö (2013) Berry characteristics of some early table grape cultivars. Res J Agric Sci 6(2):65–70
Keller M (2015) The science of grapevines, 2nd edn. Academic Press, San Diego, CA, United States
Keppel H (2001) Erfahrungen und ausgewählte Ergebnisse elektrochemischer Untersuchungen unter Berücksichtigung des P‑Wertes. In: BTQ, Inst. F. Obst-und Gartenbau (ed) Tagungsband der 8. Internationalen Tagung elektrochemischer Qualitätstest Universität für Bodenkultur in Wien, 22–24.02.2001
Kishino AA, Roberto SR (2007) Implantação do Pomar. In: Kishino AY, de Carvalho SLC, Roberto SR (eds) Viticultura Tropical. O Sistema de produçã do Paraná. Instituto Agronômico do Paraná, Londrina, pp 141–169
Kok D (2017) Assessment of electrochemical attribute and monoterpene content of twelve aromatic grape cultivars (V. vinifera L.) grown under the ecological conditions of Northwestern Turkey. Oxid Commun 40(1–II):557–564
Kok D (2018a) Berry growth and biochemical characteristics of cv. Trakya Ilkeren (V. vinifera L.) as influenced by various doses of pre-harvest hydrogen peroxide and phenylalanine applications. Erwerbs-Obstbau 60(1):47–53
Kok D (2018b) Grape growth, anthocyanin and phenolic compounds content of early ripening cv. Cardinal table grape Ilkeren (V. vinifera L.) as affected by various doses of foliar biostimulant applications with gibberellic acid. Erwerbs-Obstbau 60(3):253–259
Kok D, Bal E (2017) Compositional differences in phenolic compounds and anthocyanin contents of some table and wine grape (V. vinifera L.) varieties from Turkey. Oxid Commun 40(2):648–656
Kok D, Bal E, Celik S (2013) Influences of various canopy management techniques on wine grape quality of V. vinifera L. cv. Kalecik Karası. Bulg J Agric Sci 19(6):1247–1252
Kristic M, Moulds G, Panagiotopoulos B, West S (2003) Growing quality grapes to winery specifications: quality measurement and management options for grape growers. Winetitles, Adelaide
Looney NE (1993) Improving fruit size, appearance and other aspects of fruit crop quality with plant bioregulating chemicals. Acta Hortic 329:120–127
Maoz I, Beno Moualem D, Kaplunov T, Lewinsohn E, Lichter A (2016) Uneven distribution of flavor components in table grapes. Aust J Grape Wine Res 22:343–349
Nelson KE (1985) Harvesting and handling California table grapes for market. Bulletin 1913. University of California Press, DANR Publications, Oakland
Parrado J, Bautista J, Romero EJ, Garcia Martinez AM, Friaza V, Tejada M (2008) Production of a carob enzymatic extract: potential use as a biofertilizer. Bioresour Technol 99:2312–2318
Peppi MC, Fidelibus MW (2008) Effects of forchlorfenuron and abscisic acid on the quality of ‘Flame Seedless’ grapes. HortScience 43(1):173–176
Peppi MC, Fidelibus MW, Dokoozlian NK (2006) Abscisic acid application timing and concentration affect firmness, pigmentation and color of ‘Flame Seedless’ grape. HortScience 41(6):1140–1445
Plessis BW (2008) Cellular factors that affect table grape berry firmness. Master of AgriSciences at Stellenbosch University, South Africa
Sandhu AK, Gray DJ, Lu J, Gu L (2011) Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chem 126:982–988
Shiraishi M, Fujishima H, Chijiwa H (2010) Evaluation of table grape genetic resources for sugar, organic acid and amino acid composition of berries. Euphytica 174(1):1–13
Singleton VL, Timberlake CF, Kea L (1978) The phenolic cinnamates of white grapes and wine. J Sci Food Agric 29(4):403–410
Tyagi S, Sahay S, Imran M, Rashmi K, Mahesh SS (2017) Pre-harvest factors influencing the postharvest quality of fruits: a review. Curr J Appl Sci Technol 23(4):1–12
Velimirov A (2004) Integrative methods of product quality assessment in connection with the p‑value-determination (3 examples: food preference test, sensory evaluation and self-decomposition test). Hortic Sci 31(1):17–21
Wei YZ, Hu FC, Hu GB, Li XJ, Huang XM, Wang HC (2011) Differential expression of anthocyanin biosynthetic genes in relation to anthocyanin accumulation in the pericarp of Litchi chinensis Sonn. Plos One 6(4):e19455
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. Kok declares that he has no competing interests.
Rights and permissions
About this article
Cite this article
Kok, D. Manipulating Anthocyanin Accumulation and Phenolic Compounds Profile of cv. Trakya Ilkeren Table Grape (V. vinifera L.) by Using Foliar Biostimulant and Abscisic Acid Applications. Erwerbs-Obstbau 63 (Suppl 1), 15–21 (2021). https://doi.org/10.1007/s10341-021-00575-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-021-00575-5