Abstract
Grape growers may occasionally face difficulties in grape skin coloration and low total soluble solids content in growing of early ripening table grape cultivars. In order to overcome these difficulties, it can be utilized from foliar biostimulants treatments. The purpose of this study, carried out 2015 growing season in Tekirdağ, Turkey, was to compare the effects of doses of two different biostimulants, enhancing table grape quality characteristics of cv. Tarsus Beyazı, cv. Trakya Ilkeren and cv. Yalova Incisi. For this aim, it was focused on three different doses for both biostimulants, including 0, 1750 and 3500 ppm (respectively, Control, BSt A-1 and BSt A-2) for first biostimulant and 0, 3500 and 4500 ppm (respectively, Control, BSt B-1 and BSt B-2) for second biostimulant. As a result, present research demonstrated that BSt B-2 and BSt A-2 treatments especially enhanced most of table grape quality characteristics in cv. Tarsus Beyazı, cv. Trakya Ilkeren and cv. Yalova Incisi.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant biostimulants are diverse substances and microorganisms improving plant growth (Calvo et al. 2014) and are biological molecules working by increasing specific metabolic and physiological expression in plants (Crouch and Vanstaden 1993).
Biostimulants can be of natural or synthetic origin and consist of different organic and inorganic components. Free amino acids, seaweed and fruit extracts, effective microorganism, humic substances and chitosan are classified as natural biostimulants (Calvo et al. 2014). However, synthetic biostimulants are composed of plant growth regulators, phenolic compounds, inorganic salts, essential elements and other substances having stimulating characteristics for plants (Przybysz et al. 2014).
Biostimulants may be soil or leaf-applied depending on their composition and desired results (Kunicki et al. 2010).
Biostimulants have various beneficial effects, including enhancement of root growth and increase of fruits and seeds harvested (Zurawicz et al. 2004), increases degree of fruit ripening (Fornes et al. 2002).
Plant biostimulants are also known to enhance fruit size, appearance and quality by having direct effects on fruit growth and development or indirectly by regulating crop load, plant vigor and canopy architecture (Looney 1993).
Turkey is leading country with the high potential of table grape production in the world and is the second largest producer of table grapes with 2,132,602 tons of table grape production after China (Anonymous 2014).
Mediterranean region of Turkey mainly attracts attention with the growing of early ripening table grape cultivars, while early ripening table grapes can be also grown in other regions of Turkey at certain rate. Early ripening grape cultivars commonly grown in the appropriate ecologies of Turkey can be given as cultivars of Ora, Prima, Trakya Ilkeren, Uslu, Tarsus Beyazı, Yalova Incisi, Flame Seedless, Superrior Seedless and Ergin Çekirdeksizi (Kamiloğlu 2014; Söylemezoğlu et al. 2015).
Grape growers can experience problems of low total soluble solids content and insufficient coloration of grape skin in early table grape growing (Kok et al. 2010).
In commercial grape growing, different methods such as canopy management techniques, sprinkler cooling, spraying of various biostimulants and plant growth regulators are used for enhancing quality characteristics of grapes (Winkler et al. 1974; Kok et al. 2010, 2013; Kok 2011, 2016a, 2016b; Kok and Bal 2016, 2017a, 2017b).
The purpose of present study was to evaluate effects of various doses of two different biostimulants on yield and table grape quality characteristics of early ripening cv. Trakya Ilkeren, cv. Yalova Incisi and cv. Tarsus Beyazı.
Materials and Methods
Plant Material and Study Area
This study was performed during the 2015 growing season in a commercial vineyard (lat. 40°59′40.37″N; long. 27°31′54.21″E; 149 m. a. s. l.) in Tekirdağ, Turkey by using 12-year-old cv. Tarsus Beyazı, cv. Trakya Ilkeren and cv. Yalova Incisi grapevines grafted on 5BB rootstock.
In available research, it was utilized from three different early ripening table grape cultivars, including cv. Tarsus Beyazı (white color), cv. Trakya Ilkeren (blue-black color) and Yalova Incisi (white color). All grapevines of these three cultivars were trained to guyot training system and were spaced 2.5 m × 1.5 m (intra and inter row). The vineyard was in charge of local standard viticulture practices for cultivar and region. It was also applied a standard disease control program for fungal diseases.
The climatic characteristics of current study area are mostly mild and means of annual temperature, sunshine duration per day, relative humidity, total precipitation were respectively 15.5 °C, 5.0 h, 80.9% and 850.8 mm for 2015 year. On the other hand, general attribute of vineyard soil in research area is clay-loam with pH of 7.51.
Biostimulant Treatments and Treatment Times
In current study, effects of different doses of two biostimulants (BSt A and BSt B) on yield and quality characteristics of early ripening table grape cultivars were evaluated. Ingredients of both biostimulant were presented in Table 1. Biostimulants were applied to grapevines at the stage of grapes pea-size (7 mm diameter) by repeating three times with 10-day interval. For this purpose, it was chosen three different doses of 0, 1750 and 3500 ppm (Control, BSt A-1 and BSt A-2) for first biostimulant and three different doses of 0, 3500 and 4500 ppm (Control, BSt B-1 and BSt B-2) for second biostimulant.
Measurement of Yield and Quality Parameters
In present study, grape length (cm), grape width (cm), grape weight (g), grape firmness (g mm−1) and cluster length (cm), cluster width (cm), cluster weight (g) were measured as yield parameters. Furthermore, total soluble solids content (%), titratable acidity (g L−1), juice pH, p-value (µW), total phenolic compounds content (mg GAE kg−1 fw), color parameters (L*, a* and b* values) were determined as quality parameters.
Harvest Time and Preparation of Grape Sampling
During the 2015 growing season, early ripening grapes on the grapevines of cv. Tarsus Beyazı, cv. Trakya Ilkeren and cv. cv. Yalova Incisi were continuously observed and were harvested when the control grapes reached total soluble solids content of 13% for white grape cultivars and 16% for colored grape cultivar.
After the grapes were harvested, samples of 250-grape were collected from each treatment and were finally used to determine total soluble solids content, total acidity, juice pH, p-value of juice. Besides, it was utilized from 300-grape samples to evaluate total phenolic compounds content. All grape samples were stored at −25 °C up to analysis of total phenolic compounds content. Prior to the analysis, grape samples were removed from −25 °C, allowed to thaw overnight at 4 °C and then homogenized in a commercial laboratory blender for 20 s.
Measurement of Grape Firmness
Grape firmness of table grapes was measured with an analog penetrometer (FT 02, Wagner Instruments, Riverside, USA) and measurement results were expressed as g mm−1.
Calculation of p-Value
P-value, including redox potential (mV), pH, p-value (μW) and resistivity (Ώ) were formulated with an equation declared by Hoffmann (1991). In this research, p-values in grape juice samples from different foliar spray treatments were calculated according to equation informed above.
Color Measurement
The evaluation of skin color was performed by using CIELab color system in which the L*, a*, b* values, detected by reflectance spectrophotometry, describe a three-dimensional color space, where L* is the vertical axis and defines the lightness, from completely opaque (0) to completely transparent (100); a* and b* are the horizontal axes and define, in order of the redness (or −a* of greenness), and the yellowness (or −b* of blueness) (Bakker et al. 1986).
In this study, forty grapes, four replicates of ten grapes, were chosen randomly from different sections of the clusters at harvest times of grape cultivars for skin color measurement. The color values, including L*, a*, b* were analyzed by Hunter Lab D‑9000 Colorimeter.
Analysis of Total Phenolic Compounds Content
Total phenolic compounds content was found out by using spectrophotometric methods explained by Singleton et al. (1978). Results of analysis were expressed as milligrams of gallic acid equivalent per kilogram of fresh weight (mg GAE kg−1 fw).
Statistical Analysis
All statistical analyses were performed by using TARIST statistical software. Means submitted to analysis of variance were separated by Fisher’s Least Significant Difference (LSD) multiple comparison test (p < 0.05) when the ANOVA test was significant.
Results and Discussion
Yield Parameters
Table 2 shows effects of two different biostimulant doses on yield components of three early ripening table grape cultivars. One of the major factors, determining table grape quality is grape size (Strydom 2014). In present study, both biostimulant treatments significantly affected grape lengths of cv. Tarsus Beyazı and cv. Trakya Ilkeren except for cv. Yalova Incisi (P < 0.05). While the highest grape length means were respectively determined for BSt A-1 treatments in cv. Tarsus Beyazı (24.68 cm) and cv. Trakya Ilkeren (19.30 mm) and for Control in Yalova Incisi (23.59 mm), the lowest means were 14.70 mm for BSt A-2 treatment in cv. Tarsus Beyazı, 17.32 mm for BSt B-2 treatment in cv. Trakya Ilkeren and 22.27 mm for BSt A-1 treatment in cv. Yalova Incisi (Table 2).
Data obtained from effects of biostimulant doses on grape width of cultivars shown in Table 2 displayed that biostimulant doses had significant effects on grape width of cultivars (P < 0.05). Grape width increased from 13.93 (BSt B-2) to 18.16 mm (Control) in cv. Tarsus Beyazı, from 17.89 (BSt B-2) to 19.93 mm (Control) in cv. Trakya Ilkeren; from 17.15 (BSt A-1) to 19.35 mm (BSt B-2) in cv. Yalova Incisi (Table 2).
Grape weights of cultivars were significantly influenced by biostimulant doses (P < 0.05). The results of grape weight in cultivars in Table 2 demonstrated that the highest means were 4.90 g for Control treatment in cv. Tarsus Beyazı, 5.40 g for Control treatment in cv. Trakya Ilkeren and 6.38 g for BSt B-2 treatment in cv. Yalova Incisi and the lowest means were recorded as 3.04 g for BSt B-2 treatment in cv. Tarsus Beyazı, as 4.04 g for BSt B-2 treatment in cv. Trakya Ilkeren and as 4.61 g for BSt A-1 in cv. Yalova Incisi (Table 2).
The firmness of table grapes is one of the major factors, determining their eating quality of grapes (Plessis 2008). Table 2 shown that the biostumulant doses had significant effects on grape firmness of cultivars (P < 0.05). While BSt B-2 treatment was causing the highest grape firmness in cv. Tarsus Beyazı (657.91 g mm−1) and cv. Trakya Ilkeren (868.75 g mm−1), the highest grape firmness mean was obtained for Control (860.41 g mm−1) in cv. Yalova Incisi. The lowest grape firmness means were obtained from Control treatments in cv. Tarsus Beyazı (381.87 g mm−1) and cv. Trakya Ilkeren (503.75 g mm−1) and from BSt B-1 treatment in cv. Yalova Incisi (529.99 g mm−1) (Table 2).
Hanoock (2008) informs that cluster size is crucial for acceptance of table grapes and cluster attributes of grapes are influenced by numerous factors. In available study, means of cluster length presented in Table 2 were only found to be significant in cv. Trakya Ilkeren and cv. Yalova Incisi (P < 0.05). Grapevines of cv. Tarsus Beyazı treated with biostimulants doses shown variations from 11.95 (Control) to 18.40 cm (BSt A-1). However, grapevines subjected to different biostimulant doses exhibited the highest cluster length means for BSt A-1 treatment (23.71 cm) in cv. Trakya Ilkeren and for BSt A-1 treatment (20.77 cm) in cv. Yalova Beyazı. The lowest cluster length means were recorded as 19.26 cm for BSt B-1 treatment in cv. Trakya Ilkeren and as 15.27 cm for BSt A-2 treatment in cv. Yalova Incisi (Table 2).
Results in Table 2 indicated that cluster width was significantly increased by biostimulant doses in cv. Tarsus Beyazı except for cv. Trakya Ilkeren and cv. Yalova Incisi (P < 0.05). BSt A-2 treatment resulted in the highest cluster width for BSt A-2 treatment (15.81 cm) and the lowest mean was 7.30 cm for Control treatment in cv. Tarsus Beyazı. While the highest cluster width means were 16.79 cm for BSt A-1 treatment in cv. Trakya Ilkeren and 11.07 cm for BSt B-1 treatment in cv. Yalova Beyazı; the lowest means were obtained from Control treatment (15.22 cm) in cv. Trakya Ilkeren and from BSt A-2 treatment (8.22 cm) in cv. Yalova Beyazı (Table 2).
Data pertaining to effects of biostimulant doses on cluster weight of grape cultivars shown significant increase in cv. Tarsus Beyazı (P < 0.05) whereas cluster weights were not influenced by treatments of biostimulant in cv. Trakya Ilkeren and cv. Yalova Incisi (Table 2). The highest increase in cluster weight was observed in BSt A-2 treatment of cv. Tarsus Beyazı (435.06 g), in BSt A-1 treatment of cv. Trakya Ilkeren (573.31 g) and in BSt B-2 treatment of cv. Yalova Incisi (308.75 g). The lowest means of cluster weight were obtained from BSt B-1 treatment (146.12 g) in cv. Tarsus Beyazı, from Control treatment (381.12 g) in cv. Trakya Ilkeren and from BSt A-2 treatment (169.45 g) in cv. Yalova Incisi (Table 2).
Quality Parameters
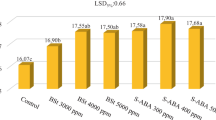
Effects of different biostimulant doses on quality parameters of three early ripening table grape cultivars are presented in Fig. 1. In grape growing, total soluble solids are accepted as an indicator of ripeness and most of commercial table grape cultivars are considered mature when total soluble solids content ranges from 13 to 18%. As displayed in Fig. 1, biostimulant doses had significant effects on total soluble solids contents of cv. Trakya Ilkeren and cv. Yalova Incisi except for cv. Tarsus Beyazı (P < 0.05). In present study, means of total soluble solids content ranged from 13.35 (Control) to 14.50% (Bst B-2) in cv. Tarsus Beyazı. On the other hand, while the highest total soluble solids content means were 17.70% for BSt B-2 treatment in cv. Trakya Ilkeren and 15.01% for BSt B-2 treatment in cv. Yalova Incisi; the lowest means were recorded as 16.07% for Control treatment in cv. Trakya Ilkeren and as 13.02% for Control treatment in cv. Yalova Incisi (Fig. 1).
The predominant nonvolatile organic acids in grapes are tartaric acid and malic acid, accounting for 90% of titratable acidity of grape juice and the acidity of grape juice has a direct effect on grape sensory quality and physical, biochemical and microbial stability (Boulton et al. 1998). Regarding effects of biostimulant doses on titratable acidity of cultivars represented in Fig. 2, significant differences were only observed in cv. Yalova Incisi (P < 0.05). Titratable acidity in grapes of cultivars from grapevines treated with Control treatment caused in the highest titratable acidity in cv. Tarsus Beyazı (6.29 g L−1), cv. Trakya Ilkeren (7.02 g L−1) and cv. Yalova Incisi (7.22 g L−1) and the lowest means were obtained from BSt B-2 treatment (5.15 g L−1) in cv. Tarsus Beyazı, from BSt B-2 treatment (6.18 g L−1) in cv. Trakya Ilkeren and from BSt B-2 treatment (5.52 g L−1) in cv. Yalova Incisi (Fig. 2).
The color and taste of grapes are influenced by juice pH and increases in pH values of grapes were observed towards harvest period of grape cultivars (Çelik 2011). Fig. 3 displays the results of pH juice in grapes of cultivars from biostimulant treatments and there were significant differences among the biostimulant doses in pH means of cv. Yalova Incisi (P < 0.05). In current study, pH means changed from 3.19 (Cotrol) to 3.49 (BSt B-2) in cv. Tarsus Beyazı, from 3.54 (Control) to 3.69 (BSt B-2) in cv. Trakya Ilkeren and from 3.28 (Control) to 3.47 (BSt B-2) in cv. Yalova Incisi (Fig. 3).
Grape quality is important for the consumer and one of the methods used for determining of numerous fruits and grape quality is calculation of p-value an electrochemical parameter of quality. This method exhibits that products with lower p-value have higher quality (Hoffmann 1991; Kara et al. 2008, 2012; Kok 2016b; Kok and Bal 2016, 2017a, 2017b). In point of p-values from biostimulant doses illustrated in Fig. 4, there were no significant differences among the cultivars except for cv. Yalova Incisi (P < 0.05). Although p-values varied according to biostimulant doses illustrated in Fig. 4, the highest p-value means were successively 137.21, 100.43 and 126.86 µW for Control treatments in all cultivars and the lowest p-values were obtained from BSt B-2 treatments in cv. Tarsus Beyazı (105.84 µW) and from BSt A‑1, BSt A‑2, BSt B‑1 and BSt B‑2 treatments in cv. Yalova Incisi (respectively, 115.50, 106.28, 115.81 and 107.03 µW) (Fig. 4).
Grapes, particularly the colored cultivars, comprise phenolic compounds in their skin and seeds (Poudel et al. 2008). Phenolic compounds contribute color and sensory attributes like bitterness and astringency of grape juice and wine (Isci et al. 2015). The statistical analysis indicated in Fig. 5 that total phenolic compounds contents of cultivars were influenced by biostimulant doses (P < 0.05). In existing study, BSt B-2 treatments lead to the highest total phenolic compounds contents in cv. Tarsus Beyazı (3469.70 mg GAE kg−1 fw), cv. Trakya Ilkeren (4611.94 mg GAE kg−1 fw) and cv. Yalova Incisi (2826.46 mg GAE kg−1 fw). However, the lowest total phenolic compounds contents were respectively obtained from Control treatment (3191.36 mg GAE kg−1 fw) in cv. Tarsus Beyazı; from Control (4190.14 mg GAE kg−1 fw) and BSt B‑1 (4234.42 mg GAE kg−1 fw) treatments in cv. Trakya Ilkeren; from Control (2453.50 mg GAE kg−1 fw), BSt A‑1 (2541.91 mg GAE kg−1 fw) and BSt B‑1 (2513.67 mg GAE kg−1 fw) treatments in cv. Yalova Incisi (Fig. 5).
Skin color has a key role in organoleptic qualities of grape, affecting the market value of table grapes (Liang et al. 2011). CIELab color system has been broadly used to evaluate skin color of grape and other fruit species (Carreno et al. 1995; Lancaster et al. 1997). The L*, a* and b* values from effects of different biostimulant doses on grape cultivars are presented in Figs. 6, 7 and 8. As displayed in Fig. 6, it was observed that there were significant differences among the biostimulant doses in terms of L* values (P < 0.05) and L* values ranged from 35.35 (BSt A-2) to 39.04 (BSt B-1) in cv. Tarsus Beyazı, from 20.60 (BSt A-2) to 23.48 (BSt B-2) in cv. Trakya Ilkeren and from 34.36 (BSt B-2) to 38.17 (Control) in cv. Yalova Incisi (Fig. 6).
Fig. 7 demonstrates that biostimulant doses have significant effects on a* values of cultivars except for cv. Yalova Incisi (P < 0.05). The a* values of cv. Trakya Ilkeren as red cultivar were the highest, changing from 0.52 (Control) to 1.77 (BST B-2) while a* values of cv. Tarsus Beyazı and cv. Yalova Incisi as green-yellow grape cultivars were respectively the lowest, changing from −3.04 (BSt A-2) to −1.53 (BSt B-1) in cv. Tarsus Beyazı and changing from −2.38 (Control) to −1.28 (BSt B-1) treatment in cv. Yalova Incisi (Fig. 7).
As presented in Fig. 8, b* values were not significantly affected by biostimulant doses in cv. Trakya Ilkeren and cv. Yalova Beyazı except for cv. Tarsus Beyazı (P < 0.05). The b* values of cv. Tarsus Beyazı and cv. Yalova Incisi as green-yellow cultivars were successively the highest, varying from 11.35 (BSt A-2) to 17.77 (BSt B-1) in cv. Tarsus Beyazı and varying from 12.15 (BSt A-2) to 14.85 (BSt B-1) in cv. Yalova Incisi while b* values of cv. Trakya Ilkeren were the lowest, varying from −1.04 (Control) to −0.37 (BSt A-2) (Fig. 8).
Conclusions
In growing of early ripening table grape, problems derived from uneven skin coloration and low total soluble solids content can be encountered by grape growers. Results of present study revealed that foliage sprayed both biostimulants could mostly improve quality components of cv. Tarsus Beyazı, cv. Trakya Ilkeren and cv. Yalova Incisi although dependent on the treatment dose. In current study, table grape quality parameters of these early ripening grape cultivars were especially favorably affected by both biostimulant treatments in the following order of biostimulant doses BSt B-2 > BSt A-2 > BSt A-1 > BSt B-1 > Control.
References
Anonymous (2014) Food and agriculture organization of the United Nations. http://www.fao.org/faostat/en/#data/QC. Accessed 20 Feb 2017
Bakker J, Bridle P, Timberlake F (1986) Tristimulus measurement (CIELAB 76) of port wine colour. Vitis 25:67–78
Boulton RB, Singleton VL, Bisson LF, Kunkee RE (1998) Principles and practices of winemaking. Springer, Aspen; New York
Calvo P, Nelson L, Klopper JW (2014) Agricultural uses of plant biostimulants. Plant Soil 383:3–41
Carreno J, Martinez A, Almela L, Fernandez-Lopez JA (1995) Proposal of an index for the objective evaluation of the color of red table grapes. Food Res Int 28:373–377
Çelik S (2011) Viticulture (Ampelology), 3rd edn. vol I. Avcı Ofset, Istanbul
Crouch I, Vanstaden J (1993) Evidence for the presence of plant growth regulators in commercial seaweed products. Plant Growth Regul 13:21–29
Fornes F, Almela V, Abad M, Agusti M (2002) Low concentrations of chitosan coating reduce water spot incidence and delay peel pigmentation of Clementine mandarine fruit. J Sci Food Agric 85(7):1105–1112
Hanoock JF (2008) Temperate fruit crop breeding: Germplasm to genomics. University of Michigan, Michigan
Hoffmann M (1991) Elektrochemische Merkmale zur Differenzierung von Lebensmitteln. In: Meier-Ploeger A, Vogtmann H (eds) Lebensmittelqualitat – Ganzheitliche Methoden und Konzepte – Alternative Konzepte – vol 66. Deukalion. CF Müller, Karlsruhe, pp 67–86
Isci B, Gokbayrak Z, Keskin N (2015) Effects of cultural practices on total phenolics and vitamin c content of organic table grapes. S Afr J Enol Vitic 36(2):191–194
Kamiloğlu Ö (2014) Berry quality characteristics of some early table cultivars. Res J Agric Sci 7(1):58–63
Kara Z, Akay A, Ateş F, Yağmur B (2008) The P‑value as A tool for quality measurements in grape industry. 31. World Congress of Vine and Wine, 6. General Assembly of the O.I. V., Growing Techniques. Sustainability and Specificity of Production, Verona, 15–20 June 2008
Kara Z, Ateş F, Sabir A (2012) Some quality parameters investigated in Sultani Çekirdeksiz (Vitis vinifera L.) clones during ripening period. 47th Croatian and 7th International symposium on Agriculture, Opatija-Croatia, 13–17 February 2012 (Hrvatska)
Kok D (2011) Influences of pre- and post-verasion cluster thinning treatments on grape composition variables and monoterpene levels of Vitis vinifera L. Cv. Sauvignon Blanc. J Food Agric Environ 9(1):22–26
Kok D (2016a) Effects of foliar seaweed and humic acid treatments on monoterpene profile and biochemical properties of cv. Riesling berry (V. vinifera L.) throughout the maturation period. J Tekirdag Agric Fac 13(2):67–74
Kok D (2016b) Variation in total phenolic compounds, anthocyanin and monoterpene content of ‘Muscat Hamburg’ table grape variety (V. vinifera L.) as affected by cluster thinning and early and late period basal leaf removal treatments. Erwerb Obstbau 58(4):241–246
Kok D, Bal E (2016) Seedless berry growth and bioactive compounds of cv. ‘Recel Uzümü’ (V. vinifera L.) as affected by application doses and times of pre-harvest Thidiazuron. Erwerb Obstbau 58(4):253–258
Kok D, Bal E (2017a) Electrochemical properties and biochemical composition of cv. Shiraz wine grape (V. vinifera L.) depending on various dose and application time of foliar microbial fertilizer treatment. Erwerb Obstbau. https://doi.org/10.1007/s10341-017-0319-9
Kok D, Bal E (2017b) Chemical and non-chemical thinning treatments influence berry growth and composition of cv. Shiraz wine grape (V. vinifera L.). Erwerb Obstbau. https://doi.org/10.1007/s10341-017-0321-2
Kok D, Bal E, Celik S, Ozer C, Karauz A (2010) The influences of different doses on table quality characteristics of cv. Trakya Ilkeren (Vitis vinifera L.). Bulg J Agric Sci 16(4):429–435
Kok D, Bal E, Celik S (2013) Influences of various canopy management techniques on wine grape quality of V. vinifera L. cv. Kalecik Karası. Bulg J Agric Sci 19(6):1247–1252
Kunicki E, Grabowska A, Sekara A, Wojciechowska R (2010) The effect of cultivar type, time of cultivation and biostimulant treatment on the yield of spinach (Spinacia oleracea L.). Folia Hortic 22:9–13
Lancaster JE, Lister CE, Reay PF, Triggs CM (1997) Influence of pigment composition on skin color in a wide range of fruit and vegetables. J Am Soc Hortic Sci 122:594–598
Liang Z, Sang M, Fan P, Wu B, Wang L, Yang S, Li S (2011) CIELAB coordinates in response to berry skin anthocyanins and their composition in Vitis. J Food Sci 76(3):490–497
Looney NE (1993) Improving fruit size, appearance and other aspects of fruit crop quality with plant bioregulating chemicals. Acta Hortic 329:120–127
Plessis BW (2008) Cellular Factors That Affect Table Grape Berry Firmness. Master‘s thesis, Stellenbosch University, South Africa
Poudel PR, Tamura H, Kataoka I, Mochioka R (2008) Phenolic compounds and antioxidant activities of skin and seeds of five wild grapes and two hybrids native to Japan. J Food Compost Anal 21:622–625
Przybysz A, Gawronska H, Gajc-Wolska J (2014) Biological mode of action of a nitrophenolates-based biostimulant: case study. Front Plant Sci 5:1–15
Singleton VL, Timberlake CF, Kea L (1978) The phenolic cinnamates of white grapes and wine. J Sci Food Agric 29:403–410
Söylemezoğlu G, Kunter B, Akkurt M, Sağlam M, Ünal A, Buzrul S, Tahmaz H (2015) Methods for improving of viticulture and production targets. VIII. Technical Congress of Turkey Agricultural Engineering, Ankara, 12–16. January 2015, pp 606–629
Strydom J (2014) The effect of foliar potassium and seaweed products in combination with a leonardite fertigation product on Flame Seedless grape quality. S Afr J Enol Vitic 35(2):283–291
Winkler A, Cook J, Kliewer W, Lider L (1974) General viticulture. University of California Press, Berkeley
Zurawicz E, Mazny A, Basak A (2004) Productivity stimulation in strawberry by application of plant bioregulators. Plant Growth Regul 653:155–160
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. Kok and E. BAL declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kok, D., BAL, E. Changes in Yield and Quality Characteristics of Some Early Ripening Table Grape Cultivars (V. vinifera L.) in Response to Different Doses of Distinct Biostimulant Treatments. Erwerbs-Obstbau 60 (Suppl 1), 11–19 (2018). https://doi.org/10.1007/s10341-018-0374-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-018-0374-x