Abstract
Acca sellowiana (Berg.) Burr. is a native Myrtaceae from southern Brazil and Uruguay, now the subject of a domestication and breeding program. Biotechnological tools have been used to assist in this program. The establishment of a reliable protocol of somatic embryogenesis has been pursued, with a view to capturing and fixing genetic gains. The rationale behind this work relies on the fact that deepening comprehension of the general metabolism of zygotic embryogenesis may certainly improve the protocol for somatic embryogenesis. Thus, in the present work we studied the accumulation of protein, total sugars, starch, amino acids, polyamines (PAs), IAA and ABA, in different stages of A. sellowiana zygotic embryogenesis. Starch is the predominant storage compound during zygotic embryo development. Increased synthesis of amino acids in the cotyledonary stage, mainly of asparagine, was observed throughout development. Total free PAs showed increased synthesis, whereas total conjugated PAs were mainly observed in the early developmental stages. IAA decreased and ABA increased with the progression from early to late embryogenesis. Besides providing basic information on the morphophysiological and biochemical changes of zygotic embryogenesis, the results here obtained may provide adequate strategies towards the modulation of somatic embryogenesis in this species as well as in other woody angiosperms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acca sellowiana (Berg.) Burr. is a native fruit species from southern Brazil and northern Uruguay. In New Zealand, Australia, the USA and even certain countries in Europe, it has been commercially cultivated since the beginning of the twentieth century (Thorp and Bieleski 2002). Recently, efforts have been made in Brazil to launch a domestication program with this species.
Conventional techniques for the vegetative propagation of A. sellowiana based on cuttings and grafting, are difficult due to the negative effects of phenolic compounds (Dal Vesco and Guerra 2001). Micropropagation techniques have been employed to overcome such problems, with studies being focused on the establishment of reliable protocols for somatic embryogenesis (Canhoto and Cruz 1996; Dal Vesco and Guerra 2001; Stefanello et al. 2005; Cangahuala-Inocente et al. 2007). One of the main limitations of these protocols is associated with the low rate of somatic embryo conversion to plantlets. These constraints are mainly a result of the lack of knowledge on the physiological and biochemical changes that occur during development and maturation of the zygotic embryo.
Zygotic embryogenesis is a complex and highly organized process that plays a central role in the life cycle of higher plants. It can usually be divided into two main steps, namely, (1) an initial morphogenetic phase which is characterized by cell division and the onset of cell differentiation, followed by (2) a maturation phase associated with the accumulation of major storage products and preparation for seed desiccation, dormancy and germination (Mordhorst et al. 1997; Sallandrouze et al. 2002).
Both the synthesis and accumulation of storage compounds play a central role in zygotic embryogenesis (Merkle et al. 1995). Storage proteins are the source of amino acids for seed germination (Misra et al. 1993). A special class of storage compounds, the LEA proteins, play an important role in seed dehydration (Wise and Tunnacliffe 2004). Apart from proteins, carbon is stored in lipids and starch. The levels of protein, lipids, and starch vary between angiosperms species (Dam et al. 2009). Soluble sugars, such as glucose and sucrose, are involved with the regulation of developmental processes occurring from embryo development to seed maturation (Gibson 2005). Amino acids are important in nitrogen metabolism and protein synthesis, as well as in the transition from heterotrophy to autotrophy (Ortiz-Lopez et al. 2000).
The polyamines, aliphatic amines with a positive charge in neutral pH, play a basic role in cell proliferation and differentiation (Bouchereau et al. 1999; Puga-Hermida et al. 2003; Baron and Stasolla 2008), and in protein synthesis and responses to water stress in plants (Bais and Havishankar 2002; Kusano et al. 2007). Taken together, these substances are reliable biochemical markers of zygotic and somatic embryo quality.
From a physiological point of view, auxins are involved in cell division and expansion, and differentiation of the vascular system (Liu et al. 1993). These hormones are also associated with regulation of the embryonic patterns of histodifferentation (Fischer-Iglesias and Nauhaus 2001; Bassuner et al. 2007). ABA is another hormone which plays a major role in the embryo, by preventing precocious germination (Kermode 1995) and promoting the accumulation of storage compounds (Cailloux et al. 1996), and thus, embryo maturation (Black 1991). In somatic embryogenesis ABA reduces the frequency of embryo malformation (Etienne et al. 1993) and the occurrence of secondary or repetitive embryogenesis (Nuutila et al. 1991).
The present work aimed to study the dynamics of biochemical and physiological changes during the development of A. sellowiana zygotic embryos, such as the levels of total proteins, amino acids, carbohydrates, polyamines, IAA and ABA. The accumulation patterns of certain of these substances in specific stages of seed development were also assayed.
Materials and methods
Plant material
Biological material was compiled together with the A. sellowiana germplasm collection at the Epagri São Joaquim Experimental Station (28°18′ Latitude S, 49°56′ Longitude W), Santa Catarina, in the south of Brazil, from November 2004 to March 2005. About 3,800 flowers were emasculated followed by manual pollination. Ovules from non-pollinated flowers were considered as the zero-time limit. Fruits were collected after 21 and 30 days, then every 15 days until reaching fruit maturation which occurred after 120 days (Fig. 1). The fruits were transported in plastic boxes with dry ice. Using a stereoscope (Olympus SZH10) the seeds were extracted from the fruits, and stored at −20°C.
Development of the seed of A. sellowiana after pollination directed. 0 DAP: floral button at the stage balloon, 21 DAP: formation of the zygotic embryo with successive cell divisions causing a pro-embryo, 30 DAP: zygotic embryo at the globular stage, 45 DAP: zygotic embryo at the heart stage, 60 DAP: zygotic embryo at the torpedo stage with endosperm still liquid, 75 DAP: zygotic embryo at the cotyledonary stage with presence of endosperm, 90, 105 and 120 DAP: zygotic embryo at the cotyledonary stage without presence of endosperm. Bar = 1 mm
Histological analysis
Samples were fixed for 24 h in a 0.2 M phosphate buffer (pH 7.3) containing 2.5% paraformaldehyde. After fixation, these were dehydrated in a graded ethanol series and embedded in historesin (Leica), as described by Arnold et al. (1975). 5 μm thick sections were sliced with a rotary microtome (Slee Technik Cut 4055) and fixed onto slides by heating. The sections were stained with 0.5% toluidine blue O (TBO) (C.I. 52040) in a 0.2 M phosphate buffer (pH 6.8) for 1 min (O’Brien et al. 1965). Photographs were taken with a standard Olympus BX 40 microscope and Olympus SZH10 stereoscope.
Soluble proteins
For each collecting date 300 mg of seeds were extracted. The seeds were stored in eppendorff tubes and stored at −20°C until the processing. Total soluble protein extraction was performed according to Cangahuala-Inocente et al. (2009). Three repetition of samples (300 mg fresh weight-FW each) were macerated at 4°C with 1 ml of extraction buffer (pH 7.0) containing 50 mM sodium phosphate dibasic, 0.2 M β-mercaptoethanol, 17.3 mM sodium dodecyl sulfate (SDS) and 1 mM phenylmethylsulfonyl fluoride (PMSF), to then be centrifuged to 4°C by 20 min at 8,000 rpm. The supernatant containing total soluble proteins was removed and the pellet stored at −20°C. Soluble proteins were sedimented at 0°C by adding two volumes of 100% ethanol into the supernatant and then centrifuging 4°C by 20 min at 10,000 rpm. The sedimented proteins were solubilized in 50 mM sodium phosphate dibasic (pH 7.0). Protein content was determined by the Bradford (1976) method, using bovine serum albumin as standard.
Total sugar and starch
The extraction of total soluble sugars was performed according to Shannon (1968). The pellet from the protein extraction was macerated using 2 ml methanol–chloroform–water (MCW) (12:5:3), centrifuged for 10 min at 2,000 rpm. The supernatant was recovered and the pellet was re-extracted using 2 ml MCW. One part chloroform and 1.5 part water were added for each four parts of the supernatant, followed by centrifuging for 10 min at 2,000 rpm, from which two phases were obtained. The upper aqueous phase was removed for dosage using anthrone at 0.2%, in accordance with Umbreit et al. (1964).
The extraction and determination of starch levels were based on the procedures of McCready et al. (1950). The pellets used in the total soluble sugar extraction were ground with 1 ml of 30% perchloric acid and centrifuged for 15 min at 10,000 rpm. The supernatant containing starch was removed and the pellets were re-extracted twofold. The supernatants were combined and the pellets eliminated. For dosage was using anthrone at 0.2%. The sugar and starch concentrations were calculated using glucose as standard. The absorbance was read in UV–VIS UV-1203 spectrophotometer (Shimadzu) at 620 nm.
Amino acid
Amino acid determination was carried out according to Santa-Catarina et al. (2006). Three biological samples (200 mg FW) of each developmental stage were ground, individually, in 6 ml of 80% (v/v) ethanol and evaporated in a ‘speed vac’ centrifuge concentrator (ThermoSavant, Milford, USA). Samples were re-suspended in 2 ml of Milli’Q type water and centrifuged at 20,000g for 10 min. The supernatant was filtered through a 20 μm membrane. Amino acids were derivatizated with o-phthaldialdehyde (OPA) and identified by HPLC using a C-18 reverse phase column (SUPELCOSIL™, 5 μm particle size, L × I.D. 25 cm × 4.6 mm). The gradient was developed by mixing increasing proportions of 65% methanol with a buffer solution (50 mM sodium acetate, 50 mM sodium phosphate, 20 ml l−1 methanol, 20 ml l−1 tetrahydrofuran and pH 8.1 adjusted with acetic acid). The gradient of 65% methanol was programmed to 20% over the first 32 min, from 20 to 100% between 32 and 71 min, and 100% between 71 and 80 min, at a 1 ml min−1 flow and 40°C. Fluorescence excitation and emission wavelengths of 250 and 480 nm, respectively, were used for amino acid detection. Peak areas and retention times were measured by comparison with known quantities of standard amino acids.
Polyamines
Putrescine (Put), Spermidine (Spd) and Spermine (Spm) were defined according to Silveira et al. (2004a). Three biological samples (300 mg FW) of each developmental stage were ground, individually, in 3 ml of 5% (v/v) perchloric acid. After 1 h, extracted samples were centrifuged for 20 min at 15,000g and 0°C. In each case, the supernatant containing free polyamines was removed and the pellets re-extracted. Supernatants were then combined and the pellets eliminated. Free polyamines were determined directly from the supernatant. Conjugated polyamines were extracted by hydrolyzing 200 μl of supernatant with 200 μl of 12 N HCl for 18 h at 110°C. The samples were dried under nitrogen. The conjugated polyamines were solubilized in 200 μl of 5% Perchloric acid.
Free and conjugated polyamines were derivatizated by dansyl chloride made up in acetone at a concentration of 5 mg ml−1. A 40 μl aliquot of the sample was added to 100 μl of dansyl chloride, 20 μl of 0.05 mM diaminoheptane (internal standard) and 50 μl of saturated sodium carbonate. In sequence, the samples were incubated in the dark for 50 min at 70°C. The toluene phase was collected and dried under nitrogen. Finally, dansylated polyamines were solubilized in 200 μl of acetonitrile.
Twenty μl of the dansylated polyamines were separated by reverse phase HPLC in a C-18 reverse phase column (Shimadzu SHIM-PACK CLC-ODS, 5 μm particle size, L × I.D. 25 cm × 4.6 mm). The gradient was developed by mixing increasing proportions of absolute acetonitrile to 10% acetonitrile in water (pH 3.5). The gradient of absolute acetonitrile was programmed at 65% over the first 10 min, from 65 to 100% between 10 and 13 min and at 100% between 13 and 21 min. The flow was 1 ml min at 40°C. The fluorescence detector was set at 340 nm (excitation) and 510 nm (emission). A mixture of putrescine (Put), spermidine (Spd) and spermine (Spm) was used as standard.
IAA and ABA determination
IAA and ABA contents were determined according to Santa-Catarina et al. (2006). Three biological samples (1,000 mg FW) of each developmental stage were ground, individually, in a 5 ml extraction buffer (80% ethanol + 1% polyvinylpyrrolidone-40). [3H]IAA and [3H]ABA were added to the samples as internal radioactive standards. After 90 min of incubation, samples were centrifuged during 15 min at 15,500g, and 4°C. Supernatants were concentrated in a ‘speed vac’ centrifuge concentrator (ThermoSavant, Milford, USA) at 45°C, until reaching 20% of the initial volume (1 ml). Volumes were then raised (Milli’Q water type) to 3 ml and the pH adjusted to 2.5 using HCl (1 N). The samples were partitioned twice by using ethyl ether as an organic solvent. Organic layers were combined and the aqueous residue eliminated by freezing the base of the tubes during 5 s in liquid nitrogen, with transference of the organic phase to a clean tube. The organic phase containing IAA and ABA was dried in a ‘speed vac’ at 45°C, dissolved in 200 μl of 100% methanol, transferred to micro tubes and after stored at −80°C until analysis. Aliquots (40 μl) of stored samples were analyzed by reverse phase HPLC, by using a C-18 reverse phase column (Shimadzu SHIM-PACK CLC-ODS, 5 μm particle size, L × I.D. 25 cm × 4.6 mm). The gradient was developed by mixing increasing proportions of absolute methanol to 10% methanol plus 0.5% acetic acid in water. The IAA content was defined by using a fluorescence detector at 280 nm (excitation) and 350 nm (emission) and the ABA content by means of a UV–VIS detector at 254 nm. A mixture of IAA and ABA was used as a standard. Fractions containing IAA and ABA were collected and analyzed in the Packard Tri-Carb liquid scintillation counter to estimate losses and data validation.
Data analysis
Data were analyzed by ANOVA (P < 0.05) followed by the Student-Newman-Keuls (SNK) test, using Statistica version 7.0 software. In the event of ANOVA and SNK tests not being used, the mean of three replicates and standard error were then applied to analyze data.
Results and discussion
Formation of zygotic embryos
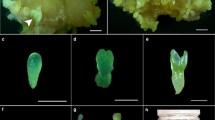
A. sellowiana ovules are anatropous, developing asynchronously (Fig. 2a) with axillary placenta. The nucellus presents layers of cells with prominent nuclei (Fig. 2b). Members of the order Myrtales, which includes the Myrtaceae, have a crassinucellate ovule, the micropyle being formed by external and internal teguments, with two layers of cells, ephemeral or absent antipodal cells and a nuclear-type endosperm (Tobe and Raven 1983).
Histological sections of the process of zygotic embryogenesis of A. sellowiana. a Ovule of the immature fruit 0 DAP, stained with AT-O, b the opening of the micropyle detail and the presence of the teguments protection, c formation of the pro-embryo in the immature fruit, d cells in the suspensor and pro-embryo, e immature fruit at 30 DAP, observing the zygotic embryo in globular stage, f details of the zygotic embryo in globular stage and the presence of the outer tegument of protection. Bar = 10 μM. Mi micropyle, teg tegument, ca chalaza, nu nucellus, fu funicular, ex-teg outer tegument, in-teg internal tegument, en endosperm, ze zygotic embryo, sus suspensor, pro-en pro-embryo, glo globular stage
Twenty one days after pollination (DAP), the first cellular division, leading to the formation of the pro-embryo (Fig. 2c) with embryonal and suspensor cells (Fig. 2d), was noted. According to Dodeman et al. (1997), several structural alterations occur in the pro-embryo, mostly associated with vacuole organization, nucleus location, the presence of organelles and cell wall changes. After 30 DAP the apical cells of the pro-embryo originated a spherical mass of cells, corresponding to the globular stage (Fig. 2e, f). The endosperm of the nuclear position was constituted of a cell layer with conspicuous nuclei (Fig. 2e). In Arabidopsis thaliana the embryo in the globular stage stems from a sequence of divisions of embryonal cells, the embryo soon acquiring a heart shape (Mansfield and Briarty 1991).
Forty five DAP, histological sections at heart stage embryos showed a well-defined proto-dermal layer (Fig. 3a, b). Sixty days after pollination, the embryo was in the torpedo-stage with an extended apical–basal axis (data not shown). According to Goldberg et al. (1994), dramatic changes occur in the transition from globular to torpedo-stage embryos. Among other features, cotyledons arise from two lateral domains, this resulting in bilateral symmetry.
Histological sections of the process of zygotic embryogenesis of A. sellowiana. a Immature fruit at 45 DAP looking up the zygotic embryo in stage cordiforme, b zygotic embryo in detail the heart stage and the presence of protoderm, c longitudinal section of the zygotic embryo at the initial cotyledonary stage to 75 DAP, d longitudinal section the zygotic embryo at the cotyledonary stage at 90 DAP, e details of the root meristem, f detail of the apical meristem. Note that the cells of the leaves cotyledonary are somewhat thickened. Bar = 10 μM. ex-teg outer tegument, he heart stage, protoder protoderm, cot cotyledon, procam procambium, ap-me apical meristem, ra-me radicle meristem, cap root cap
The early cotyledonary stage was observed at 75 DAP (Fig. 3c) showing rudimentary cotyledonary leaves surrounded by the endosperm. At 90 DAP, the endosperm present in seed had almost been consumed (Fig. 3d). In addition, at this stage cells in the apical (Fig. 3e) and basal (Fig. 3f) meristems were observed at the end of the hypocotyls—radicle axis. Starting from 105 to 120 days, fruit development was concluded with maturation. Concomitantly, embryos showed a thickened cotyledons with storage compounds (see Fig. 1).
Protein and carbohydrates
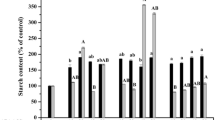
Total protein levels during embryo development ranged from 0.24 to 0.39 mg g FW−1 (Fig. 4). Storage proteins provide free amino acids to be used for early embryonal stages until reaching plantlet autotrophy (Prewein et al. 2006). During zygotic embryogenesis of Ocotea catharinensis, proteins were only detected at high concentrations in the late developmental stages, peaking at the mature embryo stage (Santa-Catarina et al. 2006). In Araucaria angustifolia zygotic embryogenesis, the embryonic axis protein content increased until the cotyledonary stage, with further stabilization in the mature seed (Silveira et al. 2008).
Total soluble sugars ranged from 1.45 to 2.23 mg g FW−1 during zygotic embryo development (Fig. 4). Starch content remained quite stable during embryo development, the highest value (24.5 mg g FW−1) being observed at 21 DAP (Fig. 4). This phase is coincident with fertilization and zygote formation. Similar results were reported by Pescador et al. (2008) with this same species. Starch and sugar contents play a major role in seed development through the supply of compounds to be consumed during transition from seed heterotrophy to plantlet autotrophy (Merkle et al. 1995). In Quercus robur, starch accumulated gradually in the late embryonal developmental stages (Prewein et al. 2006). Studies using an Arabidopsis mutant showed the role of sugars in the regulation of genetic expression, proliferation and cell death, in seedling-growth, leaf expansion and senescence, and in seed development (Smeekens 2000; Gibson 2005; Rolland et al. 2006).
Histochemical analysis of mature A. sellowiana zygotic embryos (120 DAP) revealed a low starch-content and a high content of protein and lipidic bodies in cotyledon cells (data not shown). It is commonly accepted that the main storage compounds during seed development are proteins, carbohydrates and lipids, and that the ratio of this composition is different in each species (Bewley and Black 1994). Thus, Caesalpinia peltophoroides seed cotyledons presented about 50% of lipids, 32% of soluble carbohydrates, 7.7% of starch and 6.8% of soluble proteins (Corte et al. 2006). On the other hand, the predominant reserve was starch in seed cotyledons of Anthyrium andraeaman, whereas endosperm reserves were mainly constituted of starch and proteins (Matsumoto et al. 1998).
Amino acids
Amino acids are important primary assimilation products in nitrogen metabolism (Ortiz-Lopez et al. 2000). In addition to the synthesis of proteins, amino acids directly or indirectly control various aspects of plant growth and development (Coruzzi and Last 2000).
In the present work, amino acid content was enhanced after 45 days from the pollination period, when the embryos had already reached the heart stage (Table 1). At this stage, amino acids may be used for the synthesis of specific proteins associated with histodifferentiation, since this is the moment when the embryo begins to establish its radial symmetry, vascularization and formation of the cotyledons.
The total amino acid content peaked at 75 DAP (Table 1), a period in which the zygotic embryo was in the cotyledonary stage. These results confirm those obtained during embryonic development of A. angustifolia (Astarita et al. 2003a), Pinus taeda (Silveira et al. 2004b) and O. catharinensis (Santa-Catarina et al. 2006).
A decrease in amino acid levels was observed from 90 to 105 DAP (Table 1). This could be ascribed to their consumption for the synthesis of various, mainly reserve, proteins. According to Weber et al. (1997) the transport of amino acid to the cotyledons can be initially passive, although an additional system of active absorption can be established in late developmental stages, when large amounts of proteins are stored to ensure seedling development. A decrease in total amino acid levels in the mature embryo was also observed in O. catharinensis (Santa-Catarina et al. 2006).
Asparagine was the prevalent amino acid, with a peak (Table 1) in the late embryonal stages (75–90 DAP), when the cotyledons were fully developed, fully suggesting its involvement in the mobilization of embryo reserves. This result confirms that reported by Feirer (1995) and Santa-Catarina et al. (2006) during seed development of P. strobus and. O. catharinensis, respectively. Asparagine plays a central role in nitrogen storage and transport in plants, which is further facilitated by its biochemical properties (Lea et al. 2007).
Glutamine, aspartic acid, γ-aminobutyric acid (Gaba) and alanine are accumulated at lower levels than asparagine (Table 1). Glutamines and glutamic acid are precursors of other amino acids (Radwanski and Last 1995). In addition, exogenous glutamine is commonly supplemented to the basal culture medium in several protocols of somatic embryogenesis, including A. sellowiana (Dal Vesco and Guerra 2001). In the present work alanine showed high levels at 45, 75 e 90 DAP, and the IAA levels showed an inverse proportion comparatively to alanine. This suggests that synthesis and degradation of this amino acid could be associated with the IAA synthesis when this auxin is higly required. The l-alanine and ε-lysine conjugates were also found to be useful for induction and development of Oenothera leaf callus and in tomato cell-suspension culture, two systems which require highly active sources of auxin (Magnus et al. 1992).
Unlike the rest, Gaba is a non-proteic amino acid which results from glutamic acid decarboxylation (Satya-Naraian and Nair 1990), normally being accumulated in response to 2,4-D, or under stress conditions (Snedden and Fromm 1998). In the present work, Gaba was the fourth largest amino acid accumulated, mostly from the 75 to 90 DAP, decreasing thereafter (Table 1). The presence of these amino acids at such levels suggests its role in A. sellowiana embryogenesis. This amino acid was also accumulated in the early somatic embryogenesis of carrots as shown by Kamada and Harada (1984).
Polyamines (PAs)
The total PAs level was low at zero-time, increasing significantly up to 21 DAP. In subsequent stages (30, 45 and 60 DAP), increased levels of conjugated PAs were observed, these progressively decreasing until embryos reached maturity (Fig. 5a). Conversely, total free PA levels increased according to progression to further developmental stages, peaking by 105 DAP (Fig. 5a). This variation was different from that observed in O. catharinensis where the levels of free PAs were higher than those of conjugated PAs, both types of PAs reaching high levels in mature zygotic embryos (Santa-Catarina et al. 2006). The metabolic role of conjugated PAs has not yet been fully elucidated. Bais and Havishankar (2002) suggested that free PA levels in plants can be regulated by the formation of reversible conjugated PAs. It has also been suggested that a combination of PAs with cinnamic acid and phenols may regulate the pool of free PAs in plant cells (Mader and Hanke 1997).
The mean concentration a total polyamines (μg/g) of fresh weight (FW), b ratio of PAs: Put (Spd + Spm)−1 and c free PAs (μg/g) of fresh weight (FW) during the development and maturation of the zygotic embryo of A. sellowiana (n = 3). Values followed by different letters, inside of parameters indicating significant differences according to the SNK test (P < 0.05)
The PA ratio [Put.(Spd + Spm)−1] was low both at zero-time and 21 DAP (Fig. 5b), corresponding, respectively, to fertilization and formation of the zygotic embryo. Geoffriau et al. (2006) showed the involvement of PAs in the gynogenesis of Allium cepa, where the Put/Spd-Spm ratio was low during flowering. In the present work, the proportion of PAs [Put.(Spd + Spm)−1] was high during embryo development (30, 45 and 60 DAP), corresponding, respectively, to the globular, heart and torpedo stages, the highest being detected at the heart stage then decreasing until the cotyledonary stage (Fig. 5b). These results are in agreement with those reported in A. angustifolia (Astarita et al. 2003b), P. taeda (Silveira et al. 2004b), and O. catharinensis (Santa-Catarina et al. 2006), Thus, the PA ratio could be considered as a reliable marker of embryonal development in A. sellowiana.
In the present work, at zero-time and at the time of fertilization and formation of the zygotic embryo (DAP 21), free Spd was predominant as compared to other PAs (Fig. 5c). It has been shown that high Spd levels are associated with induction and floral development (Aribaud and Martin-Tanguy 1994). Spd was the most abundant free PA in Brassica rapa, followed by Put and Spm (Puga-Hermida et al. 2003). These authors also pointed out that free PAs content did not change when the flower was still closed, changing the profile only after synthesis of carotenoids in petals.
In the present work, similar patterns of variation between free PAs, mainly for Put at all stages, were noted in subsequent developmental stages (Fig. 5c). This could be attributed to Put conversion to Spd and Spm by means of Spd and Spm synthases, as suggested by Puga-Hermida et al. (2003). At 60 DAP the ratio was quite the same, although levels were lower, ranging further (105 and 120 DAP) for Spd (Fig. 5c).
It is important to note that arginine and ornithine are direct precursors, whereas methionine is an indirect precursor, of PAs biosynthetic routes in plants (Antognoni et al. 1998; Bouchereau et al. 1999). In the present study, these amino acids were those that presented the highest levels at 75 and 90 DAP, the later corresponding to the cotyledonary stage. Accordingly, the accumulation of these amino acids as detected in this developmental stage could be due to the synthesis of PAs, as revealed by analysis. These PAs could be important during maturation of zygotic embryos.
IAA and ABA
The role of IAA in zygotic embryogenesis is well-known (Ribnicky et al. 2002; Bassuner et al. 2007), and is mainly associated with cell division and elongation, and differentiation of the vascular system (Gaspar et al. 1996). Many of its effects are dependent on transport across tissues and organs (Fischer-Iglesias et al. 2001; Friml et al. 2003).
In the present work, IAA and ABA levels between the heart and cotyledonary stages were inversely proportional (Fig. 6). Dynamic changes in these hormones could be associated with changes in the patterns of histodifferentiation and the establishment of embryonal radial and axial symmetry, as suggested by (Michalczuck et al. 1992). The dynamics of variation at IAA levels follows the same pattern observed in the development of seeds, in which high IAA levels occur during embryo development, to then decrease in mature seeds (Bewley and Black 1994). In the present work, the highest IAA levels were observed in the torpedo stage (60 DAP), followed by a continuous decrease (Fig. 6). The same pattern of IAA accumulation was observed in seeds of O. catharinensis (Santa-Catarina et al. 2006). High IAA levels were found in Quercus robur at the heart stage, with a decrease in more advanced developmental stages (Prewein et al. 2006). In A. angustifolia seeds, high IAA levels occurred in tissues of the embryonic axis and in the early stages of development (Astarita et al. 2003c).
In P. glauca and P. taeda the high levels of IAA observed during initial seed development were linked with growth of the zygotic embryo (Kong et al. 1997; Silveira et al. 2004b). In orthodox seeds, as in the case of A. sellowiana, reduction in hormone levels in mature seeds is a common feature caused by the high degree of seed dehydration. These hormones can be conjugated for further release during seed germination (Kong et al. 1997).
Tryptophan is considered the main precursor of IAA biosynthesis (Bandurski et al. 1995). The biosynthesis of tryptophan is important for establishing embryo polarity in early stages of development (Astarita et al. 2003c). In the present work, a decrease in tryptophan levels in the torpedo stage (60 DAP) was observed (Table 1), this occurring concomitantly with the IAA peak, thus suggesting that tryptophan was consumed in IAA biosynthesis.
ABA also plays a major role in embryonal development. Among other effects ABA prevents early germination (Kermode 1995). In the present study, the highest levels of ABA (Fig. 6) were observed at the cotyledonary stage (90, 105 and 120 DAP). The patterns of ABA accumulation in the present work are common in most angiosperms, which show an increase in ABA content during seed development and a decline in the mature seed (Bewley and Black 1994). In embryos of Quercus robur, ABA peaked at the cotyledonary stage and then decreased progressively. Concomitant with ABA, the water content declined as the embryo reached the maximum size (Prewein et al. 2006). Rock and Quatrano (1995) showed that at the end of barley zygotic embryogenesis, a decrease in IAA levels and an increase in ABA levels was observed. This ran parallel with the accumulation of storage compounds. In the present case, similar features were observed regarding the dynamics of IAA and ABA accumulation.
Conclusions
In this study relevant information about the biochemical and physiological changes occurring during the formation and development of the zygotic embryo of A. sellowiana was acquired. Starch is the predominant storage compound during embryonal development. All amino acids are synthesized during embryogenesis with an increased accumulation in the heart and cotyledonary stages. Asparagine is the major amino acid observed. Free PAs synthesis during early developmental stages, as well as the accumulation of PAs conjugates in the cotyledonary stage, may be considered as reliable biochemical markers of embryonal development in A. sellowiana. IAA and ABA levels were inversely proportional between the heart and cotyledonary stages, suggesting their involvement with histodifferentiation patterns, mainly the establishment of embryonal symmetries. The information here obtained will help to explain the biochemical and physiological changes that occur during zygotic embryogenesis and may assist in the development of improved somatic embryogenesis protocols in this and other woody plant species (Fig. 7).
References
Antognoni F, Fornalè S, Grimmer C, Komor E, Bagni N (1998) Long-distance translocation of polyamines in phloem and xylem of Ricinus communis L. plants. Planta 204:520–527. doi:10.1007/s004250050287
Aribaud M, Martin-Tanguy J (1994) Polyamine metabolism, floral initiation and floral development in chrysanthemum (Chrysanthemum morifolium Ramat.). Plant Growth Regul 15:23–31. doi:10.1007/BF00024673
Arnold CD, Mitrenga D, Mayresbach H (1975) Gefriertro und einbsttung in glycolmethacrylat (GMA)—Ergehnisse histochemischer reaktionen. Acta histochem 14:271–277
Astarita LV, Floh EIS, Handro W (2003a) Free amino acid, protein and water content changes associated with seed development in Araucaria angustifolia. Biol Plant 47:53–59. doi:10.1023/A:1027376730521
Astarita LV, Handro W, Floh EIS (2003b) Changes in polyamines content associated with zygotic embryogenesis in the Brazilian pine, Araucaria angustifolia (Bert.) O. Ktze. Rev Bras Bot 26:163–168. doi:10.1590/S0100-84042003000200003
Astarita LV, Floh EIS, Handro W (2003c) Changes in IAA, tryptophan and activity of soluble peroxidases associated with zygotic embryogenesis in Araucaria angustifolia (Brazilian pine). Plant Growth Regul 39:113–118. doi:10.1023/A:1022542618945
Bais HP, Havishankar GA (2002) Role of polyamines in the ontogeny of plants and their biotechnological applications. Plant Cell Tissue Organ Cult 69:1–34. doi:10.1023/A:1015064227278
Bandurski RS, Cohen JD, Slovin JP, Reinecke DM (1995) Hormone biosynthesis and metabolism. In: Davies PJ (ed) Plant hormone. Physiology, biochemistry and molecular biology. Kluwer, Dordrecht, pp 39–65
Baron K, Stasolla C (2008) The role of polyamines during in vivo and in vitro development. In Vitro Cell Dev Biol Plant 44:384–395. doi:10.1007/s11627-008-9176-4
Bassuner BM, Lam R, Lukowitz W, Yeung EC (2007) Auxin and root initiation in somatic embryos of Arabidopsis. Plant Cell Rep 26:1–11. doi:10.1007/s00299-006-0207-5
Bewley JD, Black M (1994) Seeds: physiology of development and germination, 2nd edn. Plenum Press, New York
Black M (1991) Abscisic acid in germination and dormancy. In: Davies WJ, Jones HJ (eds) Abscisic acid physiology and biochemistry. Bios Scientific Publishers, Oxford, pp 81–98
Bouchereau A, Aziz A, Larher F, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125. doi:10.1016/S0168-9452(98)00218-0
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Cailloux F, Julien-Guerrier J, Linissier L, Coudret A (1996) Long-term somatic embryogenesis and maturation of somatic embryos in Hevea brasiliensis. Plant Sci 120:185–196. doi:10.1016/S0168-9452(96)04491-3
Cangahuala-Inocente GC, Dal Vesco LL, Steinmacher DA, Torres A, Guerra MP (2007) Improvements in somatic embryogenesis protocol in Feijoa (Acca sellowiana (Berg) Burret): induction, conversion and synthetic Seeds. Sci Hortic (Amsterdam) 11:228–234. doi:10.1016/j.scienta.2006.10.030
Cangahuala-Inocente GC, Steiner N, Maldonado SB, Guerra MP (2009) Patterns of protein and carbohydrate accumulation during somatic embryogenesis of Acca sellowiana. Pesq agropec bras 44:217–224
Canhoto JM, Cruz GS (1996) Feijoa sellowiana Berg (pineapple guava). In: Bajaj YPS (ed) Tree IV. Biotechnology in agriculture on forestry. Springer, Berlin, pp 156–172
Corte VB, Lima De, e Borges EE, Pontes CA, De Almeida Leite IT, Ventrella MC, Mathias AA (2006) Mobilização de reservas durante a germinação das sementes e crescimento das plântulas de Caesalpinia peltophoroides Benth.(Leguminosae-caesalpinoideae). Rev Arvore 30(6):941–949
Coruzzi G, Last R (2000) Amino acids. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiologists, Rockville, pp 358–410
Dal Vesco LL, Guerra MP (2001) The effectiveness of nitrogen sources in Feijoa (Feijoa sellowiana Berg) somatic embryogenesis. Plant Cell Tissue Organ Cult 64:19–25. doi:10.1023/A:1010635926146
Dam S, Laursen BS, Ornfelt JH, Jochimsen B, Stærfeldt HH, Friis C, Nielsen K, Goffard N, Besenbacher S, Krusell L, Sato S, Tabata S, Thogersen IB, Enghild JJ, Stougaard J (2009) The proteome of seed development in the model legume Lotus japonicus. Plant Physiol 149:1325–1340. doi:10.1104/pp.108.133405
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48:1493–1509
Etienne H, Sotta B, Montoro P, Miginiac E, Carron MP (1993) Relation between exogenous growth regulators and endogenous indole-3-acetic acid and abscisic acid in expression of somatic embryogenesis in Hevea brasiliensis (Mull. Arg.). Plant Sci 88:91–96. doi:10.1016/0168-9452(93)90113-E
Feirer RP (1995) The biochemistry of conifer embryo development: amino acids, polyamines, and storage proteins. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants. v.1. Kluwer, Dordrecht, pp 317–336
Fischer-Iglesias C, Nauhaus G (2001) Zygotic embryogenesis hormonal control of embryo development. In: Bhojwani SS, Soh WY (eds) Current trends in the embryology of angiosperms. Kluwer, Dordrecht, pp 223–247
Fischer-Iglesias C, Sundberg B, Neuhaus G, Jones AM (2001) Auxin distribution and transport during embryonic pattern formation in wheat. Plant J 26:115–129. doi:10.1046/j.1365-313x.2001.01013.x
Friml J, Vieten A, Sauer M, Weijers D, Scwarz H, Hamann T, Offringa R, Jurgens G (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426:147–153. doi:10.1038/nature02085
Gaspar T, Kevers C, Penel C, Greppin H, Reid DM, Thorpe TA (1996) Plant hormones and growth regulators in plant tissue culture. In Vitro Cell Dev Biol Plant 32:272–289. doi:10.1007/BF02822700
Geoffriau E, Kahane R, Martin-Tanguy J (2006) Polyamines are involved in the gynogenesis process in onion. Physiol Plant 127:119–129. doi:10.1111/j.1399-3054.2006.00597.x
Gibson SI (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8:93–102. doi:10.1016/j.pbi.2004.11.003
Goldberg RB, Paiva G, Yadegari R (1994) Plant embryogenesis: zygote to seed. Science 266:605–614. doi:10.1126/science.266.5185.605
Kamada H, Harada H (1984) Changes in endogenous amino acids compositions during somatic embryogenesis in Daucus carota. Plant Cell Physiol 25:27–38
Kermode AR (1995) Regulatory mechanisms in the transition from seed development to germination: interaction between the embryo and the seed environment. In: Kigel J, Galili G (eds) Seed development and germination. Marcel Dekker, New York, pp 273–332
Kong L, Attree SM, Fowke LC (1997) Changes of endogenous hormone levels in developing seeds, zygotic embryos and megagametophytes in Picea glauca. Physiol Plant 101:23–30. doi:10.1111/j.1399-3054.1997.tb01815.x
Kusano T, Yamaguchi K, Berberich T, Takahashi Y (2007) Advances in polyamine research in 2007. J Plant Res 120:345–350. doi:10.1007/s10265-007-0074-3
Lea PJ, Sodek L, Parry MAJ, Shewry PR, Halford NG (2007) Asparagine in plants. Ann Appl Biol 150:1–26. doi:10.1111/j.1744-7348.2006.00104.x
Liu CM, Xu ZM, Chua NH (1993) Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell 5:621–630
Mader JC, Hanke DE (1997) Polyamine sparing may be involved in the prolongations of cell division due to inhibition of phenyl-propanoid synthesis in cytokinin starved soybean cells. J Plant Growth Regul 16:89–93. doi:10.1007/PL00006983
Magnus V, Nigović B, Hangarter RP, Good NE (1992) N - (indol-3-ylacetyl) amino acids as sources of auxin in plant tissue culture. J Plant Growth Regul 11:19–28. doi:10.1007/BF00193839
Mansfield SG, Briarty LG (1991) Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Can J Bot 69:461–476. doi:10.1139/b91-063
Matsumoto TK, Kuehnle AR, Webb DT (1998) Zygotic embryogenesis in anthurium (araceae). Am J Bot 85:1560–1568. doi:10.2307/2446482
McCready RM, Guggolz J, Silveira VE, Owens HS (1950) Determination of starch an amylose in vegetables. Anal Chem 22:1156–1158. doi:10.1021/ac60045a016
Merkle SA, Parrott WA, Flinn BS (1995) Morphogenic aspects of somatic embryogenesis in plants. Dordrecht. Kluwer Acad Publishers Cap 5:155–203
Michalczuck L, Cooke TD, Cohen JD (1992) Auxin levels at different stages of carrot somatic embryogenesis. Phytochemistry 4:1097–1103. doi:10.1016/0031-9422(92)80241-6
Misra S, Attree SM, Leal I, Fowke LC (1993) Effect of abscisic acid, osmoticum, and desiccation on synthesis of storage proteins during the development of white spruce somatic embryos. Ann Bot (Lond) 71:11–22. doi:10.1006/anbo.1993.1002
Mordhorst AP, Toonen MAJ, De Vries SC (1997) Plant embryogenesis. Crit Rev Plant Sci 16:535–576. doi:10.1080/713608156
Nuutila AM, Kurten U, Kauppinen V (1991) Optimization of sucrose and inorganic nitrogen concentrations for somatic embryogenesis of birch (Betula pendula Roth.) callus cultures: a statistical approach. Plant Cell Tissue Organ Cult 24:73–77. doi:10.1007/BF00039733
O’Brien TP, Feder N, McCully ME (1965) Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59:368–373. doi:10.1007/BF01248568
Ortiz-Lopez A, Chang HC, Bush DR (2000) Amino acid transporters in plants. Biochim Biophys Acta 1465:275–280. doi:10.1016/S0005-2736(00)00144-9
Pescador R, Kerbauy GB, Kraus JE, Ferreira WM, Guerra MP, Figueiredo-Ribeiro C (2008) Changes in soluble carbohydrates and starch amounts during somatic and zygotic embryogenesis of Acca sellowiana (Myrtaceae). In Vitro Cell Dev Biol Plant 44:289–299. doi:10.1007/s11627-008-9118-1
Prewein C, Endemann M, Reinöhl V, Salaj J, Sunderlikova V, Wihelm E (2006) Physiological and morphological characteristics during development of pedunculate oak (Quercus robur L.) zygotic embryos. Trees (Berl) 20:53–60. doi:10.1007/s00468-005-0012-8
Puga-Hermida MI, Gallardo M, Matilla AJ (2003) The zygotic embryogenesis and ripening of Brassica rapa seeds provokes important alterations in the levels of free and conjugated abscisic acid and polyamines. Physiol Plant 117:279–288. doi:10.1034/j.1399-3054.2003.00033.x
Radwanski ER, Last RL (1995) Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell 7:921–934
Ribnicky DM, Cohen JD, Hu W, Coke TJ (2002) An auxin surge following fertilization in carrots: a mechanism for regulating plant totipotency. Planta 214:505–509. doi:10.1007/s004250100639
Rock CD, Quatrano RS (1995) The role of hormones during seed development. In: Davies PJ (ed) Plant hormones. Kluwer, Dordrecht, pp 671–697
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709. doi:10.1146/annurev.arplant.57.032905.105441
Sallandrouze A, Faurobert M, Maâtaoui ME (2002) Characterization of the development stages of cypress zygotic embryos by two-dimensional electrophoresis and by cytochemistry. Physiol Plant 114:608–618. doi:10.1034/j.1399-3054.2002.1140415.x
Santa-Catarina C, Silveira V, Balbuena TS, Viana AM, Estelita MEM, Handro W, Floh EIS (2006) IAA, ABA, polyamines and free amino acids associated with zygotic embryo development of Ocotea catharinensis. Plant Growth Regul 49:237–247. doi:10.1007/s10725-006-9129-z
Satya-Naraian V, Nair PM (1990) Metabolism, enzymology and possible roles of 4-aminobutyrate in higher plants. Phytochemistry 29:367–375. doi:10.1016/0031-9422(90)85081-P
Shannon JC (1968) A procedure for the extraction and fractionation of carbohydrates from immature Zea mays kernels. Res Bull (Sun Chiwawitthaya thang Thale Phuket) 842:1–8
Silveira V, Floh EIS, Handro W, Guerra MP (2004a) Effect of plant growth regulators on the cellular growth and levels of intracellular protein, starch and polyamines in embryogenic suspension cultures of Pinus taeda. Plant Cell Tissue Organ Cult 76:53–60. doi:10.1023/A:1025847515435
Silveira V, Balbuena TS, Santa-Catarina C, Floh EIS, Guerra MP, Handro W (2004b) Biochemical changes during zygotic embryogenesis in Pinus taeda L. Plant Growth Regul 44:147–156
Silveira V, Santa-Catarina C, Balbuena TS, Moraes FMS, Ricart CAO, Sousa MV, Guerra MP, Handro W, Floh EIS (2008) Endogenous abscisic acid and protein contents during seed development of Araucaria angustifolia. Biol Plant 52(1):101–104. doi:10.1007/s10535-008-0018-3
Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51:49–81. doi:10.1146/annurev.arplant.51.1.49
Snedden WA, Fromm H (1998) Calmodulin, calmodulin-related proteins and plant responses to the environment. Trends Plant Sci 3:299–304. doi:10.1016/S1360-1385(98)01284-9
Stefanello S, Dal Vesco LL, Ducroquet JPHJ, Nodari RO, Guerra MP (2005) Somatic embryogenesis from floral tissues of feijoa (Feijoa sellowiana Berg). Sci Hortic (Amsterdam) 105:117–126. doi:10.1016/j.scienta.2004.11.006
Thorp G, Bieleski R (2002) Feijoas: origins, cultivation and uses. David Baterman, New Zealand, p 87
Tobe PH, Raven H (1983) The embryology of Axinandra zeylanica (Crypteroniaceae) and the relationships of the genus. Bot Gaz 144(3):426–432. doi:10.1086/337393
Umbreit WW, Burris RH, Stauffer JF (1964) Manometric techniques. Burgess Publishing Co, Minneapolis
Weber H, Borisjuk L, Wobus W (1997) Sugar import and metabolism during seed development. Trends Plant Sci 2:169–174. doi:10.1016/S1360-1385(97)85222-3
Wise JM, Tunnacliffe A (2004) POPP the question: what do LEA proteins do? Trends Plant Sci 9:13–17. doi:10.1016/j.tplants.2003.10.012
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cangahuala-Inocente, G.C., Silveira, V., Caprestano, C.A. et al. Dynamics of biochemical and morphophysiological changes during zygotic embryogenesis in Acca sellowiana (Berg.) Burr.. Plant Growth Regul 59, 103–115 (2009). https://doi.org/10.1007/s10725-009-9393-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-009-9393-9