Abstract
The invasive, Halyomorpha halys (Hemiptera: Pentatomidae), is a severe economic insect pest native to East Asia. A strong effort has been made to identify natural egg parasitoids of H. halys in invaded regions, but parasitism rates reported from these studies have been inconsequentially low. To determine the species composition, phenology, and efficiency of egg parasitoids in the native region of H. halys, we deployed fresh and frozen sentinel H. halys egg masses from March through December in Kyoto, Japan. Our findings provide valuable insights on the abundance and parasitism rates of native H. halys parasitoids in Japan. A total of seven parasitoid species emerged from the sentinel egg masses, but Trissolcus japonicus had the highest parasitism rate of all parasitoids recovered (84% on fresh egg masses) and maintained the largest portion of the total parasitoid species composition (60% on fresh egg masses). The early season parasitoid community in Kyoto, Japan, is dominated by T. japonicus, with the first parasitism activity occurring in March. Throughout the course of the field study, T. japonicus also sustained a significantly higher parasitism rate on fresh H. halys eggs than frozen. The results from this research help expand the understanding of parasitoids in the native region of H. halys and hold importance for the future development of biological control programs against this invasive pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Information regarding the species richness and phenology of Halyomorpha halys parasitoids in the invasive pest's native range is lacking
-
We conducted an intensive survey of egg parasitoids in Kyoto, Japan using sentinel H . halys egg masses
-
Seven egg parasitoid species belonging to three genera were recovered
-
Overall, Trissolcus japonicus was the most abundant parasitoid species, and maintained the highest parasitism rates
-
These results support the implementation of T. japonicus in potential biological control programs against H . halys.
Introduction
The enemy release hypothesis states that the rapid increase in abundance and distribution that invasive species may experience upon arrival to a new region is associated with their escape from natural controlling agents in their native range (Keane and Crawley 2002). In most cases, few existing natural enemies will attack an adventive pest with much success, and the endemic species that do so are typically opportunistic generalists with a wide range of hosts (Hoddle 2004). Once established, invasive pests can cause major environmental damage and severe economic loss in agricultural industries (Pimentel et al. 2000; Messing and Wright 2006). An essential component of any successful management strategy against an invasive pest species is gaining a fundamental understanding of the pest’s biology, behavior, and natural control in its native range (Evans and Speight 2004).
The brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae), is an invasive economic insect pest native to subtropical and temperate regions of East Asia including Japan, China, and Korea (Zhu et al. 2012a; Lee et al. 2013). Hitchhiking as a by-product of international commerce is the main mode of unintended introduction of the pest to foreign areas, as H. halys can stowaway undetected in internationally bound cargo such as machinery, used vehicles, and personal baggage (Hoebeke and Carter 2003; Gariepy et al. 2014; Kriticos et al. 2017). This invasive pest has already established populations in North America, South America, and Europe (Hoebeke and Carter 2003; Wermelinger et al. 2008; Fogain and Graff 2011; Faúndez and Rider 2017), and climatic models have forecasted multiple regions as amenable for potential future invasions (Zhu et al. 2012a; Kriticos et al. 2017). Upon arrival to an uninvaded area, H. halys is known to spread rapidly through human activity and can establish populations throughout all climatically suitable locations in which they are exposed (Haye et al. 2015a). Monitoring and trapping H. halys is difficult due to the pest’s exceptional mobility (Nielsen et al. 2013). This pest also has hundreds of different host crops that it is able to feed on to complete development (Lee et al. 2013; Rice et al. 2014). Furthermore, native biological control from natural enemies has been ineffective from field assessments conducted in invaded regions (Haye et al. 2015b; Ogburn et al. 2016; Dieckhoff et al. 2017). As a result, the economic damage inflicted by H. halys to agricultural industries in invaded areas has been severe due to damaged and thus unmarketable fruit and low crop yields (Nielsen and Hamilton 2009; Kuhar et al. 2012).
Chemical control remains the most prevalent form of management against H. halys in its non-native range (Leskey et al. 2012a; Kuhar and Kamminga 2017). However, pesticides are seen as a short-term solution as they can be expensive both monetarily and environmentally (Hull and VanStarner 1983; Lee et al. 2014). Frequency of insecticide applications targeting H. halys has increased by nearly four times dating back to when the pest first began inflicting economic injury in invaded regions (Leskey et al. 2012a). The increased use of insecticides throughout the field season has also led to secondary pest outbreaks in agricultural settings (Rice et al. 2014). In addition, H. halys has been shown to develop tolerance after exposure to several insecticides in laboratory trials (Leskey et al. 2012b). To sustainably mitigate the agricultural threat this adventive pest imposes in regions of invasion, the development of a robust, multi-faceted integrated pest management approach is necessary.
Biological control has emerged at the forefront of explored sustainable measures to manage H. halys. Laboratory experiments have determined generalist predators have varying levels of success in providing control against different life stages of H. halys (Morrison et al. 2016; Pote and Nielsen 2017; Kamiyama et al. 2021a). The implementation of parasitoid wasps as a measure of control has shown more promise, as research efforts have focused on understanding the parasitism efficacy and environmental effects of prospective egg parasitoids (Lee et al. 2013; Rice et al. 2014). Field surveys exploring the presence of H. halys parasitoids using sentinel and naturally laid H. halys egg masses have been conducted in several invaded regions (Abram et al. 2017). Parasitoids from the genera Trissolcus, Telenomus, Anastatus, Ooencyrtus, and Gyron have been documented to successfully parasitize sentinel H. halys egg masses in the USA (Herlihy et al. 2016; Dieckhoff et al. 2017; Tillman et al. 2020). Similarly, a wide range of parasitoids from the genera Trissolcus, Telenomus, Anastatus, Ooencyrtus, and Acroclisoides have been recorded parasitizing sentinel egg masses in Switzerland and Italy (Haye et al. 2015b; Roversi et al. 2016; Moraglio et al. 2020). However, the parasitism rates inflicted by these indigenous egg parasitoids from North America and Europe are generally low, below 5% across these field studies (Abram et al. 2017; Dieckhoff et al. 2017).
Research conducted in the native range of H. halys—specifically China and Japan—has determined parasitoids primarily from the Trissolcus genus as the main H. halys egg parasitoids (Arakawa and Namura 2002; Lee et al. 2013), although species in the Telenomus, Ooencyrtus, and Anastatus genera have also been identified in successfully parasitizing H. halys egg masses (Zhang et al. 2017; Avila et al. 2021). Field assessments conducted in kiwifruit in central China revealed a native H. halys parasitoid species composition of 41% Trissolcus japonicus and 48% T. cultratus (Avila et al. 2021), whereas surveys conducted in mixed fruit orchards in northern China displayed a species composition consisting of over 90% T. japonicus (Zhang et al. 2017). The field parasitism rate of T. japonicus on H. halys ranges from 50 to 80% as determined by sentinel egg mass deployment in China (Yang et al. 2009; Zhang et al. 2017) and is over 90% in laboratory experiments (Haye et al. 2015b). Programs testing T. japonicus as an appropriate biological control agent against H. halys are under evaluation worldwide (Hedstrom et al. 2017; Charles et al. 2019; Milnes and Beers, 2019; Lara et al 2019; Haye et al. 2020; Saunders et al. 2021).
The native range of currently known H. halys parasitoids and the overall species composition is poorly understood and projected to be underestimated due to a lack of field sampling in Asia (Avila and Charles 2018; Yonow et al. 2021). Other gaps in knowledge pertaining to H. halys parasitoids in Asia also exist. For example, there is limited published information available describing behaviors such as the overwintering habits and seasonal phenology of parasitoids in the native range of H. halys. The presented research therefore aims to clarify the seasonal phenology and components of biological control against H. halys in its native range by surveying weekly for egg parasitoids in Japan. The results from this study not only establish the first phenological assessment of H. halys egg parasitoids in Japan, but are also expected to improve the biological knowledge of the parasitoid richness and community structure in the native range of H. halys. A better understanding of the parasitoid biology in the native range of H. halys will have implications for the development and refinement of pending classical biological control methods.
Materials and methods
Insect colonies
Halyomorpha halys used to produce egg masses for the field experiments were reared at Kyoto University in Uji, Japan. The insects were originally field collected from Tokyo, Japan. The colonies of H. halys were kept in plastic containers (40 cm × 15 cm × 20 cm) (Daiso, Taizo Sangyo Co., Ltd., Hiroshima, Japan) fitted with mesh lids and were provided a mixture of green beans (Phaseolus vulgaris), carrots (Daucus carota ssp. sativus), and raw almonds (Prunus dulcis). All colony containers were thoroughly checked for egg masses daily to ensure the age of each egg mass was known. The H. halys colonies were maintained at a temperature of 24 ± 1 °C and a relative humidity of roughly 70% in a temperature-controlled room.
Sentinel egg mass study
Three fresh and three frozen sentinel egg cards were clipped to foliage (1 m above the ground) near Kyoto University, Uji Campus in Kyoto, Japan (34°54′39" N, 135°48′09" E) every week from March 22, 2020, to December 18, 2020. Egg masses of varying age between 0 and 24 h were fixed to 3 cm × 2 cm pieces of cardstock via double-sided sticky tape (Lara et al. 2019). Exposed parts of the tape were covered with small pieces of paper towel to ensure parasitoids in the field would not get stuck, hindering potential parasitism attempts. Cold-treated egg cards were placed in a − 20 °C freezer and frozen for at least one week before field deployment (Tillman et al. 2020). Sentinel cards were left in the field for 72 h and then returned to the laboratory. Upon their return, egg masses were transferred to a clear plastic snap cap vial (4.0 cm × 2.3 cm) (SKS Science, Watervliet, NY, USA). Daily observations were made of the returned egg masses for one month to observe H. halys nymph or parasitoids emergence (Dieckhoff et al. 2017). Sentinel egg masses were then allowed another two months of incubation time, in which egg masses were checked three times per week to ensure potential developing parasitoids were provided sufficient time to develop and emerge. Any unemerged eggs from the sentinel egg masses were then dissected to determine the contents of the eggs: developing nymph, developing parasitoid, or undetermined (aborted egg with undifferentiated contents) (Kaser et al. 2018; Costi et al. 2020). All egg masses were kept at a temperature of 24 ± 1 °C and a relative humidity of roughly 70% in a temperature-controlled room.
Twenty control fresh egg masses were constructed in the same manner as the fresh sentinel field egg masses and then reared in a laboratory setting in order to compare H. halys nymphal emergence rates between the field-deployed and laboratory-reared egg mass groups. Twenty control fresh egg masses were also set up to compare the parasitism rates between fresh field-deployed sentinel egg masses and the laboratory-conducted controls. In these control parasitism experiments, only T. japonicus were tested since they were the only parasitoid recovered from the field in high enough numbers (see Results). Trissolcus japonicus colony lines were established by providing a female parasitoid emerged from a fresh sentinel egg mass with a fresh H. halys egg mass 24 h of age in a clear plastic snap cap vial. Female T. japonicus were removed following 48 h of exposure to the egg mass, and the parasitism rates were determined (see statistical analysis section). The control egg masses were reared at a temperature of 24 ± 1 °C and a relative humidity of roughly 50% in a temperature-controlled room.
Parasitoid identification
Initial identification of emerged parasitoid wasp genera was determined morphologically through the presence/absence of key features (Hirashima and Yamagishi 1981; Zhang et al, 2005; Talamas et al. 2017; Chen et al. 2019). The species of emerged parasitoid wasps was then confirmed through molecular identification for each sentinel egg mass that was successfully parasitized in the field (i.e., at least one fully emergent adult parasitoid). Genomic DNA was isolated from a whole parasitoid using QIAamp DNA extraction kit (QIAGEN, Germantown, MD, USA) per the manufacturer’s instructions. Following parasitoid DNA extraction, the barcode region of the mitochondrial gene cytochrome oxidase I (COI) was amplified with universal insect PCR primers LCO-1490 and HCO-2198 (Folmer et al. 1994). The PCR products, ranging from 615 to 650 base pairs, were purified using a FastGene PCR extraction kit (Nippon Genetics Co., Ltd., Tokyo, Japan) and then subjected to Sanger sequencing (Eurofin Genomics Co., Ltd., Tokyo, Japan). Both forward and reverse sequences were manually trimmed and aligned using MEGA X (Kumar et al. 2018). All parasitoid CO1 sequences were then compared against existing sequences in the GenBank database using the similarity search from the Basic Local Alignment Search Tool (BLAST) (http://www.ncbi.nlm.nih.gov/BLAST) to confirm the species identity of each specimen. A representative sequence from each determined parasitoid species was submitted to the GenBank database, and accession numbers were obtained.
Temperature data
Temperature data throughout the duration of the field study were obtained for the location 34°53′24" N, 135°48′00" E in Uji, Japan (Meteoblue 2021), 2 km from the location of the deployed sentinel egg masses. The data provided daily, as well as monthly minimums, maximums, and average temperatures. The temperature data were used to help relate field parasitism activity with seasonality in Kyoto.
Statistical analysis
Sentinel egg mass data were separated by season (spring, summer, and fall), unless otherwise noted (e.g., all data pooled together). The first sampling event, March 22, through May 30, was designated as “spring,” June 7 through August 28, was designated as “summer,” and September 6 through December 20 was designated as “fall.” When determining the parasitism levels of the native parasitoids, only sentinel egg masses with emerged parasitoids were included for analysis (Zhang et al. 2017). Parasitism rate was calculated by dividing the number of successfully emerged parasitoids by the total number of eggs from an egg mass. When determining nymphal emergence levels from fresh sentinel egg masses, all fresh egg masses were included for analysis (parasitized and non-parasitized). Nymph emergence rate was calculated by dividing the total number of emerged H. halys nymphs by the total number of eggs from an egg mass. Developmental days for parasitoids were calculated by summing the total number of days from the first day a sentinel egg mass was deployed in the field until parasitoid emergence from the egg mass. The summed days were then added for each emerged parasitoid and finally divided by the total number of parasitoids that emerged from each parasitized egg mass to give the average developmental days.
Generalized linear models (GLMs) with a logit link function were fit to the data using the glm function in the lme4 package from the RStudio program (v.3.4.1) (R Core Team 2018). The GLMs estimated the effect seasonality (spring, winter, fall), egg mass type (fresh, frozen), and the seasonality x type interaction had on the parasitism rate and parasitoid species composition measured from the sentinel H. halys egg masses. Analyses were run on pooled data (all seasons and both egg mass types combined), and also data separated by season and egg mass type.
A two-way ANOVA was run to determine the effect the different egg mass types had on the developmental days between the different species of recovered parasitoids. A Tukey’s HSD post hoc test was then used to determine differences in the means between the variables at p < 0.05. A Welch two-sample t test was run to determine: (1) the difference in parasitism rates between fresh and frozen sentinel egg masses for T. japonicus and T. cultratus, (2) the difference in the H. halys nymphal emergence rates between the laboratory-reared and field-deployed fresh sentinel egg masses, and (3) the difference in T. japonicus parasitism rates between fresh sentinel and laboratory-reared control egg masses at p < 0.05.
Results
In total, 228 fresh and frozen sentinel H. halys egg masses (5343 eggs) were deployed in Kyoto, Japan, over 38 weeks from March through December 2020. The majority of eggs (71.3%) remained unemerged after retrieval, meaning neither H. halys nymphs nor parasitoids emerged from the eggs. Successful parasitism occurred on 16.2% of all the sentinel egg masses deployed. Of the successfully parasitized egg masses, 543 parasitoids emerged from 930 eggs (58.4%) (Table 2).
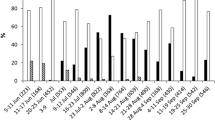
The first record of successful parasitism from the sentinel field H. halys egg cards occurred in early spring from a fresh egg mass (Fig. 1). The second record of successful parasitism occurred eight weeks later in late spring from both fresh and frozen egg masses. Parasitism was regularly recorded on sentinel egg masses from late spring until final field parasitism in early fall. The average daily temperature during the 3-day period (March 19−22) the first parasitized sentinel egg mass was in the field ranged between 10 and 13 °C, with a maximum temperature of 21 °C (Supp. Fig. 1). Throughout the year, average daily temperatures rose from early spring until late summer and then began to lower until late fall (Supp. Fig. 2).
Nymphal H. halys emerged from the fresh sentinel egg masses every week from early spring to late fall (Fig. 2). Nymphal emergence rate varied weekly, but was typically lowest in early spring and late fall. The highest nymphal emergence rate for a deployed set of fresh sentinel egg masses was in mid-summer (90.4%). No H. halys nymphs successfully emerged from the fresh egg masses deployed in the field after late November. The average nymphal emergence rate from the fresh field egg masses was 27.7%, which was significantly lower than the average nymphal emergence rate from laboratory-reared control H. halys egg masses (90.5%) (t = 14.02, p < 0.005). Dissections of the unemerged sentinel eggs revealed 20 unemerged parasitoids from the fresh egg masses and 41 unemerged parasitoids from the frozen egg masses. Of the unemerged fresh eggs, 1.4% were developing parasitoids, 82.9% were developing H. halys nymphs, and 15.7% were undetermined. Of the unemerged frozen eggs, 1.6% were developing parasitoids and 98.4% were undetermined.
Morphological analysis identified parasitoids from three genera, Trissolcus, Anastatus, and Ooencyrtus. Molecular analysis confirmed the species of five of the emerged parasitoids, four from the genera Trissolcus and one from Anastatus. A BLAST search displayed the best similarity score (%) of the CO1 barcode sequence (base pairs in length) for the parasitoids, and a representative sequence was submitted to GenBank for each species. Trissolcus japonicus yielded a 650-bp sequence (accession number: MZ466407) with 99% sequence identity scores against most previously published T. japonicus sequences (e.g., MN615626.1). Trissolcus cultratus yielded a 615-bp sequence (accession number: MZ456341) with 99% sequence identity scores against most previously published T. cultratus sequences (e.g., AB971829). Trissolcus mitsukurii yielded a 628-bp sequence (accession number: MZ466409) with 99% sequence identity scores against most previously published T. mitsukurii sequences (e.g., MT671789.1). Trissolcus comperei yielded a 640-bp sequence (accession number: MZ466408) with 99% sequence identity scores against most previously published T. comperei sequences (e.g., MN615656.1). Anastatus japonicus yielded a 641-bp sequence (accession number: MZ433261) with 99% sequence identity scores against most previously published A. japonicus sequences (e.g., MZ433261.1). Molecular analysis could not reliably confirm the species for one other parasitoid: Trissolcus sp. 631-bp sequence (accession number: MZ503514) with only 90% sequence identity scores against most previously published T. plautiae sequences (e.g., MN615614.1). Molecular analysis was not performed on Ooencyrtus sp. due to a low amount of successfully emerged specimens.
The first parasitoid species to successfully emerge from a fresh sentinel egg mass was T. japonicus (early spring), followed by Trissolcus sp. (early summer), then T. cultratus and T. mitsukurii (mid-summer), A. japonicus (late summer), and finally T. comperei (late summer) (Fig. 3). Similarly, T. japonicus was also the first parasitoid species to emerge from a frozen sentinel egg mass (late spring), followed by T. cultratus (mid-summer), T. comperei (mid-summer), T. mitsukurii (late summer), and lastly Ooencyrtus sp. (late summer) (Fig. 4). The only parasitoid that parasitized a sentinel egg mass (fresh or frozen) during each season was T. japonicus.
When pooling the data, seasonality did not have a significant effect on the parasitism rate found on the sentinel H. halys egg masses (Z = 0.84; p = 0.40), but egg mass type did have a significant effect on parasitism rate (Z = 2.31; p = 0.02) (Table 1). The pooled data also showed the parasitism rate was significantly higher on fresh sentinel egg masses (73.3%) than frozen (39.6%) (t = 3.69, p < 0.005). The parasitism rates were significantly higher on fresh sentinel egg masses compared to frozen for T. japonicus (t = 5.023, p < 0.001), and the parasitism rates were similar between fresh and frozen egg masses for T. cultratus (t = 1.059, p = 0.339) (Table 2). Also, the average parasitism rate of T. japonicus on the fresh sentinel egg masses across the entire field season (84.1%) was similar with the parasitism rate of T. japonicus on the control egg masses in laboratory conditions (95.0%) (t = 1.62, p = 0.131). However, T. mitsukurii produced numerically the highest parasitism rate on fresh sentinel egg masses (100%). All four identified Trissolcus species produced average parasitism rates over 70% on fresh egg masses across the entire field season, and Trissolcus sp. had a parasitism rate of 21% on fresh egg masses across the entire field season. Trissolcus mitsukurii had numerically the highest parasitism rate on frozen sentinel egg masses across the entire field season (69.6%).
Similarly, pooling the data showed that seasonality did not have a significant on the parasitoid species composition found on the sentinel H. halys egg masses (Z = 1.18; p = 0.24), but egg mass type did have a significant effect on species composition (Z = 2.93; p = 0.003) (Table 3). Trissolcus japonicus, T. cultratus, T. mitsukurii, and T. comperei all successfully emerged from both fresh and frozen sentinel egg masses (Table 4). Anastatus japonicus and Trissolcus sp. only emerged from fresh egg masses, and Ooencyrtus sp. only emerged from a frozen egg mass. Trissolcus japonicus was the only parasitoid recovered in the spring. The most abundant parasitoid from the fresh egg masses was T. japonicus in the summer (42.2%) and A. japonicus in the fall (58.8%). Across the entire field season, T. japonicus was the most abundant parasitoid on fresh egg masses (59.7%), followed by T. cultratus (20.3%). All other parasitoids made up under 10% of the species composition from fresh egg masses. Trissolcus japonicus was also the most abundant parasitoid on frozen egg masses cross the entire field season (49.0%), followed by T. cultratus (26.1%), then T. mitsukurii (21.6%). All other parasitoids made up under 5% of the species composition from frozen egg masses.
Developmental days prior to adult parasitoid emergence were the highest for Anastatus japonicus on fresh egg masses compared to all other parasitoid species (F = 5.599, p = 0.001) (Supp. Table 1).
Discussion
The results of this research elucidate the seasonal phenology and species composition of native parasitoids which target H. halys egg masses in Kyoto, Japan. Such information is essential for the future development and refinement of classical biological control programs against H. halys in invaded regions (Lara et al. 2019). The seasonal phenology of H. halys egg parasitoids, as measured by our study, peaks in mid-summer and ceases in early fall. The population activity trend demonstrated by the parasitoids is comparable to the seasonal population dynamics of H. halys in other parts of Japan, which also peaks in mid-summer and drops off in the early fall (Tsutsumi 2003; Kamiyama et al. 2021b). Similar to previous studies conducted in the native range of H. halys, T. japonicus demonstrated a high capacity for parasitizing H. halys (Yang et al. 2009; Zhang et al. 2017). The field parasitism rate of T. japonicus on fresh H. halys egg masses was determined to be 84% (Table 2), higher than the reported field parasitism rates found in China by Yang et al. (2009) (50%) and Zhang et al. (2017) (50−80%). Parasitoids T. cultratus and T. mitsukurii also displayed relatively high parasitism rates on the fresh sentinel H. halys egg masses throughout the field season (71% and 100%, respectively). Trissolcus cultratus has been documented parasitizing sentinel H. halys egg masses in Switzerland, although they were only able to emerge from dead and frozen eggs (Haye et al. 2015b).
Remarkably, the first parasitism activity from our assessment occurred on March 22 from a T. japonicus parasitized fresh sentinel H. halys egg mass, months before populations of H. halys in Japan are expected to become active (Tsutsumi 2003; Lee et al. 2013; Kamiyama et al. 2021b). This is the first record of successful parasitism by any parasitoid species on H. halys eggs as early as March. Field studies conducted in central Italy, northern China, and the southeastern USA invariably reported the first parasitism of H. halys in May (Roversi et al. 2016; Zhang et al. 2017; Tillman et al. 2020). These former surveys, however, did not begin sampling for parasitoids until May, meaning earlier parasitism is feasible in other regions. The minimum threshold temperature for T. japonicus development is estimated at 12.2 °C when reared on H. halys eggs (Yang et al. 2009), and 12.4 °C when reared on Glaucias subpunctatus eggs, another phytophagous Pentatomidae native to Japan (Kuki et al. 2019). March temperatures in Kyoto did meet this developmental temperature threshold; however, the second record of successful parasitism occurred on May 15, nearly two months after the first record. There was no sign of parasitoid activity between the first two parasitism records as no developing parasitoids were found from the dissections of the unemerged sentinel egg masses during this time, supporting the notion that the first female T. japonicus breaking diapause in the early spring was more of an anomaly.
The first parasitism in March resulted in both male and female progeny, evidencing the original female T. japonicus was mated prior parasitism (Kuki et al. 2019). It is unknown whether the female was mated prior to overwintering or after breaking diapause. Other insects such as leaf beetles and grasshoppers are able to mate before entering diapause and lay fertile eggs after overwintering without mating again in the spring (Stevens and McCauley 1989; Zhu et al. 2012b). Trissolcus biproruli (Hymenoptera: Scelionidae), a parasitoid of spined citrus bug, demonstrated successful parasitism up to three weeks following initial mating (James 1988). Furthermore, laboratory and semi-field experiments conducted by James (1988) revealed that adult T. biprorufi do not appear to enter a reproductive diapause while overwintering, and are capable of winter parasitism if exposed to suitable environmental conditions. Yet, further research investigating the overwintering habits of T. japonicus is necessary to confirm if the initial female T. japonicus parasitizes the sentinel H. halys egg mass without prior spring mating.
The parasitism rate on the fresh H. halys sentinel egg masses was significantly higher than the frozen egg masses in Kyoto, Japan, which differed from previous studies that reported similar parasitism rates between fresh and frozen sentinel egg masses in the southeastern USA, and higher rates on frozen than fresh in the eastern USA (Herlihy et al. 2016; Tillman et al. 2020). Additionally, field studies performed in Europe showed that native European parasitoids can successfully emerge from frozen H. halys egg masses, but fail to develop on fresh egg masses (Haye et al. 2015b; Roversi et al. 2016). Egg parasitoids must overcome defensive chemical compounds on the host’s eggs, as well as the immune response of the developing host embryo in order to achieve successful parasitism (Tognon et al. 2017). Parasitoids generally accomplish this by probing their ovipositor and injecting venom and/or symbiotic viruses into the host’s eggs (Strand et al. 1985; Zhu et al. 2018; Abram et al. 2019). Endemic parasitoids often lack the ability to overcome an invasive host’s defensive response to complete successful parasitism (Haye et al. 2015b; Roversi et al. 2016). However, cold-sterilization has been reported to disable a host’s natural defensive mechanisms, resulting in higher parasitism rates (Haye et al. 2015b). In the present study, T. cultratus had similar parasitism rates between fresh and frozen sentinel egg masses, whereas T. japonicus had a higher parasitism rate on fresh than frozen. Egg parasitoids have limited resources and a short time period when host eggs are viable for parasitism, so female parasitoids depend heavily on chemical volatiles from adult hosts and egg masses (Conti and Colazza 2012; Bertoldi et al. 2019; Malek et al. 2021). The suitability for parasitoid development decreases with egg mass freezing (Wong et al. 2020); however, the chemical effect of cold-sterilizing egg masses is currently unknown.
Trissolcus japonicus was the dominant early season parasitoid emerging from sentinel H. halys egg masses (100% of the total spring parasitoid species composition), indicating the parasitoid faces little competition or simply outcompetes other egg parasitoids in the spring. Over the course of the entire field season, the parasitoid species richness in Kyoto expands, as T. japonicus made up 59.7% of the overall parasitoid species complex from fresh egg masses, followed by T. cultratus (20.3%). Our results are similar to research done in central China, which determined the two most abundant parasitoids attaching fresh H. halys egg masses were T. japonicus (41% relative abundance) and T. cultratus (48% relative abundance) (Avila et al. 2021). Zhang et al. (2017) reported a H. halys parasitoid species composition in northern China dominated by T. japonicus (90% of total species composition), with no other parasitoid species reaching 5%. In the spring in Kyoto, the H. halys parasitoid species composition detected from our assessment is similar to the T. japonicus heavy distribution reported from northern China, whereas over the course of an entire field season, the species composition is more comparable to that of central China. Parasitoids from the genus Trissolcus dominate the overall parasitoid species composition in the native range of H. halys, but sentinel egg mass studies in invaded regions including Europe and the USA typically show more even distributions among Anastatus sp., Ooencyrtus sp., Telenomus sp., and Trissolcus sp. (Roversi et al. 2016; Abram et al. 2017; Dieckhoff et al. 2017; Zhang et al. 2017; Moraglio et al. 2020; Tillman et al. 2020; Avila et al. 2021). Regardless, the parasitism rate of H. halys egg masses by native parasitoid species in invaded regions is inconsequentially low, ranging between 2 and 7% (Talamas et al. 2015; Dieckhoff et al. 2017). Also, the invasive H. halys can act as an evolutionary trap for indigenous parasitoids in regions of recent invasion, as generalist parasitoids may unsuccessfully attempt to parasitize the eggs of the H. halys, leading to a decrease in the native egg parasitoid numbers (Abram et al. 2014; Costi et al. 2020). It is worth noting that in our study only one specimen per parasitized egg mass was sent in for molecular analysis, meaning H. halys egg masses that were potentially hyperparasitized by multiple species may have been overlooked (Konopka et al. 2017).
In the current study, there is evidence that factors from the field have a negative effect on the nymphal emergence rates of the fresh sentinel H. halys egg masses. Nymphal emergence rates of the fresh egg masses in the field were reduced by approximately 75% in comparison with the laboratory-reared controls. Studies which highlight the life cycle of H. halys denote the developmental temperature threshold for eggs to be within 15 °C and 33 °C (Nielsen et al. 2008). Overnight temperatures in March and April, as well as November and December, routinely fell below the lower-temperature developmental limit for eggs (Supp. Fig. 2), indicating that ambient temperature in Kyoto may have been the culprit for the low nymph emergence rates early and late in the season. However, when temperatures were maintained within the developmental threshold, nymphal emergence rates remained lower than the controls, suggesting different reasons for the reduced field hatch rates. We speculate biotic factors, aside from successful parasitism, as the main facilitator of the reduced H. halys field emergence. In the sentinel egg masses, unemerged eggs (neither H. halys nymphs or parasitoids emerged) were the most abundant on returned sentinel cards. One possible explanation for the high amount of unemerged eggs is that these eggs may have been visited by parasitoids and parasitism was attempted, but not successful in terms of producing progeny. Non-reproductive parasitism has commonly been observed in several groups of insects including Pentatomidae in field experiments (Abram et al. 2019; Kamiyama et al. 2019; Costi et al. 2020). Pseudoparasitism is one such phenomenon of parasitoid-induced host egg abortion, in which physical disruption and/or infection during the parasitism effort leads to host mortality, but ultimately no parasitoids emerge from the host egg (Abram et al. 2016, 2019). Although only about 1.5% of the unemerged sentinel eggs from our study revealed developing parasitoids, non-reproductive parasitism is often difficult to identify and quantify even through egg dissections (Jones et al. 2014; Cornelius et al. 2016).
This research reveals the native seasonal phenology and species composition of H. halys parasitoids in Kyoto, Japan, and offers support for a classical biological control approach as a form of management against the pest. Although this study highlights the high parasitism rate and abundance of T. japonicus, further research is still necessary to gain a better understanding of the phenology and behavior of parasitoids in the native range of H. halys, and how this may translate to invaded regions. We acknowledge the amount of caution and level of assuredness that must be attained before implementation of a classical biological control scheme. Therefore, we present these results to fill gaps in knowledge and supplement the groundwork for potential future programs in invaded regions.
Author contributions
MTK conceived and designed the research. MTK conducted the research and analyzed the data. MTK and CCSY wrote the manuscript. KM, TH, and CCYS secured funding for the research. All authors read and approved the manuscript.
Availability of data and material
All the sequences have been deposited in GenBank with accession numbers provided in the main text. All parasitoid samples can be made accessible upon request to the first and corresponding authors.
Code availability
All codes will be made available upon request.
References
Abram PK, Gariepy TD, Boivin G, Brodeur J (2014) An invasive stink bug as an evolutionary trap for an indigenous egg parasitoid. Biol Invasions 16:1387–1395. https://doi.org/10.1007/s10530-013-0576-y
Abram PK, Brodeur J, Burte V, Boivin G (2016) Parasitoid-induced host egg abortion: an underappreciated component of biological control services provided by egg parasitoids. Biol Control 98:52–60. https://doi.org/10.1016/j.biocontrol.2016.04.002
Abram PK, Hoelmer KA, Acebes-Doria A et al (2017) Indigenous arthropod natural enemies of the invasive brown marmorated stink bug in North America and Europe. J Pest Sci 90:1009–1020. https://doi.org/10.1007/s10340-017-0891-7
Abram PK, Brodeur J, Urbaneja A, Tena A (2019) Nonreproductive effects of insect parasitoids on their hosts. Annu Rev Entomol 64:259–276. https://doi.org/10.1146/annurev-ento-011118-111753
Arakawa R, Namura Y (2002) Effects of temperature on development of three Trissolcus spp. (Hymenoptera: Scelionidae), egg parasitoids of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Entomol Sci 5:215–218. https://doi.org/10.1303/aez.2004.177
Avila GA, Charles JG (2018) Modelling the potential geographic distribution of Trissolcus japonicus: a biological control agent of the brown marmorated stink bug, Halyomorpha halys. BioControl 63:505–518. https://doi.org/10.1007/s10526-018-9866-8
Avila GA, Chen J, Li W, Alavi M, Mi Q, Sandanayaka M, Zhang F, Zhang J (2021) Seasonal abundance and diversity of egg parasitoids of Halyomorpha halys in kiwifruit orchards in China. Insects 12:428. https://doi.org/10.3390/insects12050428
Bertoldi V, Rondoni G, Brodeur J, Conti E (2019) An egg parasitoid efficiently exploits cues from a coevolved host but not those from a novel host. Front Physiol 10:746. https://doi.org/10.3389/fphys.2019.00746
Charles JG, Avila GA, Hoelmer KA, Hunt S, Gardner-Gee R, MacDonald F, Davis V (2019) Experimental assessment of the biosafety of Trissolcus japonicus in New Zealand, prior to the anticipated arrival of the invasive pest Halyomorpha halys. Biocontrol 64:367–379. https://doi.org/10.1007/s10526-019-09949-x
Chen YM, Gibson GAP, Peng LF, Iqbal A, Zang LS (2019) Anastatus Motschulsky (Hymenoptera, Eupelmidae): egg parasitoids of Caligula japonica Moore (Lepidoptera, Saturniidae) in China. ZooKeys 881:109–134. https://doi.org/10.3897/zookeys.881.34646
Conti E, Colazza S (2012) Chemical ecology of egg parasitoids associated with true bugs. Psyche. https://doi.org/10.1155/2012/651015
Cornelius M, Dieckhoff C, Vinyard BT, Hoelmer KA (2016) Parasitism and predation on sentinel egg masses of the brown marmorated stink bug (Hemiptera: Pentatomidae) in three vegetable crops: importance of dissections for evaluating the impact of native parasitoids on an exotic pest. Environ Entomol 45:1536–1542. https://doi.org/10.1093/ee/nvw134
Costi E, Wong WHL, Cossentine J, Acheampong S, Maistrello L, Haye T, Talamas EJ, Abram PK (2020) Variation in levels of acceptance, developmental success, and abortion of Halyomorpha halys eggs by native North American parasitoids. Biol Control 151:104396. https://doi.org/10.1016/j.biocontrol.2020.104396
Dieckhoff C, Tatman KM, Hoelmer KA (2017) Natural biological control of Halyomorpha halys by native egg parasitoids: a multi-year survey in northern Delaware. J Pest Sci 90:1143–1158. https://doi.org/10.1007/s10340-017-0868-6
Evans HF, Speight MR (2004) Health and protection | Integrated pest management practices. In: Burley J (ed) Encyclopedia of forest sciences. Elsevier Ltd, Oxford, pp 318–333
Faúndez EI, Rider DA (2017) The brown marmorated stink bug Halyomorpha halys (Stål, 1855) (Heteroptera: Pentatomidae) in Chile. Arq Entomolóxicos 17:305–307
Fogain R, Graff S (2011) First records of the invasive pest, Halyomorpha halys (Hemiptera: Pentatomidae), in Ontario and Quebec. J Entomol Soc Ont 142:45–48
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Gariepy TD, Haye T, Fraser H, Zhang J (2014) Occurrence, genetic diversity, and potential pathways of entry of Halyomorpha halys in newly invaded areas of Canada and Switzerland. J Pest Sci 87:17–28. https://doi.org/10.1007/s10340-013-0529-3
Haye T, Gariepy T, Hoelmer K, Rossi J-P, Streito J-C, Tassus X, Desneux N (2015a) Range expansion of the invasive brown marmorated stinkbug, Halyomorpha halys: an increasing threat to field, fruit and vegetable crops worldwide. J Pest Sci 88:665–673. https://doi.org/10.1007/s10340-015-0670-2
Haye T, Fischer S, Zhang J, Gariepy T (2015b) Can native egg parasitoids adopt the invasive brown marmorated stink bug, Halyomorpha halys (Heteroptera: Pentatomidae), in Europe? J Pest Sci 88:693–705. https://doi.org/10.1007/s10340-015-0671-1
Haye T, Moraglio ST, Stahl J, Visentin S, Gregorio T, Tavella L (2020) Fundamental host range of Trissolcus japonicus in Europe. J Pest Sci 93:171–182. https://doi.org/10.1007/s10340-019-01127-3
Hedstrom C, Lowenstein D, Andrews H, Bai B, Wiman N (2017) Pentatomid host suitability and the discovery of introduced populations of Trissolcus japonicus in Oregon. J Pest Sci 90:1169–1179. https://doi.org/10.1007/s10340-017-0892-6
Herlihy MV, Talamas EJ, Weber DC, Falabella P (2016) Attack and success of native and exotic parasitoids on eggs of Halyomorpha halys in three Maryland habitats. PLoS ONE 11(3):e0150275. https://doi.org/10.1371/journal.pone.0150275
Hirashima Y, Yamagishi K (1981) Redescriptions of the types of some Japanese Scelionidae preserved in the United States National Museum (Hymenoptera, Proctotrupoidea). J Fac Agr Kyushu Univ 25:153–159.https://doi.org/10.5109/23726
Hoddle MS (2004) Restoring balance: using exotic species to control invasive exotic species. Conserv Biol 18:38–49. https://doi.org/10.1111/j.1523-1739.2004.00249.x
Hoebeke ER, Carter ME (2003) Halyomorpha halys (Stål) (Heteroptera: Pentatomidae): a polyphagous plant pest from Asia newly detected in North America. Proc Entomol Soc Wash 105:225–237
Hull LA, VanStarner R (1983) Impact of four synthetic pyrethroids on major natural enemies and pests of apple in Pennsylvania. J Econ Entomol 76:122–130. https://doi.org/10.1093/jee/76.1.122
James DG (1988) Fecundity, longevity, and overwintering of Trissolcus biproruli Girault (Hymenoptera: Scelionidae) a parasitoid of Biprorulus bibax Breddin (Hemiptera: Pentatomidae). J Aust Ent Soc 27:297–301. https://doi.org/10.1111/j.1440-6055.1988.tb01177.x
Jones AL, Jennings DE, Hooks CRR, Shrewsbury PM (2014) Sentinel eggs underestimate rates of parasitism of the exotic brown marmorated stink bug Halyomorpha halys. Biol Control 78:61–66. https://doi.org/10.1016/j.biocontrol.2014.07.011
Kamiyama MT, Schreiner Z, Guédot C (2019) Diversity and abundance of natural enemies of Drosophila suzukii in Wisconsin, USA fruit farms. BioControl 64:665–676. https://doi.org/10.1007/s10526-019-09966-w
Kamiyama MT, Matsuura K, Yoshimura T, Yang CCY (2021a) Predation of the brown marmorated stink bug, Halyomorpha halys by the Japanese acrobat ants, Crematogaster matsumurai and Crematogaster osakensis. Biol Control. https://doi.org/10.1016/j.biocontrol.2021.104570
Kamiyama MT, Matsuura K, Yoshimura T, Yang CCY (2021b) Improving invasive species management using predictive phenology models: an example from brown marmorated stink bug (Halyomorpha halys) in Japan. Pest Manag Sci. https://doi.org/10.1002/ps.6589
Kaser JM, Akotsen-Mensah C, Talamas EJ, Nielsen AL (2018) First Report of Trissolcus japonicus parasitizing Halyomorpha halys in North American agriculture. Fla Entomol 101:680–683. https://doi.org/10.1653/024.101.0406
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170. https://doi.org/10.1016/S0169-5347(02)02499-0
Konopka JK, Haye T, Gariepy T, Mason P, Gillespie D, McNeil JN (2017) An exotic parasitoid provides an invasional lifeline for native parasitoids. Ecol Evol 7:277–284. https://doi.org/10.1002/ece3.2577
Kriticos DJ, Kean JM, Phillips CB, Senay SD, Acosta H, Haye T (2017) The potential global distribution of the brown marmorated stink bug, Halyomorpha halys, a critical threat to plant biosecurity. J Pest Sci 90:1033–1043. https://doi.org/10.1007/s10340-017-0869-5
Kuhar TP, Kamminga KL, Whalen J, Dively GP, Brust G, Hooks CRR, Hamilton G, Herbert DA (2012) The pest potential of brown marmorated stink bug on vegetable crops. Plant Health Prog. https://doi.org/10.1094/PHP-2012-0523-01-BR
Kuhar TP, Kamminga K (2017) Review of the chemical control research on Halyomorpha halys in the USA. J Pest Sci 90:2021–2031. https://doi.org/10.1007/s10340-017-0859-7
Kuki D, Wada Y, Tsunashima A, Itoyama K (2019) Developmental properties and parasitism capacity of the egg parasitoid Trissolcus japonicus (Hymenoptera: Scelionidae), reared on eggs of Glaucias subpunctatus (Hemiptera: Pentatomidae). Appl Entomol Zool 54:307–312. https://doi.org/10.1007/s13355-019-00627-z
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lara JR, Pickett CH, Kamiyama MT, Figueroa S, Romo M, Cabanas C, Bazurto V, Strode V, Briseno K, Lewis M, Oliva J, Hernandez G, Hoddle MS (2019) Physiological host range of Trissolcus japonicus in relation to Halyomorpha halys and other pentatomids from California. Biocontrol 64:513–528. https://doi.org/10.1007/s10526-019-09950-4
Lee D-H, Short BD, Joseph SV, Bergh JC, Leskey TC (2013) Review of the biology, ecology, and management of Halyomorpha halys (Hemiptera: Pentatomidae) in China, Japan, and the Republic of Korea. Environ Entomol 42:627–641. https://doi.org/10.1603/EN13006
Lee D-H, Short BD, Nielsen AL, Leskey TC (2014) Impact of organic insecticides on the survivorship and mobility of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) in the laboratory. Fla Entomol 97:414–421. https://doi.org/10.1653/024.097.0211
Leskey TC, Short BD, Butler BR, Wright SE (2012a) Impact of the invasive brown marmorated stink bug, Halyomorpha halys (Stål), Mid-Atlantic tree fruit orchards in the United States: case studies of commercial management. Psyche 2012:1–14. https://doi.org/10.1155/2012/535062
Leskey TC, Lee D-H, Short BD, Wright SE (2012b) Impact of insecticides on the invasive Halyomorpha halys (Hemiptera: Pentatomidae): analysis of insecticide lethality. J Econ Entomol 105:1726–1735. https://doi.org/10.1603/EC12096
Malek R, Kaser JM, Anfora G, Ciolli M, Khrimian A, Weber DC, Hoelmer KA (2021) Trissolcus japonicus foraging behavior: Implications for host preference and classical biological control. Biol Control. https://doi.org/10.1016/j.biocontrol.2021.104700
Messing RH, Wright MG (2006) Biological control of invasive species: solution or pollution. Front Ecol Environ 4:132–140. https://doi.org/10.1890/1540-9295(2006)004[0132:BCOISS]2.0.CO;2
Meteoblue (2021) Weather archive Uji In: https://www.meteoblue.com/en/weather/ historyclimate/climatemodelled/uji_japan_1849372. Accessed 20 Jan 2021
Milnes JM, Beers EH (2019) Trissolcus japonicus (Hymenoptera:Scelionidae) causes low levels of parasitism in three North American pentatomids under field conditions. J Insect Sci 19:4. https://doi.org/10.1093/jisesa/iez074
Moraglio ST, Tortorici F, Pansa MG, Castelli G, Pontini M, Scovero S, Visentin S, Tavella L (2020) A 3-year survey on parasitism of Halyomorpha halys by egg parasitoids in northern Italy. J Pest Sci 93:183–194. https://doi.org/10.1007/s10340-019-01136-2
Morrison WR, Mathews CR, Leskey TC (2016) Frequency, efficiency, and physical characteristics of predation by generalist predators of brown marmorated stink bug (Hemiptera: Pentatomidae) eggs. Biol Control 97:120–130. https://doi.org/10.1016/j.biocontrol.2016.03.008
Nielsen AL, Hamilton GC, Matadha D (2008) Developmental rate estimation and life table analysis for Halyomorpha halys (Hemiptera: Pentatomidae). Environ Entomol 37:348–355. https://doi.org/10.1093/ee/37.2.348
Nielsen AL, Hamilton GC (2009) Seasonal occurrence and impact of Halyomorpha halys (Hemiptera: Pentatomidae) in tree fruit. J Econ Entomol 102:1133–1140. https://doi.org/10.1603/029.102.0335
Nielsen AL, Holmstrom K, Hamilton GC, Cambridge J, Ingerson-Mahar J (2013) Use of black light traps to monitor the abundance, spread, and flight behavior of Halyomorpha halys (Hemiptera: Pentatomidae). J Econ Entomol 106:1495–1502. https://doi.org/10.1603/EC12472
Ogburn EC, Bessin R, Dieckhoff C, Dobson R, Grieshop M, Hoelmer KA, Mathews C, Moore J, Nielsen AL, Poley K, Pote JM, Rogers M, Welty C, Walgenbach JF (2016) Natural enemy impact on eggs of the invasive brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), in organic agroecosystems: a regional assessment. Biol Control 101:39–51. https://doi.org/10.1016/j.biocontrol.2016.06.002
Pimentel D, Lach L, Zunigia R, Morrison D (2000) Environmental and economic costs of nonindigenous species in the United States. Bioscience 50:53–65. https://doi.org/10.1641/0006-3568(2000)050[0053:EAECON]2.3.CO;2
Pote JM, Nielsen AL (2017) Life stage specific predation of Halyomorpha halys (Stål) by generalist predators. Biol Control 114:1–7. https://doi.org/10.1016/j.biocontrol.2017.07.007
R Core Team (2018) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing
Rice KB, Bergh CJ, Bergmann EJ, Biddinger DJ, Dieckhoff C, Dively G, Fraser H, Gariepy T, Hamilton G, Haye T, Herbert A, Hoelmer K, Hooks CR, Jones A, Krawczyk G, Kuhar T, Martinson H, Mitchell W, Nielson AL, Pfeiffer DG, Raupp MJ, Rodrigues-Saona C, Shearer P, Shrewsbury P, Venugopal PD, Whalen J, Wiman NG, Leskey TC, Tooker JF (2014) Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J Integr Pest Manag 5:A1–A13. https://doi.org/10.1603/IPM14002
Roversi PF, Binazzi F, Marianelli L, Costi E, Maistrello L, Sabbatini-Peverieri G (2016) Searching for native egg-parasitoids of the invasive alien species, Halyomorpha halys Stål (Heteroptera Pentatomidae) in Southern Europe. Redia 99:63–70. https://doi.org/10.19263/REDIA-99.16.01http://dx.doi.org/
Saunders TE, Avila GA, Holwell GI (2021) Pre-emptive host-specificity testing of Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae) reveals high parasitism levels against the endemic New Zealand alpine shield bug in laboratory no-choice tests. Austral Entomol 60:455–460. https://doi.org/10.1111/aen.12532
Stevens L, McCauley DE (1989) Mating prior to overwintering in the imported willow leaf beetle, Plagiodera versicolora (Coleoptera: Chrysomelidae). Ecol Entomol 14:219–223. https://doi.org/10.1111/j.1365-2311.1989.tb00772.x
Strand MR, Quarles JM, Meola SM, Vinson SB (1985) Cultivation of teratocytes of the egg parasitoid Telenomus heliothidis (Hymenoptera: Scelionidae). In Vitro Cell Dev Biol 21:361–367. https://doi.org/10.1007/BF02623466
Talamas EJ, Herlihy MV, Dieckhoff C, Hoelmer KA, Buffington ML, Bon M-C, Weber DC (2015) Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. J Hymenopt Res 43:119–128. https://doi.org/10.3897/JHR.43.4661
Talamas EJ, Buffington ML, Hoelmer K (2017) Revision of Palearctic Trissolcus Ashmead (Hymenoptera, Scelionidae). J Hymenopt Res 56:3–185. https://doi.org/10.3897/jhr.56.10158
Tillman G, Toews M, Blaauw B, Sial A, Cottrell T, Talamas E, Buntin D, Joseph S, Balusu R, Fadamiro H, Lahiri S, Patel D (2020) Parasitism and predation of sentinel eggs of the invasive brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), in the southeastern US. Biol Control. https://doi.org/10.1016/j.biocontrol.2020.104247
Tognon R, Aldrich JR, Buffington ML, Talamas EJ, Sant’Ana J, Zalom FG (2017) Halyomorpha halys (Heteroptera: Pentatomidae) egg surface chemicals inhibit North American Telenomus and Trissolcus (Hymenoptera: Scelionidae) parasitism. Biol Control 114:39–44. https://doi.org/10.1016/j.biocontrol.2017.07.014
Tsutsumi T (2003) Fruit tree stink bug- interesting ecology and clever prevention. Rural Culture Association, Tokyo (In Japanese, ISBN-10 is 4540021257)
Wermelinger B, Wyniger D, Forster B (2008) First records of an invasive bug in Europe: Halyomorpha halys Stal (Heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Bull Soc Entomologique Suisse 81:1–8
Wong WHL, Walz MA, Oscienny AB, Sherwood JL, Abram PK (2020) An effective cold storage method for stockpiling Halyomorpha halys (Hemiptera: Pentatomidae) eggs for field surveys and laboratory rearing of Trissolcus japonicus (Hymenoptera: Scelionidae). J Econ Entomol 114:571–581. https://doi.org/10.1093/jee/toaa307
Yang Z-Q, Yao Y-X, Qiu L-F, Li Z-X (2009) A new species of Trissolcus (Hymenoptera: Scelionidae) parasitizing eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with comments on its biology. Ann Entomol Soc Am 102:39–47. https://doi.org/10.1603/008.102.0104
Yonow T, Kriticos DJ, Ota N, Avila GA, Hoelmer KA, Chen H, Caron V (2021) Modelling the potential geographic distribution of two Trissolcus species for the brown marmorated stink bug Halyomorpha halys. Insects 12:491. https://doi.org/10.3390/insects12060491
Zhang YZ, Wie L, Huang DW (2005) A taxonomic study of Chinese species of Ooencyrtus insecta: Hymenoptera: Encyrtidae). Zool Stud 44:347–360
Zhang J, Zhang F, Gariepy T, Mason P, Gillespie D, Talamas E, Haye T (2017) Seasonal parasitism and host specificity of Trissolcus japonicus in northern China. J Pest Sci 90:1127–1141. https://doi.org/10.1007/s10340-017-0863-y
Zhu G, Bu W, Gao Y, Liu G (2012a) Potential geographic distribution of brown marmorated stink bug invasion (Halyomorpha halys). PloS ONE 7(2):e31246. https://doi.org/10.1371/journal.pone.0031246
Zhu D-H, Cui S-S, Fan YS, Liu Z (2012b) Adaptive strategies of overwintering adults: reproductive diapause and mating behavior in a grasshopper, Stenocatantops splendens (Orthoptera: Catantopidae). Insect Sci 20:235–244. https://doi.org/10.1111/j.1744-7917.2011.01493.x
Zhu F, Cusumano A, Bloem J, Weldegergis BT, Villela A, Fatouros NE, van Loon JJA, Dicke M, Harvey JA, Vogel H, Poelmea EH (2018) Symbiotic polydnavirus and venom reveal parasitoid to its hyperparasitoids. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1717904115
Acknowledgements
We express the deepest gratitude and sympathy to the family, friends, colleges, and collaborators of Dr. Tsuyoshi Yoshimura from Kyoto University, a co-author who tragically passed away during this research. We would like to thank the Earth Corporation Ako Plant and the Hohto Shoji Co. for providing funding (to CCSY) as well as insects for this research. We also thank Prof. Chow-Yang Lee from University of California, Riverside, for supplying essential tools for our field work. Funding was also provided by the Japanese Government Monbukagakusho (MEXT) (to MTK) Scholarship and the Virginia Tech Faculty Start-up Research Fund (to CCSY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors report no potential conflict of interest.
Additional information
Communicated by Tim Haye.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kamiyama, M.T., Matsuura, K., Hata, T. et al. Seasonal parasitism of native egg parasitoids of brown marmorated stink bug (Halyomorpha halys) in Japan. J Pest Sci 95, 1067–1079 (2022). https://doi.org/10.1007/s10340-021-01455-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-021-01455-3