Abstract
Characterizing the olfactory responses of insect pests is critical for developing biological control options and pest management strategies in the field. Such responses form the basis for evaluating interactions between plants and insects, as well as providing evidence to support the use of non-crop plant types in pest suppression tactics. To evaluate the potential aversion or attraction of Bemisia tabaci MED/Q to volatiles from various plants, behavioral responses of adult whitefly were observed in Y-type olfactometer tests (n = 30 individuals per trial, with 3 replicate trials per treatment combination; n = 4230 individuals in total). We quantified the potential repellent effects of the essential oils of Thymus pulegioides (‘thyme’) and Artemisia absinthium (‘wormwood’) on B. tabaci MED/Q. The essential oil of T. pulegioides as well as three of the four major subcomponents tested (thymol 36.18%, p-Cymene 10.85% and thymol methyl ether 7.45%, but not Carvacrol 13.43%) had significant repellent effects on B. tabaci MED/Q. Similarly, the essential oil of A. absinthium, as well as the two major subcomponents tested (Linalool 23.41% and (−)-β-Pinene 27.88%), had marginally significant repellent effects on B. tabaci MED/Q. In tests across increasing concentrations of these volatile compounds, repellent effects were typically only significant at the two highest concentrations tested. Overall, these results demonstrate that major constituents of certain aromatic plant oils have a strong repellent effect and contact toxicity on B. tabaci MED/Q. These findings have important implications for more environmentally friendly biological control options using aromatic plants to repel target pests in production crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Key message
-
Aromatic plants thyme and wormwood have potential to be effective repellents for whiteflies.
-
The constituents of essential oils had repellent activity and contact toxicity on whiteflies.
-
The results provide new aromatic plant products for environmentally friendly pest control.
Introduction
Plant volatiles provide an important basis for host selection by herbivorous insects, and during the course of evolution, plants have produced repellent volatile substances to deter feeding damage (De Moraes et al. 2001; Paré and Tumlinson 1999). With recent advances in phytochemical separation and purification technology (Altemimi et al. 2017), the potential use of plant essential oils for pest management has attracted increasing attention (Chaieb et al. 2018; Benelli et al. 2019a; Benelli et al. 2019c; Ikbal and Pavela 2019; Soares et al. 2019; Peres et al. 2020; Shah et al. 2020), particularly in the context of feeding-deterrent mechanisms for serious agricultural pests (Isman 2020). Insect olfactory responses to plant volatiles will be helpful in testing the viability of using non-crop plant types in pest suppression tactics.
The silverleaf whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), is one of the most devastating agricultural pests worldwide, primarily in tropical and subtropical habitats. It not only causes direct feeding damage from sap sucking, but also causes indirect damage as an effective vector of up to 100 plant pathogenic viruses (Taggar and Gill 2016). The highly invasive B. tabaci Mediterranean (MED) form, also known as biotype Q or MED/Q, predominates in many regions of the world and causes severe crop damage and economic losses in countries such as China and India (Kumar et al. 2019; Rao et al. 2011). Bemisia tabaci feeds mainly on the undersides of crop leaves, where it is not readily affected by conventional spraying of insecticides. Consequently, farmers must use excessive applications of chemical insecticides in order to control populations. As a result, there have been widespread reports of insecticide resistance in B. tabaci MED/Q in recent years that have weakened the prospects for effective management using conventional practices (Yao et al. 2017; Zheng et al. 2017). There is currently a urgent need to identify effective natural alternative products and environmentally friendly pest control methods, with a lower risk to the environment and non-target species than the chemical pesticides widely used worldwide (Desneux et al. 2007; Khederi et al. 2019). The application of aromatic plant oils has been studied extensively for use in pest control, including their application against B. tabaci MED/Q, as potential alternatives to synthetic pesticides. Using these aromatic plants as a component of habitat manipulation strategies within agroecosystems has been identified as one of the most effective solutions in conservation biological control (CBC) (Carvalho et al. 2017; Gurr et al. 2017; Hatt et al. 2019; Khan et al. 2008).
Thymus pulegioides L. (Lamiaceae) is widely distributed in Mediterranean regions of Europe and typically occurs in meadows and on undisturbed soils. This species is less commercially important than other thyme species, such as T. vulgaris and T. zygis (Lawrence 1992). However, it has known insect repellent properties, with one constituent thymol being an active ingredient in pesticide products registered for use as insecticides, animal repellents and fungicides (Ansari et al. 2010; Bekircan et al. 2014; Park et al. 2017). Moreover, T. pulegioides has been used for its antiseptic and anti-inflammatory properties and has been reported to have antioxidant action, lipid peroxidation inhibition and free radical scavenging activity (Fernandes et al. 2010). Similarly, Artemisia species (Asteraceae) are known for their pharmacological, repellent, antifeedant and insecticidal properties (Bachrouch et al. 2015; Negahban et al. 2006, 2007; Schmitz 1999). Artemisia absinthium L., is a small perennial shrub that has been used as an herbal medicine throughout Europe, North America, Asia and South Africa (He et al. 2009). A. absinthium oil exhibited strong fumigant toxicity against Rhyzopertha dominica (Coleoptera: Bostrichidae) adults, a stored product pest and high fumigant activity against Spodoptera littoralis (Lepidoptera: Noctuidae), a greenhouse pest (Dhen et al. 2014). Previous studies have shown the repellent and anti-ovipositional effects of the essential oils of T. vulgaris and A. camphorata on several species of whitefly including Trialenrodes vaporariorum (Westwood), B. tabaci (MEAM1, also called biotype B) and Aleuroclava jasmini (Takahashi) (Aroiee et al. 2005; Baldin et al. 2013; Khederi et al. 2019; Yang et al. 2010). A. absinthium oil possesses antitrypanosomal, antiplasmodial, analgesic and antidepressant and antioxidant (Tariku et al. 2011).
Given the importance and widespread availability of these two plant species, and their potential use in conservation biological control, the primary objective of our research was to evaluate the repellent effectiveness of plant volatile extracts of T. pulegioides and A. absinthium, as well as their main constituent chemical components, and contact toxicity against B. tabaci MED/Q. We then tested the sensitivity of B. tabaci responses to varying concentrations of the major volatile components. Such information will be important in the context of developing more environmentally friendly pest management strategies for B. tabaci MED/Q.
Materials and methods
Plant material
The T. pulegioides plants used for the extraction of essential oils were collected from a commercial grower at Hulun Buir State Farm (49º12′ N, 119º73′ E, Hulun Buir, China). The A. absinthium plants were collected from the aromatic plant garden at the Institute of Botany, Chinese Academy of Sciences (Beijing, China).
Insect collection and rearing
A population of B. tabaci was supplied by the Institute of Plant & Environment Protection, Beijing Academy of Agriculture and Forestry Science. They were reared on cotton (Gossypium hirsutum L. var. ‘Shiyuan 321’) seedlings in ventilated mesh cages in a greenhouse compartment (26 ± 2 ℃, RH 60 – 70%, L:D = 14:10 photoperiod). The biotype of the B. tabaci population was confirmed using a cleaved-amplified polymorphic sequence marker for cytochrome oxidase I (mtCOI), as described by Khasdan et al. (Khasdan et al. 2005). PCR amplification was performed using primers C1-J-2159 and L2-N-3014 (Frohlich et al. 1999), which was followed by digestion with VspI, generating a clear polymorphism biotype MED/Q.
Extraction of essential oils, gas chromatography and mass spectrometry
When flowering (mid-July 2016 for T. pulegioides and late-August 2016 for A. absinthium) the aboveground parts of plants (including flowers, stems and leaves) were collected, air-dried and finely ground and subjected to 3 h steam distillation using a Clevenger-type apparatus (British type), according to the procedure described in the European Pharmacopoeia (Anonymous 1996) to produce the essential oils in a yield of 0.27% and 0.67% (v/w) based on the dry weight of the samples from T. pulegioides and A. absinthium, respectively. The distilled essential oils were dried over anhydrous sodium sulphate, and after filtration, stored in sealed vials at 4℃ until further analysis.
The essential oils were analyzed by gas chromatography–mass spectrometry (GC–MS) techniques. GC–MS analysis was carried out using a gas chromatograph Agilent-Technologies 7890 N (Agilent-Technologies, Palo Alto, CA, the USA) equipped with a split-splitless injector, Agilent-Technologies 7683 autosampler and an Agilent HP5-MS fused silica column (5% phenyl-methylpolysiloxane, 30 m × 0.25 mm i.d., film thickness 0.25 μm). Injector temperature was 250℃, and the heating program was as follows: 40 °C for 2 min, linear ramp at a rate of 4 ℃ min−1 to 260 ℃, and then ramp to 310 ℃ at 60 ℃ min−1, before being held at 310℃ for 15 min. The transfer line temperature was 280 ℃. Helium was used as carrier gas at a flow rate of 1.0 mL min−1 through the column. Samples were diluted in hexane with a ratio of 1:100, and 1 μL was injected in the split mode (1:20). The GC was fitted with a quadrupole mass spectrometer with Agilent-Technologies 5973 detector. The MS conditions were as follows: ionization energy, 70 eV; electronic impact ion source temperature, 200 °C; quadrupole temperature, 150 °C; mass range, 40–600 u. Agilent masshunter 5.0 was used to analyze the mass spectra and chromatograms.
Identification of components
Most constituents of the essential oils were identified by comparison of their linear retention indices (RIs) with values in the literature. The linear retention indices were determined by injection of a hexane solution containing the C7–C40 series of n-alkanes under the same operating conditions. Further identification was made by comparison of their mass spectra with either those stored in the mass spectra databases (NIST, ver. 8.0 and Wiley 275) or with mass spectra from a home-made library. The relative concentrations of components were obtained by peak area normalization. No response factors were calculated.
Y-tube olfactometer behavioral experiments
The experiments were conducted in a dark laboratory at 26 ± 2 ℃, RH 70 ± 5%. In all experiments, the responses of the whiteflies to volatiles were tested in a closed system Y-tube olfactometer (internal diameter, 2.5 cm; stem length, 15 cm; arm lengths, 10 cm at 75° angle) (Takabayashi and Dicke 1992). A 20 W warm yellow LED located above the device provided uniform lighting. Two streams of purified air filtered through activated charcoal were each blown through a 100 mL glass container and into the arms of the olfactometer at 100 mL min−1. The base of the olfactometer was connected to a house vacuum at 200 mL min−1. Prior to the introduction of each whitefly, a 1 × 2 cm rectangular filter paper impregnated with 1 µL of either the treatment chemical or a solvent control solution (paraffin) was inserted into each Y-tube arm odor source container. Whiteflies were starved for 4 h in advance of the olfactometer test, and one adult was individually introduced at the basal end of the Y-tube. Each whitefly adult was used only once in a single test. Whitefly behavior was observed, and the odor choice was recorded when it passed a marker point half way up either one of the arms of the Y-tube. A ‘no choice’ was recorded when the whitefly did not reach the marker point within 5 min, and these data were not used in analyses. Whiteflies were tested until 30 successful choice tests had been completed for each treatment comparison. Odor sources were interchanged between the arms of the Y-tube after every five whiteflies testing, in order to account for the influence of unforeseen asymmetric aspects of the Y-tube setup. The olfactometer and glass containers were cleaned and rinsed with EtOH and deionized H2O after each set of 30 successful choice tests had been completed.
Odor sources
New filter papers with odor sources or paraffin were used for each individual whitefly. Odor sources in the experiments included the extracted essential oils of T. pulegioides and A. absinthium, as well as identified subcomponents of each essential oil: thymol 36.18%, carvacrol 13.43%, p-Cymene 10.85% and thymol methyl ether 7.45% were the main components of the essential oil of T. pulegioides. The main components of essential oil A. absinthium include (−)-β-Pinene 27.88% and Linalool 23.41%. Five concentrations (0.01, 0.1, 1, 10 and 100 µg) of thymol, carvacrol, p-Cymene, or linalool dissolved in 0.1 mL paraffin were applied in behavioral experiments. Similarly, 0.01, 0.1, 1, 10 and 100µL of thymol methyl ether, or (−)-β-Pinene diluted in 0.1 mL paraffin were used in the behavioral experiments. All compounds were commercially available from Sigma-Aldrich. Each experiment (odor source vs control) was repeated three times (i.e., 3 × 30 starved whiteflies) for a particular concentration of odor source on three different experimental days, with a new whitefly tested on each occasion (4230 successful whitefly choice tests in total).
Contact toxicity assays
The bioefficacy of essential oil components against adults of B. tabaci was evaluated on following the methods of Saad et al. 2017. The essential oils were diluted in paraffin, to achieve a concentration of 0.01, 0.1 and 0.5 (ml/L total volume). Fresh leaves from tomato plant were secured with cotton swab immersed in 10% sucrose solution and agar and put in the transparent 300-mL plastic cup. Each solution was applied to filter paper (7-cm diam) by using a micropipette, with 5 µl of essential oil, or with paraffin (control 1) or with distilled water (control 2).The filter paper was dried under a ventilated hood before attaching to the inside of a 7-cm culture plate. Nearly 20 adult whiteflies were introduced into cup, and the culture plate with the treated filter paper was covered. B. tabaci adult mortality was recorded after 1 h, 3 h, 5 h and 24 h later. (When no leg or antennal movements were observed, the insect was considered dead.) Treatment concentrations were replicated 12 times.
Statistical analysis
We tested B. tabaci MED/Q responses to the essential oil of T. pulegioides (and its four major subcomponents: thymol 36.18%, p-Cymene 10.85%, carvacrol 13.43% and thymol methyl ether 7.45%) as well as the essential oil of A. absinthium (and its two major subcomponents of (−)-β-Pinene 27.88% and linalool 23.41%) and compared these to the control response for paraffin alone, using a generalized linear mixed effects model (GLMM) in the ‘glmmTMB’ package (Brooks et al. 2017) in R 3.6.2 (R Core Team, 2019). We specified a binomial error structure (with logit link function) and specified random effects in the model for ‘Trial’ (to account for potential non-independence of replicate individuals tested in the same batch of 30 test runs), ‘Date’ (to account for potential non-independence of multiple cohorts of individuals tested on the same day), and ‘Side’ (to account for idiosyncratic differences in lighting or other conditions on different sides of the Y-tube arms).
The same model specification was used for testing B. tabaci MED/Q responses to increasing concentrations of volatile compounds (0, 0.01, 0.1, 1, 10 and 100 µg or µL) for thymol, p-Cymene, carvacrol, thymol methyl ether, (−)-β-Pinene and linalool, except in this case fixed effects were specified in the model for the interaction between odor treatment and concentration.
The contact toxicity of essential oils on mortality of B. tabaci adult was analyzed by two-way repeated measures on general linear model. Means were separated when F text was significant on the basis of Tukey’s test.
Results
B. tabaci responds to the essential oils of T. pulegioides and A. absinthium
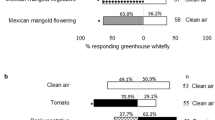
When whiteflies were given the choice between a no odor control versus the odor of either one of the two essential oils, they significantly preferred the no odor control (Fig. 1, binomial GLMM, T. pulegioides z = −4.621, P < 0.001; A. absinthium z = −5.231, P < 0.001). In both cases, ca 80% of whiteflies tested were repelled by the volatile oils of T. pulegioides and A. absinthium (Fig. 1).
Bemisia tabaci responses in Y-tube olfactometer tests to the odors of essential oils of T. pulegioides (including its four main constituents: thymol, carvacrol, p-Cymene and thymol methyl ether) and A. absinthium (including its two main constituents: (−)-β-Pinene and linalool) compared with control (paraffin) (ns, non-significant difference P > 0.1; • P < 0.1; *P < 0.05; **P < 0.01; ***P < 0.001). The color’s shades are for the different significant level. Green for T. pulegioides and blue for A. absinthium
Chemical composition of the essential oils
A total of 44 different volatile compounds were detected in the essential oil of T. pulegioides (Table 1) and 33 volatile compounds in the essential oil of A. absinthium (Table 2). Of these, thymol (36.18%) was the main constituent of T. pulegioides essential oil, followed by carvacrol (13.43%), p-Cymene (10.85%) and thymol methyl ether (7.45%). For A. absinthium, the major constituents were (−)-β-Pinene (27.88%) and linalool (23.41%).
B. tabaci responds to the major volatile components within the essential oils of T. pulegioides and A. absinthium
Of the four major constituents of T. pulegioides oil, at their naturally occurring concentrations, only thymol and thymol methyl ether showed highly significant repellent action (> 70% of white flies repelled; z = −3.783 P < 0. 001 and −3.259, P < 0.01), whereas p-Cymene had weaker, but significant, repellent effects (ca 65% of whiteflies repelled, z = −2.546 P < 0. 05), while carvacrol had no significant effect relative to the solvent control (Fig. 1).
For the two major constituents of A. absinthium oil, at their naturally occurring concentrations, both (−)-β-Pinene and linalool had marginally significant repellent effects (ca 60% of whiteflies repelled, z = −1.823 and z = −2.004, P < 0. 05), but at a much lower level of repellent action than the essential oil of A. absinthium (Fig. 1).
Effects of varying concentrations of volatile components on B. tabaci MED/Q
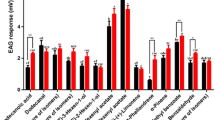
The single-compound assays across an odor concentration gradient produced significant repellent effects on B. tabaci in all compounds except carvacrol (Fig. 2b). For components of T. pulegioides oil, responses were dose-dependent, and repellent action occurred at moderate to high concentrations of 1–100 µg for thymol, and at high concentrations of 10–100 µg for p-Cymene and thymol methyl ether (Fig. 2a, c, d). At the highest concentrations > 75–80% of whitefly were repelled, comparable to the essential oil effect for T. pulegioides (Fig. 1).
Bemisia tabaci responses in Y-tube olfactometer tests to varying concentrations of odors of the major volatile components from T. pulegioides (A–D) and A. absinthium oil (E, F) compared with control (paraffin; indicated as 0 μg or μL concentration) (ns, non-significant difference P > 0.1 F; •P < 0.1; *P < 0.05; **P < 0.01; ***P < 0.001)
For components of A. absinthium oil, significant repellant effects were only seen at the highest concentration of 100 µg, for both (−)-β-Pinene and linalool (Fig. 2e, f). In particular, almost 75% of whiteflies were repelled at the highest concentration of (−)-β-Pinene, which is comparable to the essential oil effect for A. absinthium (Fig. 1). There was a weak, but non-significant, indication that whiteflies were attracted to the lowest odor dose of 0.01 µL linalool (62.22% of whiteflies attracted, z = 1.648, P < 0.10; Fig. 2f).
Contact toxicity against B. tabaci adults
Observed mortality 20 h after exposure to the T. pulegioides oils at concentrations of 0.01, 0.1 and 0.5 ml/L caused adult mortality of 53.39%, 69.18% and 84.02%, differed significantly with water and paraffin (P < 0.05) (Table 3). The does 0.01, 0.1 and 0.5 ml/L of A. absinthium oil was statistically similar with T. pulegioides oil, killed 53.47%, 63.62% and 80.23% adults, respectively (P < 0.05), when compared to the control (water and paraffin). As time goes on, the mortality of B. tabaci adults showed a significant increase when exposed to two essential oils at three concentrations (FT. pulegioides = 282.63, FA. absinthium = 99.49, all df = 4, 55, P < 0.001) (Table 4).
Discussion
Phytochemistry of aromatic essential oils
The essential oils of certain plant species can repel insect pests on crops, but their efficacy varies according to the phytochemical profile of the plant extract (Regnault-Roger et al. 2012). For example, different chemotypes of T. vulgaris have been shown to have different toxicities against the bruchid bean weevil Acanthoscelides obtectus (Regnault-Roger et al. 1993). Similarly, the curculionid maize weevil Sitophilus zeamais was found to be more sensitive to dominant compounds of farnesol (chemotype 1) or springene plus beta-farnesene (chemotype 2) from ‘fish-poison bean’ Tephrosia vogelii (Fabaceae), rather than to a mixture of the two chemotypes together (Kerebba et al. 2020). Therefore, the benefits of growing repellent plants or applying essential oil extracts can be highly variable in a crop production system depending on the chemotype used. In a management context, characterizing and isolating the dominant chemical constituents of highly repellent chemotypes could allow more efficient production and application of the active compound in pest control. While the repellent properties of essential oils are frequently attributed to the mixture ratio of constituents, the likelihood in most cases is that the main components are probably responsible for the majority of the biological effects (Regnault-Roger et al. 2012). In this study, we report significant repellent action for the essential oil extracts of T. pulegioides in repellency class V and A. absinthium in repellency class IV (Jilani and Su 1983; Wagan et al. 2018). More importantly, we identify dominant constituents of the essential oils that have exceptionally promising repellent action at higher concentrations, including thymol, p-Cymene and thymol methyl ether (from T. pulegioides essential oil) and (−)-β-Pinene (from A. absinthium essential oil). We discuss the importance of these findings for more environmentally friendly conservation biological control measures in crop production systems.
Essential oil compounds from ‘thyme’
High levels of suppression of B. tabaci populations have been reported previously for the essential oil of thyme (Kim et al. 2011). In this study, thymol (36.18%) was the main constituent of T. pulegioides essential oil, followed by carvacrol (13.43%), p-Cymene (10.85%) and thymol methyl ether (7.45%). The dominance of thymol and carvacrol chemotypes in the essential oil of thyme is not unusual (Mockute and Bernotiene 2001; Sárosi et al. 2011). In fact, thymol occurs widely in the essential oils of many species within the family Lamiaceae, such as species of Thymus, Monarda and Origanum (Tabanca et al. 2013). Previous studies have revealed that thymol has larvicide and fumigant activity against insect pests as diverse as rice weevils Sitophilus oryzae, larvae of the western corn rootworm moth, Diabrotica virgifera, and adult house flies, Musca domestica L and mosquitoes, Culex pipiens (Lee et al. 1997; Rozman et al. 2007; Traboulsi et al. 2002). Suppressive effects are related to the capacity for thymol to disrupt GABAergic pathways and inhibit the nervous systems of pests and their locomotory behavior (Oliveira et al. 2018; Priestley et al. 2003). Our results also confirmed that thymol had a significant repellent effect against B. tabaci MED/Q. Similarly, p-Cymene was reported to be effective repellents against B. tabaci MED/Q (Bleeker et al. 2009; Fang et al. 2013; Li et al. 2016; Shi et al. 2018). Our analysis here shows that p-Cymene also has repellent properties, suggesting that some different isomers of the same compound may have a similar role in repelling pests. Lastly, we report significant repellent activity of thymol methyl ether against B. tabaci MED/Q (which is a major constituent of the chemotype of T. pulegioides essential oil used in this study), which has not previously been recorded, to our knowledge.
Surprisingly, one of the main constituents of the essential oil of thyme, carvacrol, had no significant repellent effects on B. tabaci MED/Q, even at the highest concentrations tested. In previous studies, carvacrol has shown significant antifungal, larvicidal, phytotoxic and insecticidal properties against a wide range of organisms, such as mosquitoes, microorganisms and rice weevils (Benelli et al. 2019b; Caglar et al. 2007; Kordali et al. 2008; Petrović et al. 2019; Sökmen et al. 2004; Tabanca et al. 2013; Traboulsi et al. 2002). For B. tabaci more specifically, a 24-h fumigant exposure with carvacrol had lower toxicity (LC50, 0.56 μg/cm3) than thymol (LC50, 0.35 μg/cm3) or dichlorvos (LC50, 0.20 μg/cm3) against B. tabaci MED/Q (Chae et al. 2014). This indicates that B. tabaci MED/Q may be more tolerant of carvacrol than other volatiles.
Essential oil compounds from ‘wormwood’
The essential oil composition of A. absinthium has been extensively studied, and at least ten different chemotypes have been recognized previously (Sharopov et al. 2012). In this study, the major constituents of (−)-β-Pinene (27.88%) and linalool (23.41%) were markedly different from those reported for wormwood in European, Middle Eastern or other Asian locations, indicating that this is a new chemotype of wormwood that has not previously been studied (Orav et al. 2006; Sharopov et al. 2012). Almost certainly there are numerous additional chemotypes of A. absinthium that remain to be discovered. The observed differences in the constituents of A. absinthium essential oils across geographic regions may be due to genetic factors, environmental conditions such as temperature, rainfall, altitude, solar radiation or soil chemistry (amongst others), and even the phenological stage and time of harvesting of plant tissues. Here, (−)-β-Pinene had the greatest repellent action against B. tabaci MED/Q, while linalool had a weak effect, even at the highest concentration tested (100 µL). Previous research has found that structural isomers of α-Pinene did not show significant repellent effect against B. tabaci MED/Q (Du et al. 2016; Zhao et al. 2012), but no previous studies have tested the effects of β-pinene on whiteflies. Moreover, in other insect species, (−)-β-pinene exhibited only a mild repellent effect against the German cockroach Blattella germanica L., whereas some synthetic derivatives of β-pinene possessed greater repellency (Liao et al. 2017). Here, our new findings indicate that (−)-β-pinene is part of a novel suite of repellent chemicals from wormwood that could be used against B. tabaci MED/Q, with the appropriate structural isomers used at higher concentrations.
There was a weak (but not statistically significant) indication in the data that a concentration of 0.01 µg of linalool could have a slight attraction effect on B. tabaci MED/Q (62.22%, P < 0.10). This is consistent with the effect of linalool on B. tabaci MEAM1/ B at low concentrations (also 0.01µL) (Cao et al. 2008), but more evidence would be required to confirm the result in B. tabaci MED/Q. Certainly, it is known that different concentrations of odorant molecules can activate different signaling pathways, changing the pattern of olfactory receptor neuron activation and evoking different responses (Kaupp 2010; Leal 2013). Linalool has even been described as the multifunctional floral volatile, to manage floral mutualists and antagonists (Raguso 2016).
Potential for eco-friendly approaches to whitefly control
Aromatic plant oils can be developed into products that are environmentally friendly and ideally suited for use in integrated pest management programs. Here, we have demonstrated that the essential oils of T. pulegioides and A. absinthium have strong repellent effects and contact toxicity on B. tabaci MED/Q, supporting their potential use in whitefly management. Natural products, especially those from plant sources, are potentially less hazardous ‘eco-friendly’ alternatives to conventional synthetic chemical pesticides that can have high off-target impacts. The effectiveness of this approach, however, will hinge on the degree of variability in chemotype and constituent compounds of essential oil extracts and may not have the desired reliability and repeatability of action that farmers would be looking for. Often, variability in effectiveness of mixtures might stem from unrecognized synergistic effects among major and minor compounds of complex blends of several chemical constituents (Akhtar and Isman 2013; Akhtar et al. 2012; Deletre et al. 2016; Waliwitiya et al. 2009). Identifying the main chemical constituents underpinning the mode of repellent action might instead have greater utility in a crop production setting. Here, we identified four such promising compounds (m-Thymol 36.18%, p-Cymene 10.85%, thymol methyl ether 7.45% and linalool 23.41%) with promising repellent action against B. tabaci MED/Q. Of course, there is the potential problem of pest desensitization to continued use of any single compound. It was known that pests will develop resistance more slowly to an insecticide composed by several different compounds than to a single ingredient (Isman 2000). Most plants deploy multiple modes of action in a ‘multichemical defense’ against a variety of potential herbivores (Raffa 1987). These complex mixtures are likely to be more durable with respect to insects evolving resistance and developing behavioral desensitization (Pavela 2007). Therefore, having a suite of four (or more) compounds with high repellent action will provide a range of potential options for deployment (e.g., rotation of compounds, additive mixtures, or designed synthetic derivatives). However, this would require explicit testing of the most effective strategies.
Although essential oils are stronger volatile in the environment, it will disappear to control insects in a hurry. In recent year, nanoencapsulated essential oils impart development in the field and protect active ingredients of the essential oils from the solar light-induced degradation. (Peres et al. 2020), further as to lay foundations to the exploration of commercial products using essential oils. Although the specific biochemical and behavioral pathways by which the volatile monomer components influence whiteflies remain to be tested, this research has laid a solid foundation for the application of ‘push–pull strategies’ in the comprehensive prevention and control of whiteflies and has broadened the train of thought on aromatic plant oils in more environmentally friendly pest management.
Author contributions
SW, LS, ND and FZ conceived and designed the study. SL and ZQ conducted the experiments. SL and HL analyzed the data and wrote the manuscript, with input from all authors. All authors read and approved the manuscript.
Change history
20 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10340-022-01541-0
References
Adams RP (2007) Review of identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. J Am Soc Mass Spectrom 18:803–806. https://doi.org/10.1016/j.jasms.2007.01.001
Akhtar Y, Isman MB (2013) Plant natural products for pest management: the magic of mixtures. Advanced technologies for managing insect pests. Springer, Cham, pp 231–247. https://doi.org/10.1007/978-94-007-4497-4_11
Akhtar Y et al (2012) Effect of chemical complexity of essential oils on feeding deterrence in larvae of the cabbage looper. Physiol Entomol 37:81–91. https://doi.org/10.1111/j.1365-3032.2011.00824.x
Altemimi A et al (2017) Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from plant extracts. Plants 6:42. https://doi.org/10.1111/j.1365-3032.2011.00824.x
Anonymous (1996) European pharmacopoeia, 3rd edn. In. Strasburg: Council of Europe
Ansari DE, Moharramipour S, Goldasteh S (2010) Ovicidal, larvicidal and oviposition deterrency effects of essential oil from Thymus vulgaris L.(Lamiaceae) on Callosobruchus maculatus (F.)(Col., Bruchidae). J Entomol Res 2(6):73–84. https://www.sid.ir/en/journal/ViewPaper.aspx?id=208109
Aroiee H, Mosapoor S, Karimzadeh H (2005) Control of greenhouse whitefly (Trialeurodes vaporariorum) by thyme and peppermint. Curr ApplSci Technol 5:511–514. https://www.thaiscience.info/journals/Article/KLST/10424456.pdf
Bachrouch O et al (2015) Major compounds and insecticidal activities of two Tunisian Artemisia essential oils toward two major coleopteran pests. Ind Crops Prod 65:127–133. https://doi.org/10.1016/j.indcrop.2014.12.007
Baldin EL et al (2013) Plant-derived essential oils affecting settlement and oviposition of Bemisia tabaci (Genn.) biotype B on tomato. J Pest Sci 86:301–308. https://doi.org/10.1007/s13744-015-0356-8
Bekircan Ç, Cüce M, Sökmen A (2014) Antifeedant activity of the essential oils from four different lamiaceae species against Agelastica alni L. (Coleoptera: Chrysomelidae). Adv Zool Bot 2:57–62. https://doi.org/10.13189/azb
Benelli G et al (2019a) Insecticidal activity of the essential oil from Schizogyne sericea (Asteraceae) on four insect pests and two non-target species. Entomologia Generalis 39:9–18. https://doi.org/10.1127/entomologia/2019/0662
Benelli G et al (2019b) Lyme disease is on the rise—How about tick repellents? A global view. Entomologia Generalis 39:61–72. https://doi.org/10.1127/entomologia/2019/0787
Benelli G et al (2019c) Evaluation of two invasive plant invaders in Europe (Solidago canadensis and Solidago gigantea) as possible sources of botanical insecticides. J Pest Sci 92:805–821. https://doi.org/10.1007/s10340-018-1034-5
Bleeker PM et al (2009) The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol 151:925–935. https://doi.org/10.1104/pp.109.142661
Brooks ME et al (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.32614/RJ-2017-066
Caglar O et al (2007) Insecticidal effect of essential oil of Origanum acutidens against several stored product pests. Fresenius Environ Bull 16:1395–1400
Cao F et al (2008) Behavioural responses of Bemisia tabaci B-biotype to three host plants and their volatiles. Acta Entomol Sin 51:830–838. https://doi.org/10.1016/S1005-9040(08)60003-3
Carvalho MG, Bortolotto OC, Ventura MU (2017) Aromatic plants affect the selection of host tomato plants by Bemisia tabaci biotype B. Entomol Exp Appl 162:86–92. https://doi.org/10.1111/eea.12534
Chae S et al (2014) Fumigant toxicity of summer savory and lemon balm oil constituents and efficacy of spray formulations containing the oils to B- and neonicotinoid-resistant Q-Biotypes of Bemisia tabaci (Homoptera: Aleyrodidae). J Econ Entomol 107:286–292. https://doi.org/10.1603/EC13287
Chaieb I et al (2018) Chemical composition and aphicidal potential of Citrus aurantium peel essential oils. Entomologia Generalis 37:63–75. https://doi.org/10.1127/entomologia/2017/0317
De Moraes CM, Mescher MC, Tumlinson JH (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410:577–580. https://doi.org/10.1038/35069058
Deletre E et al (2016) Naturally occurring bioactive compounds from four repellent essential oils against Bemisia tabaci whiteflies. Pest Manag Sci 72:179–189. https://doi.org/10.1002/ps.3987
Desneux N et al (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106. https://doi.org/10.1146/annurev.ento.52.110405.091440
Dhen N et al (2014) Chemical composition and fumigant toxicity of Artemisia absinthium essential oil against Rhyzopertha dominica and Spodoptera littoralis. Tunisian Journal of Plant Protection 9:57–61
Du W et al (2016) A primary screening and applying of plant volatiles as repellents to control whitefly Bemisia tabaci (Gennadius) on tomato. Sci Rep 6:22140. https://doi.org/10.1038/srep22140
Fang Y et al (2013) Tomato yellow leaf curl virus alters the host preferences of its vector Bemisia tabaci. Sci Rep 3:2876. https://doi.org/10.1038/srep02876
Fernandes ÂSF et al (2010) Lipophilic and hydrophilic antioxidants, lipid peroxidation inhibition and radical scavenging activity of two Lamiaceae food plants. Eur J Lipid Sci Technol 112:1115–1121. https://doi.org/10.1002/ejlt.201000368
Frohlich D et al (1999) A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol Ecol 8:1683–1691. https://doi.org/10.1046/j.1365-294x.1999.00754.x
Gurr GM et al (2017) Habitat management to suppress pest populations: progress and prospects. Ann Rev Entomol 62:91–109. https://doi.org/10.1146/annurev-ento-031616-035050
Hatt S et al (2019) Aromatic plants of East Asia to enhance natural enemies towards biological control of insect pests. A review. Entomologia Generalis 38:275–315. https://doi.org/10.1127/entomologia/2019/062
He Z et al (2009) Chemical constituents from the aerial parts of Artemisia minor. J Nat Prod 72:1198–1201. https://doi.org/10.1021/np800643n
Ikbal C, Pavela M (2019) Essential oils as active ingredients of botanical insecticides against aphids. J Pest Sci 92:971–986. https://doi.org/10.1007/s10340-019-01089-6
Isman MB (2000) Plant essential oils for pest and disease management. Crop Prot 19:603–608. https://doi.org/10.1016/S0261-2194(00)00079-X
Isman MB (2020) Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem Rev 19:235–241. https://doi.org/10.1007/s11101-019-09653-9
Jilani G, Su HC (1983) Laboratory studies on several plant materials as insect repellants for protection of cereal grains. J Econ Entomol 76:154–157. https://doi.org/10.1093/jee/76.1.154
Kaupp UB (2010) Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci 11:188–200. https://doi.org/10.1038/nrn278
Kerebba N et al. (2020) Chemical variation and implications on repellency activity of Tephrosia vogelii (Hook f.) essential oils against Sitophilus zeamais motschulsky. Agriculture 10(5):164. https://doi.org/10.3390/agriculture10050164
Khan ZR et al (2008) Chemical ecology and conservation biological control. Biol Control 45:210–224. https://doi.org/10.1038/41681
Khasdan V et al (2005) DNA markers for identifying biotypes B and Q of Bemisia tabaci (Hemiptera: Aleyrodidae) and studying population dynamics. Bull Entomol Res 95:605–613. https://doi.org/10.1079/ber2005390
Khederi SJ et al (2019) Insecticidal effects of essential oils from two medicinal plants against Aleuroclava jasmini (Hemiptera: Aleyrodidae). Journal of Crop Protection 8:57–67
Kim SI et al (2011) Contact and fumigant toxicity of plant essential oils and efficacy of spray formulations containing the oils against B- and Q-biotypes of Bemisia tabaci. Pest Manag Sci 67:1093–1099. https://doi.org/10.1002/ps.2152
Kordali S et al (2008) Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Biores Technol 99:8788–8795. https://doi.org/10.1016/j.biortech.2008.04.048
Kumar R et al (2019) Insecticidal activity of botanical oils and other neem-based derivatives against whitefly, Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) on cotton. Int J Trop Insect Sci 39:203–210. https://doi.org/10.1007/s42690-019-00027-4
Lawrence B (1992) Chemical components of Labiatae oils and their exploitation. In: Harley RM, Reynolds T (eds) Advances in Labiatae science. The Royal Botanic Gardens, Kew, Great Britain 399–436
Leal WS (2013) Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol 58:373–391. https://doi.org/10.1146/annurev-ento-120811-153635
Lee S et al (1997) Insecticidal activity of monoterpenoids to western corn rootworm (Coleoptera: Chrysomelidae), twospotted spider mite (Acari: Tetranychidae), and house fly (Diptera: Muscidae). J Econ Entomol 90:883–892. https://doi.org/10.1046/j.1365-2583.1997.00178.x
Li S et al (2016) Behavioral responses of Bemisia tabaci MED to 13 plant volatiles. J Plant Protection 43:105–110. https://doi.org/10.13802/j.cnki.zwbhxb.2016.01.015
Liao SL et al (2017) Hydronopylformamides: modification of the naturally occurring compound (-)-β-pinene to produce insect repellent candidates against Blattella germanica. Molecules 22:1004. https://doi.org/10.3390/molecules22061004
Mockute D, Bernotiene G (2001) The α-terpenyl acetate chemotype of essential oil of Thymus pulegioides L. Biochem Syst Ecol 29:69–76. https://doi.org/10.1016/s0305-1978(00)00026-0
Negahban M, Moharramipour S, Sefidkon F (2006) Insecticidal activity and chemical composition of Artemisia sieben besser essential oil from Karaj. Iran J Asia-Pacific Entomol 9:61–66. https://doi.org/10.1016/S1226-8615(08)60276-9
Negahban M, Moharramipour S, Sefidkon F (2007) Fumigant toxicity of essential oil from Artemisia sieberi Besser against three stored-product insects. J Stored Prod Res 43:123–128. https://doi.org/10.1016/j.jspr.2006.02.002
Oliveira AP et al (2018) Essential oil of Lippia sidoides and its major compound thymol: Toxicity and walking response of populations of Sitophilus zeamais (Coleoptera: Curculionidae). Crop Prot 112:33–38. https://doi.org/10.1016/j.cropro.2018.05.011
Orav A et al (2006) Composition of the essential oil of Artemisia absinthium L. of different geographical origin. Proc Es Acad Sci Chem 55(3):155–165. https://doi.org/10.1007/s00464-014-3641-4
Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121:325–332. https://doi.org/10.1104/pp.121.2.325
Park J-H et al (2017) Insecticidal toxicities of carvacrol and thymol derived from Thymus vulgaris Lin. against Pochazia shantungensis Chou & Lu., newly recorded pest. Sci Rep 7:40902. https://doi.org/10.1038/srep40902
Pavela R (2007) Possibilities of botanical insecticide exploitation in plant protection. Pest Technol 1:47–52. http://globalsciencebooks.info/Online/GSBOnline/images/0706/PT_1(1)/PT_1(1)47-52o.pdf
Peres MC et al (2020) In natura and nanoencapsulated essential oils from Xylopia aromatica reduce oviposition of Bemisia tabaci in Phaseolus vulgaris. J Pest Sci 93:807–821. https://doi.org/10.1007/s10340-019-01186-6
Petrović M et al (2019) Assessment of toxicity and biochemical response of Tenebrio molitor and Tribolium confusum exposed to Carum carvi essential oil. Entomologia Generalis 38:333–348. https://doi.org/10.1127/entomologia/2019/0697
Priestley CM et al (2003) Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABAA receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br J Pharmacol 140:1363–1372. https://doi.org/10.1038/sj.bjp.0705542
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Raffa KF (1987) Influence of host plant on deterrence by azadirachtin of feeding by fall armyworm larvae (Lepidoptera, Noctuidae). J Econ Entomol 80:384–387. https://doi.org/10.1093/jee/80.2.384
Raguso RA (2016) More lessons from linalool: insights gained from a ubiquitous floral volatile. Curr Opin Plant Biol 32:31–36. https://doi.org/10.1016/j.pbi.2016.05.007
Rao Q et al (2011) Distribution and dynamics of Bemisia tabaci invasive biotypes in central China. Bull Entomol Res 101:81–88. https://doi.org/10.1017/S0007485310000428
Regnault-Roger C et al (1993) Insecticidal effect of essential oils from mediterranean plants upon Acanthoscelides Obtectus Say (Coleoptera, Bruchidae), a pest of kidney bean (Phaseolus vulgaris L.). J Chem Ecol 19:1233–1244. https://doi.org/10.1007/BF00987383
Regnault-Roger C, Vincent C, Arnason JT (2012) Essential oils in insect control: low-risk products in a high-stakes world. Annu Rev Entomol 57:405–424. https://doi.org/10.1146/annurev-ento-120710-100554
Rozman V, Kalinovic I, Korunic Z (2007) Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. J Stored Prod Res 43:349–355. https://doi.org/10.1016/j.jspr.2006.09.001
Saad KA et al (2017) Toxic, repellent, and deterrent effects of Citronella essential oil on Bemisia tabaci (Hemiptera: Aleyrodidae) on chili plants. J Entomol Sci 52(2):16–32. https://doi.org/10.18474/JES16-32.1
Sárosi S et al (2011) Essential oil polymorphism of Thymus pulegioides collected in Monti Pisani, Italy. Acta horticulturae 955:59–64. https://doi.org/10.17660/ActaHortic.2012.955.5
Schmitz G (1999) Phytophagous arthropod fauna (Acari, Insecta) of the mugwort species Artemisia vulgaris (Asteraceae) in Central Europe. Entomol Gen 24:145–160. https://doi.org/10.1046/j.1570-7458.1999.00427.x
Shah FM et al (2020) Action threshold development in cabbage pest management using synthetic and botanical insecticides. Entomologia Generalis 40:157–172. https://doi.org/10.1127/entomologia/2020/0904
Sharopov FS, Sulaimonova VA, Setzer WN (2012) Composition of the essential oil of Artemisia absinthium from Tajikistan. Rec Natural Prod 6:127–134. https://doi.org/10.1080/00173134.2012.668219
Shi X et al (2018) Plants pre-infested with viruliferous MED/Q cryptic species promotes subsequent Bemisia tabaci infestation. Front Microbiol 9:1404. https://doi.org/10.3389/fmicb.2018.01404
Sökmen M et al (2004) In vitro antioxidant, antimicrobial, and antiviral activities of the essential oil and various extracts from herbal parts and callus cultures of Origanum acutidens. J Agric Food Chem 52:3309–3312. https://doi.org/10.1021/jf049859g
Soares MA et al (2019) Botanical insecticide and natural enemies: a potential combination for pest management against Tuta absoluta. J Pest Sci 92:1433–1443. https://doi.org/10.1007/s10340-018-01074-5
Tabanca N et al (2013) Comparative investigation of Umbellularia californica and Laurus nobilis leaf essential oils and identification of constituents active against Aedes aegypti. J Agric Food Chem 61:12283–12291. https://doi.org/10.1021/jf4052682
Taggar GK, Gill RS (2016) Host plant resistance in Vigna sp towards whitefly, Bemisia tabaci (Gennadius): a review. Entomol Gen 36:1–24. https://doi.org/10.1127/entomologia/2016/0184
Takabayashi J, Dicke M (1992) Response of predatory mites with different rearing histories to volatiles of uninfested plants. Entomol Exp Appl 64:187–193. https://doi.org/10.1111/j.1570-7458.1992.tb01608.x
Tariku Y et al (2011) In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chem Biodivers 8(4):614–623. https://doi.org/10.1002/cbdv.201000331
Traboulsi AF et al (2002) Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest Manag Sci 58:491–495. https://doi.org/10.1002/ps.486
Wagan TA et al (2018) Effectiveness of aromatic plant species for repelling and preventing oviposition of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). J Appl Entomol 142:287–295. https://doi.org/10.1111/jen.12471
Waliwitiya R, Kennedy CJ, Lowenberger CA (2009) Larvicidal and oviposition-altering activity of monoterpenoids, trans-anithole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). Pest Manag Sci 65:241–248. https://doi.org/10.1002/ps.1675
Yang NW et al (2010) Effects of plant essential oils on immature and adult sweetpotato whitefly, Bemisia tabaci biotype B. Crop Prot 29:1200–1207. https://doi.org/10.1016/j.cropro.2010.05.006
Yao FL et al (2017) Dynamics of Bemisia tabaci biotypes and insecticide resistance in Fujian province in China during 2005–2014. Sci Rep 7:40803. https://doi.org/10.1038/srep40803
Zhao YQ et al (2012) Effects of the volatiles from different tomato varieties on host selection behavior of B-biotype Bemisia tabaci. Chin J Appl Ecol 23(09):2509–2514. https://doi.org/10.13287/j.1001-9332.2012.0347
Zheng HX et al (2017) Dynamic monitoring (B versus Q) and further resistance status of Q-type Bemisia tabaci in China. Crop Prot 94:115–122. https://doi.org/10.1016/j.cropro.2016.11.035
Acknowledgements
We thanks Raphael K. Didham (CSIRO Health & Biosecurity, Centre for Environment and Life Sciences, Australia) for his statistical analysis and improving language suggestions on an earlier version of the manuscript. This study was supported by funds from the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA23080603), National Key R&D Program of China (Grant No. 2017YFD0201006), National Natural Science Foundation of China (No. 32072479) and the Youth Scientific Funds Program of Beijing Academy of Agricultural (No. QNJJ201823).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Additional information
Communicated by Juergen Gross.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, S., Li, H., Zhou, Q. et al. Essential oils from two aromatic plants repel the tobacco whitefly Bemisia tabaci. J Pest Sci 95, 971–982 (2022). https://doi.org/10.1007/s10340-021-01412-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-021-01412-0