Abstract
The use of chemical pesticides as a main pest control strategy has been highly criticised due to environmental pollution and negative effects on natural enemies of pests. In modern farming, it is essential to implement integrated pest management approaches that seek to control insect pests without causing environmental damage, e.g. the use of companion plants. Basil and Mexican marigold are often used as companion plants to attract greenhouse whiteflies, hence reducing damage to solanaceous crops, but the mechanism and role of volatile cues in crop protection strategies are unknown. This study found that both flowering basil and marigold were preferred to tomato by the greenhouse whitefly (Trialeurodes vaporariorum) in Y-tube olfactometer bioassays. PCA revealed that some volatiles were more correlated to one stage than to another. The dominant volatile constituents of Mexican marigold are limonene, dihydrotagetone, (Z)-β-ocimene, α-pinene, (Z)-3-hexenyl acetate, and those from basil are linalool, 1,8-cineole, eugenol and β-elemene. Among these dominant compounds, 1,8-cineole and (Z)-3-hexenyl acetate elicited strong attraction in greenhouse whitefly at 0.01%, whereas (Z)-β-ocimene and linalool elicited strong repellence at 0.1% and 1% dosages. This suggested that the basil flowering stage attraction is due to 1,8-cineole. These volatiles demonstrated potential as lures or bio-repellents and could be used in a “push–pull” semiochemical approach for greenhouse whitefly management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional farming systems currently generally involve monocultures with heavy reliance on synthetic chemical insecticides (Tilman et al. 2002). This has prompted severe widespread criticism because of the associated biodiversity losses, human health issues, and environmental pollution worldwide (Niggli et al. 2007; Bengtsson et al. 2005). However, vegetation diversity has been shown to suppress pests via several causal pathways, as reviewed by Ratnadass et al. (2012): (1) pest-suppressing effects via visual and olfactory cues; (2) below-ground bottom-up allelopathic effects; (3) disruption of the spatial cycle via non-host effects; (4) disruption of the temporal cycle via crop rotation with non-host plants; (5) physiological resistance due to improved crop nutrition; (6) facilitation of top–down effects on aerial crop pests via natural enemy conservation; (7) stimulation of specific below-ground antagonists of pests; and (8) direct and indirect architectural effects (physical barrier effects, microclimate alteration). The incorporation of vegetation in agroecosystems for the purpose of pest control is also called “companion planting”, while trap cropping is encompassed in companion planting (Manson 2005).
Trap crops have been defined as “plant stands grown to attract insects to protect target crops from pest attack, preventing the pests from reaching the crop or concentrating them in a certain part of the field where they can be economically destroyed” (Hokkanen 1991) or, in broader definition, “as plant stands that are, per se or via manipulation, deployed to attract, divert, intercept, and/or retain targeted insects or the pathogens they vector to reduce damage to the main crop” (Shelton and Badenes-Perez 2006). There are different kinds of trap cropping: (1) conventional trap cropping where the trap crop has to be more attractive than the target crop to reduce pest damage to the target crop, (2) “dead-end trap cropping” where the trap crop is an attractant for insect pests but it does not support the growth of the insects, which therefore cannot survive and complete their life cycle, hence stalling pest movement to the target crop, (3) genetically engineered trap cropping where the trap cropping plant has been modified to increase their attractiveness or add a dead-end characteristic to the trap crop.
Field observations revealed that Mexican marigold (Tagetes minuta) and basil (Ocimum basilicum) attract the greenhouse whitefly (Trialeurodes vaporariorum, Westwood, Hemiptera: Aleyrodidae). The greenhouse whitefly is a worldwide pest which causes devastating vegetable and ornamental plant losses in many parts of the world, including tomato which is a vital vegetable crop with world production of about 180 billion tons (Bleeker et al. 2009, FAO 2018). This pest causes damage through the reduction of plant productivity directly by extracting phloem sap or indirectly by excreting honeydew on foliage, leading to the development of sooty molds that reduce leaf photosynthesis, and by the transmission of viruses, such as the tomato chlorosis crinivirus (Inbar and Gerling 2008; Moodley et al. 2019). Greenhouse whitefly damage has been reported to cause 5–30% losses on fresh tomatoes marketed in the sub-Saharan region (Johnson et al. 1992).
Although farmers use a set of integrated pest management tools to manage the greenhouse whitefly, chemical control is the most commonly used by many farmers (Gorman et al. 2002). However, chemical control treatments have recently been heavily criticised different agencies and governments due to their environmental impact, especially due to their direct connection to their destruction of bee colonies (Gross 2013). Moreover, greenhouse whiteflies have developed some resistance to several neonicotinoids, pyrethroids and ketoenols, thus making them ineffective for pest control (Kapantaidaki et al. 2018). This has led many producers to shift to more biological control methods which are by nature environmentally friendly, such as the use of natural enemies and pathogens of greenhouse whiteflies (Pilkington et al. 2010). This trend paved the way to a study on how companion plants influence the greenhouse whitefly behaviour and how to exploit the different volatile chemicals for the management of this pest (Schlaeger et al. 2018).
Our study was focused on two companion plants, i.e. basil and Mexican marigold, both of which have been reported to affect the greenhouse whitefly behaviour in previous studies. In two previous field experiments in Kenya, we observed that marigold significantly reduced the greenhouse whitefly population in cowpea crops, while basil and tomato intercropping led to a 68.7% reduction in the whitefly population (Diabate et al. 2019; Mutisya et al. 2016). Moreover, when tomato was intercropped with basil, its yields increased to up to 96.5 t ha−1 in Brazil (Carvalho et al. 2009). Moreover, basil essential oil, when added to yellow sticky traps, increased the attractiveness for greenhouse whiteflies by 4.8-fold (Górski 2004). These studies illustrated that marigold and basil play an important role in reducing crop losses associated with T. vaporariorum, but their mode of action is still unclear. The aim of this study was to investigate the likely involvement of olfactory cues, which could then subsequently be used to develop lures and repellents for greenhouse whitefly management. This study focused on evaluating the behavioural response of T. vaporariorum to basil and Mexican marigold volatiles at the different phenological stages and comparing their response with the high attractant tomato cultivar red beauty F1. We hypothesized that basil and marigold would be more attractant for whiteflies than tomato plants. Then, we studied the effect of the major volatile compounds of the companion plant.
Materials and methods
Plant material
Red beauty F1 tomato cultivar seeds and basil (Ocimum basilicum L.) seeds were purchased from Amiran Kenya Limited. Mexican marigold (Tagetes minuta L.) seeds were collected from plants growing in the Kenya Agricultural and Livestock Research Organization (KALRO) station in Kimbimbi, Kirinyaga County, Kenya (0°37′11.3″ S, 37°22′08.0″ E). French beans (Phaseolus vulgaris) were purchased from Kenya Seeds Company Limited. Red soil and manure (ratio 3:1 v/v) were used to raise the seedlings which were grown in a screen house (27 ± 1 °C temperature, 65 ± 5% relative humidity) at the International Center of Insect Physiology and Ecology (icipe), Duduville, Nairobi Campus, Kenya (1°13′17.9″ S 36°53′48.1″ E). Plants were raised in plastic pots (15 cm dia. × 15 cm height) free of pesticides, watered regularly and nourished with Agrofeed (Osho chemicals, Kenya), a vegetative fertilizer N:P:K (12:10:8), 2 weeks after transplanting. Basil and marigold were used for the bioassay experiment at both vegetative (2 months old) and flowering stages (3–4 months old). Red beauty F1 tomatoes in the vegetative stage were used 4 weeks after transplanting to conduct bioassays, while French beans were used for greenhouse whitefly rearing 4 weeks after transplanting.
Insects and bioassays
Greenhouse whitefly colonies were collected on tomato plants at the KALRO station and reared in a cage (40 cm × 40 cm × 50 cm) on 4-week-old potted French bean plants at the icipe laboratory. The cage was kept in a laboratory maintained at 25 ± 1 °C temperature, 50–60% relative humidity and 12:12 L:D photoperiod. Greenhouse whitefly females were allowed to oviposit for 2 days and then a new French bean plant was placed in the cage. 1–3 days prior to adult emergence, all French bean leaves bearing whiteflies at the 4th nymph stage were removed and placed inside a different cage with fresh plants where they developed into adults. The newly emerged adults were not sexed, hence both male and female insects were used to conduct the bioassays, while the insects used were 1–7 days old.
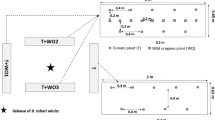
The behavioural responses of greenhouse whiteflies to tomato, basil and Mexican marigold were examined in a Y-tube olfactometer consisting of a Y-shaped glass tube (0.6 cm internal dia.; 10.5 cm arm length, 9.5 cm stem length and a 60° angle at the junction intersection). Compressed air from an electrical pump (KnF, Laboport, Lagallais, PA Sainte, France) was purified through activated charcoal and regulated by a flow meter (Aalborg, Orangeburg, NY, USA) at 50 mL/min and split into two before being pumped into Nalophan cooking bags (38 cm high × 25 cm wide, Chevalier Diffusion, F33890 Pessac-sur-Dordogne, France) containing a plant or empty (control), with clean air passing through and into the Y-tube olfactometer arms. Prior to the experiment, plastic pots bearing the test plants were covered with aluminium foil to avoid odour pollution (or excess background contaminants). The olfactory-behavioural choices of greenhouse whitefly were tested on: (a) vegetative basil versus clean air; (b) flowering basil versus clean air; (c) vegetative basil versus tomato; (d) flowering basil versus tomato (e) vegetative marigold versus clean air; (f) flowering marigold versus clean air; (g) vegetative marigold versus tomato; (h) flowering marigold versus tomato, and (i) clean air versus tomato.
The Y-tube bioassay was conducted from 9:00 to 17:00 h in a laboratory that was maintained at 25 ± 1 °C temperature and 60 ± 5% relative humidity. The Y-tube arena was positioned inside a box (20 cm × 20 cm × 30 cm) and was illuminated from above with an 8 W fluorescent lamp. Prior to the experiment, greenhouse whiteflies to be used were starved for 2 h. A greenhouse whitefly was introduced at the Y-tube stem base, then allowed for 10 min to make a choice depending on the Y-tube arm of choice. Each tested greenhouse whitefly was considered to have made a choice when it had moved halfway through the Y-tube arm towards any of the odour sources. A no-choice response was recorded when the whitefly did not make any choice within 10 min. After five insects were tested, the entire Y-tube setup was rotated 180° to avoid any asymmetry bias in the setup. 60 replications were performed for each treatment. The glass Y-tube was cleaned with 70% ethanol between every insect tested and oven-dried at the end of the day.
Headspace volatile collection
Volatile compounds from intact vegetative red beauty F1 tomato, vegetative/flowering basil, and vegetative/flowering marigold plants were collected using a headspace sampling method. The flowering stage was characterized by pedicellate flowers and small leaves. Before use, Porapak Q (50/80) 150/75 mg adsorbent (SUPELCO solutions, Bellefonte, PA, USA) was pre-cleaned with 5 ml of dichloromethane (Sigmana Aldrich, Gillingham, UK; purity ≥ 99%) to remove contaminants before drying in a stream of nitrogen. Nalophan bags were baked overnight in an oven at 100 °C before use. Volatiles of tomato, basil and Mexican marigold were collected by covering the single intact plant with the nalophan bag that was held tight around the stem with cotton wool and a rubber band to create airtight conditions while avoiding injury to the plant. Compressed air from an electrical pump (KnF, Laboport, Lagallais, PA Sainte, France) was purified through activated charcoal and pushed through nalophan bags at a 200 mL/min flow rate and pulled out through Porapak Q traps at 150 mL/min for 24 h. The difference in flow rates prevented unfiltered air from entering the system (Webster et al. 2008). Volatile collections from each of five plant replicates of tomato, basil and Mexican marigold were extracted with 150 µL dichloromethane (DCM) and concentrated to 50 µL using nitrogen gas on ice. Extracts were used immediately or stored at − 80 °C until GC–MS analysis.
Volatile analysis
Plant volatiles produced by tomato, basil and marigold were analysed using coupled gas chromatography-mass spectrometry (GC–MS) on an Agilent Technologies 7890A GC linked to a 5975 mass spectrometer which was equipped with an MSD Chemstation E.02.00.493, Wiley 9th/NIST 2008 MS library and a HP-5 MS column (30 m × 0.25 mm internal dia. × 0.25 µm film thickness) (JandW, Folsom, CA, USA). The concentrated volatiles in a 1 µL aliquot were analysed in splitless mode using helium as carrier gas at 1.2 mL/min. The oven temperature was held at 35 °C for 5 min after sample injection, then programmed at 10 °C/min to 280 °C and held for 5.5 min. Spectra were recorded at 70 eV in electron impact (EI) ionisation mode. Volatile compounds were then identified by comparison of the mass spectra data with MS libraries (Adams, Chemoecol and, NIST) and confirmed with synthetic standards and the comparison between the calculated retention indices of compounds relative to n-alkane standards (C8-C30) and retention indices from the literature (RIs obtained on HP-5 columns).
Chemicals
The synthetic standards: (Z)-3-hexenyl acetate (chemical purity 98%), (R)-(+)-limonene (96%), (S)-(-)-limonene (96%), ocimene (90%, chiral purity was not known), (+)-α-pinene (98%), 1,8-cineole (99%), (+)-linalool (97%), β-elemene (98%), eugenol (99%), and dichloromethane (99%) were obtained from Sigma-Aldrich (France). Dihydrotagetone (95%) was obtained from Santa Cruz Biotechnology (USA).
Olfactory assay with synthetic standards
We used the same Y-tube olfactometer assay as described earlier to evaluate the behavioral effects of the synthetic standards of compounds identified from basil and Mexican marigold. We tested the most abundant compounds from both basil and marigold identified in our GC–MS analysis:
limonene, dihydrotagetone, (Z)-β-ocimene, (Z)-3-hexenyl acetate, and α-pinene for Mexican marigold and linalool, 1,8-cineole, eugenol, and β-elemene for basil. Linalool and 1,8-cineole were also tenfold more abundant in flowering basil the attractant stage than in the vegetative stage. Individual compounds were tested at the following dilutions: 100 µL of the original compound was diluted with 900 µL of dichloromethane to form 10% (v/v) and then further serial dilution to obtain solutions of 0.01%, 0.1% and 1% (v/v) and each was individually tested against the control (dichloromethane). When testing the volatiles, a volume of 50 µL aliquot of each solution (i.e. 5, 50 and 500 nl/paper of active compound) was applied on filter paper and left for 30 s at 25 ± 1 °C to allow the solvent to vaporize before being placed in the test chamber. The impregnated filter paper was then placed in nalophan bags (38 cm high × 25 cm wide) connected to the olfactometer arms via PTFE tubing and used for only 1 h.
A Mexican marigold blend of the five compounds at a ratio of (27:26:20:11:16) [limonene: dihydrotagetone: (Z)-β-ocimene: α-pinene: (Z)-3-hexenyl acetate] was prepared to obtain 100 µL, which was diluted with 900 µL to obtain a 10% concentration blend which was further serial-diluted to form a 0.01%, 0.1% and 1% (v/v) blend of identified compounds. The basil blend was prepared at a ratio of (34:29:27:10) [linalool: 1,8-cineole: eugenol: β-elemene] and similar dilution procedure as used for marigold was followed to obtain blends of 1%, 0.1% and 0.01% (v/v) solution. Greenhouse whitefly responses were compared to each blend versus dichloromethane and then the blend versus vegetative tomato plants. The filter paper was replaced after 10 greenhouse whiteflies had been tested. 60 replications were performed for each treatment.
Statistical analysis
The frequency count data were subjected to a Chi-square test (χ2) with Bonferroni corrections to test the hypothesis that the greenhouse whitefly choice between a pair of odours deviates from the null model of odour source chosen with equal frequency. The null hypothesis was that greenhouse whiteflies had a 50:50 distribution across the two arms of the olfactometer. Non-respondent greenhouse whiteflies were not included in the analysis. A non‐parametric Mann–Whitney–Wilcoxon test was used to analyse differences in the emission quantity of volatiles between vegetative and flowering stages of basil and marigold plants. Moreover, a graphical approach was used to visualise chemical variations between the flowering and vegetative stages of the companion plants based on component correlations. We hypothesized that the volatile release would vary between the two stages. Principal component analysis (PCA) (“ade4” package (Dray and Dufour 2007)) was used to highlight the relationship between the vegetative and flowering of basil and marigold plants based on the emission of volatile compounds (compound peak areas from GC–MS) using a graphical approach. On the factorial map, the more two points (plant samples) were distant on the graph, the more they were considered different. On the loading plot, only the long arrows (the most correlated) were used to interpret differences between plant samples. The more a sample was towards the front of an arrow (see coordinates), the more it had a high value for this variable (volatiles compounds). All statistical analyses were conducted in the R environment (R Core Team 2018).
Results
Behavioural response of greenhouse whiteflies to clean air, tomato, basil, and Mexican marigold
We hypothesized that basil and Mexican marigold are trap crops for greenhouse whiteflies. Hence, they have to be attractant, and more attractant than tomato. In the first tests, the red beauty tomato cultivar elicited an attraction of the greenhouse whitefly compared to clean air (Fig. 1). In addition, whiteflies only preferred flowering basil relative to clean air, whereas vegetative basil did not elicit any significant behavioural response in greenhouse whiteflies. However, greenhouse whiteflies also showed a preference for marigold at both vegetative and flowering stages compared to clean air.
Behavioral response of greenhouse whiteflies to vegetative and flowering basil; and vegetative and flowering Mexican marigold vs clean air (a) and tomato (b) in a Y-tube olfactometer test. n number of responding insects, total number of insects tested per pairing was 60. Asterisks represent the significance level of x2 tests at *P < 0.05, (Chi-square with Bonferonni correction)
The flowering stages of basil and marigold were preferred by greenhouse whiteflies over tomato (Fig. 1). In contrast, the vegetative stages of both did not elicit any significant response of greenhouse whiteflies when compared to tomato.
Analysis of volatiles emitted by tomato, basil and Mexican marigold
As the behavioural responses of greenhouse whiteflies differed, we hypothesized that there were differences in the volatile release by the companion plants at different stages and by tomato plants, particularly between marigold flowering and vegetative stages as flowering basil was attractant and not in the vegetative stage. A total of 51 volatile compounds were identified from the red beauty F1 tomato cultivar, basil, and marigold (Table 1). The five most abundant volatiles of the red beauty F1 tomato cultivar in order were: α-pinene, p-cymene, 2-carene, sabinene and β-pinene. The five major volatiles of vegetative basil in order were: 2-carene, eugenol, α-pinene, bicyclogermacrene and α-humulene and of the flowering stage were: linalool, 1,8-cineole, eugenol, β-pinene and bornyl acetate. The five major volatiles of vegetative marigold in order were: α-pinene, p-cymene, limonene, dihydrotagetone and (Z)-3-hexenyl acetate, and of the flowering stage were: (Z)-tagetone, dihydrotagetone, (Z)-β-ocimene, limonene and car-3-en-2-one.
The PCA on basil explained 86.61% of the variance. According to PCA, the flowering stage was correlated with positive PC-1 values and negative PC-2 values, compounds with PC-1 > 0.7 and negative PC-2 were myrcene, linalool, 1,8-cineole, β-copaene, α-guaiene, γ-muurolene, valencene, α-bulnesene, sabinene hydrate and γ-cadinene, which contributed to the flowering stage, while the vegetative stage was correlated with zero PC-1 values, compounds with PC-1 < 0.3 were α-pinene, 2-carene, α-terpinene, p-cymene, (Z)-β-ocimene, γ-terpinene, methyl eugenol and sesquiterpene contributed to the vegetative stage (Table 2).
The PCA on marigold explained 76.63% of the variance. According to PCA, the flowering stage was correlated with positive PC-1 values, compounds with PC-1 > 0.7 were sabinene, limonene, (Z)-β-ocimene, dihydrotagetone, car-3-en-2-one, bornyl acetate, (E)-β-caryophyllene, α-humulene, germacrene D and bicyclogermacrene, which contributed to the flowering stage, while the vegetative stage was correlated with negative PC-1 values, compounds with PC-1 < 0.67 were (Z)-3-hexenol, camphene, β-pinene, p-cymene, 1,8-cineole and terpinolene, which contributed to the vegetative stage (Table 3).
Myrcene, 1,8-cineole, linalool, β-copaene, α-bulnesene and γ-cadinene were more abundant volatiles from the flowering stage of basil than from the vegetative stage, contrary to eugenol, which was less abundant (bold numbers, Table 3). (Z)-3-hexenyl acetate, camphor, α-cubebene, γ-muurolene and δ-cadinene were only detected in the flower stage. (Z)-tagetone and germacrene were more abundant in the flowering stage of marigold than in the vegative stage, in contrast, (Z)-3-hexenol and α-pinene were less abundant (bold numbers, Table 3). (Z)-3-hexenal, β-pinene, p-cymene, 1,8-cineole, (E)-β-ocimene and terpinolene were only present in the vegetative stage, contrary to α-humulene and bicyclogermacrene which were only present in the flowering stage.
Response of greenhouse whitefly to volatile compounds of basil
Based on GC–MS analysis, we tested the most abundant compound as we hypothesized they were responsible for the attraction of greenhouse whiteflies. Moreover, linalool and 1,8-cineole were also tenfold more abundant in flowering basil, i.e. the attractant stage, than in the vegetative stage. Among the four dominant compounds identified in basil, 1,8-cineole elicited attraction at two concentrations, i.e. 0.01% and 0.1% (Fig. 2); eugenol was only attractant at 0.01%; and β-elemene was only attractant at 0.1%. Linalool elicited a repellent behavioral response at all three tested concentrations.
Behavioral response of greenhouse whiteflies to five major Mexican marigold volatiles tested individually and as a blend mixed at the ratio of 27:26:20:11:16 vs. clean air or tomato tested at 0.01% (a), 0.1% (b), and 1% (c) concentration in a Y-tube olfactometer test. n number of responding insects, total number of insects tested per pairing was 60. Asterisks represent the significance level of x2 tests at *P < 0.05, (Chi-square with Bonferonni correction)
Greenhouse whiteflies were attracted to the basil blend (34:29:27:10) [linalool: 1,8-cineole: eugenol: β-elemene] at both 0.01% and 0.1% concentration rates (Fig. 2). In pairwise experiments with red beauty F1 tomato, greenhouse whiteflies were attracted to the basil blend at 0.1%, but at 0.01%. Similarly, when the basil blend + red beauty F1 tomato were jointly compared against tomato, greenhouse whiteflies were only attracted to the basil blend at 0.1%.
Response of greenhouse whiteflies to volatile compounds of Mexican marigold
Based on GC–MS analysis, we tested the most abundant compound as we hypothesized they were responsible for the attraction of greenhouse whiteflies. Among the five major volatiles produced by marigold, (Z)-3-hexenyl acetate elicited an attractive response at all tested concentrations (Fig. 3). However, greenhouse whiteflies were repelled by (Z)-β-ocimene at all tested concentrations. Limonene was also repellent to greenhouse whiteflies at 0.01% and 0.1%. α-pinene elicited a repellent behavioural response in greenhouse whiteflies at 1%. Dihydrotagetone did not elicit responses at all concentration rates.
Behavioral response of greenhouse whiteflies to four major basil volatiles, i.e. linalool, 1,8-cineole, eugenol and β-elemene tested individually and as a lend mixed at the ratio of 34:29:27:10 vs. clean air or tomato tested at 0.01% (a), 0.1% (b), and 1% (c) concentration in a Y-tube olfactometer test. n number of responding insects, total number of insects tested per pairing was 60. Asterisks represent the significance level of x2 tests at *P < 0.05, (Chi-square with Bonferonni correction)
The chemical blend [limonene: dihydrotagetone: (Z)-β-ocimene: α-pinene: (Z)-3-hexenyl acetate] (27:26:20:11:16) representing marigold was attractant at 0.1%, while at 1%, the blend was repellent compared to the control (Fig. 3). Red beauty F1 cultivar tomato plants were less preferred by greenhouse whiteflies compared to the marigold blend at 0.1%. The marigold blend was repellent at 1% compared to tomato (Fig. 3c). Joint comparison of the Mexican marigold blend + tomato to tomato showed repellence to greenhouse whiteflies at 1%.
Discussion
From the Y-tube olfactory assays, basil and Mexican marigold at flowering stages exhibited strong attraction of greenhouse whiteflies. Mexican marigold elicited a similar behavioural response at the vegetative stage. Whiteflies further showed significant attraction towards both flowering basil and marigold compared to the red beauty F1 tomato cultivar. These findings support previous field observations of a study conducted by Diabate et al. (2019), and offer an avenue for making effective use of basil and marigold as trap crops, while also using their bioactive semiochemicals for sustainable management of greenhouse whiteflies. The findings of a previous study showed that basil varieties could be potential hosts for greenhouse whiteflies, with some varieties reported to have high attractiveness, i.e. able to host > 30 eggs cm−2 (Roditakis, 1990). Intercropping basil with tomato was previously found to reduce adult whitefly populations on tomato (Carvalho et al. 2017). Contrary to our findings, greenhouse whiteflies were repelled by a floral extract of Irish lace (Tagetes filifolia) in a Y-tube olfactometer assay, mainly due to the presence of trans-anethole, a repellent compound (Camarillo and Rodriguez 2009) which was not found in the Mexican marigold study.
As basil is a highly aromatic plant that is well documented as a key herb (Pushpangadan and George 2012), volatiles identified from basil have high levels of linalool, 1–8, cineole, β-elemene and eugenol. The basil blend, when mixed at a natural ratio of [linalool: 1–8, cineole: eugenol:β-elemene] (34:29:27:10) and tested against clean air in the dual choice assay, was found to attract T. vaporariorum at both 0.1% and 0.01% concentration rates when tested against clean air and was also found to attract T. vaporariorum at 0.1% when tested against tomato. Previous studies have shown the basil major volatile constituents were linalool (3.94 mg/g), eugenol (0.896 mg/g) and 1,8-cineole (0.288 mg/g), which coincided with our findings. Linalool and 1,8-cineole were the main compounds in both vegetative and flowering stages, as also observed in different studies (Di Cesare et al. 2003; Vieira and Simon 2006; Hussain et al. 2008; Calín-Sánchez et al. 2012). Based on PCA analysis, the flowering stage released mainly linalool, myrcene,1,8-cineole, β-copaene, α-guaiene, γ-muurolene, valencene, α-bulnesene and γ-cadinene, whereas the vegetative stage released α-pinene, 2-carene, α-terpinene, p-cymene, (Z)-β-ocimene, γ-terpinene, methyl eugenol and an unidentified sesquiterpene. Linalool and 1,8-cineole were also tenfold more abundant in flowering basil, the attractant stage, than in the vegetative stage, thus suggesting they are responsible for the basil attractiveness. We found that 72.4% greenhouse whiteflies were attracted to the four component-blend of key basil compounds, while the maximum for a single compound was 67.9%. We, therefore, hypothesised that the basil blend could be used alone or in combination with physical traps, such as yellow sticky traps, in greenhouse whitefly management. However, further studies need to be carried out on the response of greenhouse whiteflies to the basil blend because creation of realistic and effective blend ratios is key but not easy due to many possible permutations which increase geometrically (Szendrei and Rodriguez-Saona 2010). Greenhouse whiteflies were attracted to either 1,8-cineole, eugenol, or β-elemene at both 0.1% and 0.01% concentration compared to clean air. These compounds are at least partly responsible for the attractiveness of basil. Sweet potato whiteflies (Bemisia tabaci) were also previously found to be attracted to 1,8-cineole and eugenol (Cao et al. 2008).
Marigold volatiles are known to contain monoterpenes, such as limonene and (Z)-ocimene and oxygenated ketones, such as dihydrotagetone, (E) and (Z)-tagetone and (Z)-ocimenone (Kumar et al. 2012). Based on PCA analysis, the Mexican marigold flowering stage (the most attractant stage) was positively correlated with limonene, (Z)-β-ocimene, dihydrotagetone, etc., which could be mainly responsible for the marigold attractiveness. However, olfactometry tests of greenhouse whiteflies against the five major compounds of marigold [limonene: dihydrotagetone: (Z)-β-ocimene: α-pinene: (Z)-3-hexenyl acetate] in their natural ratio elicited an attraction behavioural response towards the blend at 0.1%, and repellence at 1%. (Z)-3-hexenyl acetate, when tested individually on T.vaporariorum, was attractant at 0.01%, 0.1%, and 1%, indicating that it is the active compound responsible for attractiveness in the blend at 0.1% concentration. This hypothesis is supported by the findings of a study conducted on another whitefly (Bemisia tabaci) response to 3-hexenyl acetate, where the compound was found to elicit an attractant behavioural response in both Y-tube olfactometer and greenhouse assays (Li et al. 2014). Moreover, (Z)-3-hexenyl acetate, a key green leaf volatile released upon injury on plants, was found to be an attractant of greenhouse whiteflies by (Scala et al. 2013). This green leaf volatile has been reported to synergize attraction of corn earworms and codling moths to their respective sex pheromones (Light et al. 1993). The repellent nature of the volatile blend, as indicated at 1% concentration, could be due to: (1) the concentration, i.e. at high concentration some compounds become repellent (Nyasembe et al. 2012) or (2) the interaction of two or more compounds. For example, limonene and (Z)-β-ocimene, which were found to repel T. vaporariorum, can as a blend be even more repellent than the compounds alone. In a previous study, limonene dispensers were successfully used to repel T.vaporariorum from tomato and recommended for use alongside marigold plants in greenhouse trials (Conboy et al. 2019). As ocimene is an acyclic monoterpene, it is often released as a defence volatile in response to herbivory to repel pests (Bohlmann et al. 2000). (Z)-β-ocimene could also be used as a repellent stimulus to divert the pest from the crop or used in a push–pull system.
Conclusion
This study showed that different phenological stages of both basil (Ocimum basilicum L.) and Mexican marigold (Tagetes minuta L.) produce volatile cues which influence greenhouse whitefly (Trialeurode vaporariorum) behaviour. We further demonstrated that the flowering phenological stages of both plants were more attractive to whiteflies than tomato; hence, they may be good trap crop candidates for whitefly management. The attractiveness of marigold was found to be partly due to (Z)-3-hexenyl acetate, which at all tested concentrations was attractive to whiteflies while the insect’s attraction to flowering basil could be due to 1,8-cineole, eugenol and β-elemene. Moreover, the study revealed promising repellent compounds from both plants, i.e. compounds, such as limonene and (Z)-β-ocimene, from marigold and linalool from basil. The volatiles have potential as lures or bio-repellents in whitefly management.
References
Bengtsson J, Ahnström J, Weibull AC (2005) The effects of organic agriculture on biodiversity and abundance: a meta-analysis. J Appl Ecol 42:261–269. https://doi.org/10.1111/j.1365-2664.2005.01005.x
Bleeker PM, Diergaarde PJ, Ament K, Guerra J, Weidner M, Schutz S, Schuurink RC (2009) The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol 151:925–935. https://doi.org/10.1104/pp.109.142661
Bohlmann J, Martin D, Oldham NJ, Gershenzon J (2000) Terpenoid secondary metabolism in Arabidopsis thaliana: cDNA cloning, characterization, and functional expression of a myrcene/(e)-β-ocimene synthase. Arch Biochem Biophys 375:261–269. https://doi.org/10.1006/abbi.1999.1669
Calín-Sánchez Á, Lech K, Szumny A, Figiel A, Carbonell-Barrachina ÁA (2012) Volatile composition of sweet basil essential oil (Ocimum basilicum L.) as affected by drying method. Food Res Intl 48:217–225. https://doi.org/10.1016/j.foodres.2012.03.015
Camarillo G, Rodriguez C (2009) Biological activity of Tagetes filifolia (Asteraceae) on Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Rev Colomb Entomol 35:177–184
Cao F-Q, Liu W-X, Fan Z-N, Wan F-H, Cheng L-S (2008) Behavioural responses of Bemisia tabaci B-biotype to three host plants and their volatiles. Acta Entomol Sin 51:830–838
Carvalho MG, Bortolotto OC, Ventura MU (2017) Aromatic plants affect the selection of host tomato plants by Bemisia tabaci biotype. B. Entomol Exp Appl 162:86–92. https://doi.org/10.1111/eea.12534
Conboy NJA, McDaniel T, Ormerod A, George D, Gatehouse AMR, Wharton E, Tosh CR (2019) Companion planting with French marigolds protects tomato plants from glasshouse whiteflies through the emission of airborne limonene. PLoS ONE 14:1–21. https://doi.org/10.1371/journal.pone.0213071
de Carvalho LM, Nunes MUC, de Oliveira IR, de Leal M (2009) Yield of tomato in monocrop and intercropping with aromatics plants. Hort Brasil 27:458–464
Di Cesare LF, Forni E, Viscardi D, Nani RC (2003) Changes in the chemical composition of basil caused by different drying procedures. J Agric Food Chem 51:3575–3581. https://doi.org/10.1021/jf021080o
Diabate S, Martin T, Murungi LK, Fiaboe KKM, Subramanian S, Wesonga J, Deletre E (2019) Repellent activity of Cymbopogon citratus and Tagetes minuta and their specific volatiles against Megalurothrips sjostedti. J Appl Entomol 143:855–866. https://doi.org/10.1111/jen.12651
Dray S, Dufour AB (2007) The ade4 package: Implementing the duality diagram for ecologists. J Stat Softw 22:1–20. https://doi.org/10.18637/jss.v022.i04
FAO (2018) Food loss analysis: causes and solutions Case study on the tomato value chain in the Republic of Guyana. 48
Gorman K, Hewitt F, Denholm I, Devine GJ (2002) New developments in insecticide resistance in the glasshouse whitefly (Trialeurodes vaporariorum) and the two-spotted spider mite (Tetranychus urticae) in the UK. Pest Manag Sci 58:123–130. https://doi.org/10.1002/ps.427
Górski R (2004) Effectiveness of natural essential oils in the monitoring of greenhouse whitefly (Trialeurodes vaporariorum Westwood). Folia Hortic 16:183–187
Gross M (2013) EU ban puts spotlight on complex effects of neonicotinoids. Curr Biol 23:R462–R464. https://doi.org/10.1016/j.cub.2013.05.030
Hokkanen HMT (1991) Trap cropping in pest management. Annu Rev Entomol 36:119–138
Hussain AI, Anwar F, Hussain Sherazi ST, Przybylski R (2008) Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem 108:986–995. https://doi.org/10.1016/j.foodchem.2007.12.010
Inbar M, Gerling D (2008) Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Annu Rev Entomol 53:431–448. https://doi.org/10.1146/annurev.ento.53.032107.122456
Johnson MW, Caprio LC, Coughlin JA, Tabashnik BE, Rosenheim JA, Welter SC (1992) Effect of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on yield of fresh market tomatoes. J Econ Entomol 85:2370–2376. https://doi.org/10.1093/jee/85.6.2370
Kapantaidaki DE, Sadikoglou E, Tsakireli D, Kampanis V, Stavrakaki M, Schorn C, Tsagkarakou A (2018) Insecticide resistance in Trialeurodes vaporariorum populations and novel diagnostics for kdr mutations. Pest Manag Sci 74:59–69. https://doi.org/10.1002/ps.4674
Kumar R, Ramesh K, Pathania V, Singh B (2012) Effect of transplanting date on growth, yield and oil quality of Tagetes minuta L. in mid hill of North -Western Himalaya. J Essent Oil Bear Pl 15:405–414. https://doi.org/10.1080/0972060X.2012.10644068
Li Y, Zhong S, Qin Y, Zhang S, Gao Z, Dang Z, Pan W (2014) Identification of plant chemicals attracting and repelling whiteflies. Arthropod Plant Interact 8:183–190. https://doi.org/10.1007/s11829-014-9302-7
Light DM, Flath RA, Buttery RG, Zalom FG, Rice RE, Dickens JC, Jang EB (1993) Host-plant green-leaf volatiles synergize the synthetic sex pheromones of the corn earworm and codling moth (Lepidoptera). Chemoecology 4:145–152. https://doi.org/10.1007/BF01256549
Månsson PE (2005) Host selection and antifeedants in Hylobius abietis pine weevils. Doctoral thesis no.2005:16, Swedish University of Agricultural Sciences.
Moodley V, Gubba A, Mafongoya PL (2019) A survey of whitefly-transmitted viruses on tomato crops in South Africa. Crop Prot 123:21–29. https://doi.org/10.1016/j.cropro.2019.05.018
Mutisya S, Opiyo A, Martin T, Ngouajio M, Saidi M (2016) Synergistic effects of agronet covers and companion cropping on reducing whitefly infestation and improving yield of open field-grown tomatoes. Agronomy 6:42. https://doi.org/10.3390/agronomy6030042
Niggli U, Leifert C, Alföldi T, Lück L, Willer H (2007) Improving sustainability in organic and low input food production systems. In: Proceedings of the 3rd international congress of the European integrated project quality low input food (QLIF). University of Hohenheim, Germany. Research institute of organic agriculture FiBL, CH-Frick, vol 141, pp. 32–33
Nyasembe VO, Teal PE, Mukabana WR, Tumlinson JH, Torto B (2012) Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasite Vector 5:234. https://doi.org/10.1186/1756-3305-5-234
Pilkington LJ, Messelink G, van Lenteren JC, Le Mottee K (2010) “Protected biological control” biological pest management in the greenhouse industry. Biocontrol 52:216–220. https://doi.org/10.1016/j.biocontrol.2009.05.022
Pushpangadan P, George V (2012) Basil. In: Peter KV (ed) Handbook of herbs and spices. Woodhead publishing, Cambridge, pp 55–72. https://doi.org/10.1533/9780857095671.55
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ratnadass A, Fernandes P, Avelino J, Habib R (2012) Plant species diversity for sustainable management of crop pests and diseases in agroecosystems: a review. Agron Sustain Dev 32:273–303. https://doi.org/10.1007/s13593-011-0022-4
Roditakis N (1990) Host plants of greenhouse whitefly Trialeurodes vaporariorum westwood (Homoptera: Aleyrodidae) in Crete. Attractiveness and impact on whitefly life stages. Agric Ecosyst Environ 37:217–224
Scala A, Allmann S, Mirabella R, Haring MA, Schuurink RC (2013) Green leaf volatiles: a plant’s multifunctional weapon against herbivores and pathogens. Int J Mol Sci 14:17781–17811. https://doi.org/10.3390/ijms140917781
Schlaeger S, Pickett JA, Birkett MA (2018) Prospects for management of whitefly using plant semiochemicals, compared with related pests. Pest Manag Sci 74:2405–2411. https://doi.org/10.1002/ps.5058
Shelton AM, Badenes-Perez FR (2006) Concepts and applications of trap cropping in pest management. Annu Rev Entomol 51:285–308. https://doi.org/10.1146/annurev.ento.51.110104.150959
Szendrei Z, Rodriguez-Saona C (2010) A meta-analysis of insect pest behavioral manipulation with plant volatiles. Entomol Exp Appl 134:201–210. https://doi.org/10.1111/j.1570-7458.2009.00954.x
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418:671–677
Vieira RF, Simon JE (2006) Chemical characterization of basil (Ocimum spp.) based on volatile oils. Flavour Frag J 21:214–221. https://doi.org/10.1002/ffj.1513
Webster B, Bruce T, Dufour S, Birkemeyer C, Birkett M, Hardie J, Pickett J (2008) Identification of volatile compounds used in host location by the black bean aphid, Aphis fabae. J Chem Ecol 34:1153–1161. https://doi.org/10.1007/s10886-008-9510-7
Acknowledgement
This research was financially supported by ANR (16-CE32-0010-01) and the French Agricultural Research Centre for International Development (CIRAD), and materially by the International Center for Insect Physiology and Ecology (Icipe) and co-donors: UK Aid, the Swedish International Development Cooperation Agency (SIDA), and the Swiss Agency for Cooperation and Development (SDC).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Marko Rohlfs.
Rights and permissions
About this article
Cite this article
Matu, F.K., Murungi, L.K., Mohamed, S. et al. Behavioral response of the greenhouse whitefly (Trialeurodes vaporariorum) to plant volatiles of Ocimum basilicum and Tagetes minuta. Chemoecology 31, 47–62 (2021). https://doi.org/10.1007/s00049-020-00327-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-020-00327-z