Abstract
Male-produced pheromone components have been reported in the Asian longhorn beetle, Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae), but field attraction to these components has been less than overwhelming. Female ALBs were observed rhythmically extending the genitalia in a manner reminiscent of female calling behaviors in other cerambycid species. We thus hypothesized that female ALBs release volatile pheromone while performing this sex-specific behavior. A group of sesquiterpenes, including a major compound α-longipinene and several minor ones α-cubebene, α-ylangene, (−)-α-copaene, α-bergamotene, β-caryophyllene, and α-farnesene, found in genitalia extracts from virgin females elicited male antennal responses. Y-tube olfactometer assays indicated significant attraction of α-longipinene to both sexes in either the presence or absence of host volatiles. This compound was also detected in genitalia extracts from virgin males, though in much lower quantities than in females. Dose–response experiments conducted in the y-tube olfactometer and field both revealed that α-longipinene was attractive at the higher doses, but not at the lower ones. In the field, traps baited with a blend containing α-longipinene, α-cubebene, and β-caryophyllene captured significantly more ALB than solvent controls. The trap catches of α-longipinene combined with either the minor components or host compounds were both greater than those of α-longipinene alone, but the difference was not significant. These results indicate that α-longipinene is a new type of female-produced volatile pheromone in ALB, and the attraction may be synergistically enhanced by several minor components. Sesquiterpenes may play an important role in intraspecies chemical communication of this insect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
The Asian longhorn beetle is a highly risked invasive species to many parts of the world. Effective control tools are urgently needed.
-
A female-specific behavior resembling female calling behaviors in other cerambycids indicates female may release pheromone from genitalia.
-
α-Longipinene in female genitalia is attractive to both sexes.
-
α-Longipinene plays a role of aggregation pheromone. Several synergistic minor sesquiterpenes are also found in female genitalia.
-
α-Longipinene and its optimized blend with synergistic components would be effective lures for controlling this pest.
Introduction
The Asian longhorn beetle (ALB), Anoplophora glabripennis (Motschulsky) (Coleoptera: Cerambycidae: Lamiinae), is a broadly polyphagous hardwood borer originally distributed in East Asia (Lingafelter and Hoebeke 2002). Tree species in over 20 genera have been reported as hosts of ALB (Yan et al. 2008). Approximately 40% of the poplars in China were damaged by ALB, since the first reported outbreak of ALB in the 1960s through the 1990s, resulting in catastrophic economic and ecological impacts (Luo et al. 2000; Gao and Li 2001). Due to the large number of suitable host trees, the climatic similarity to its native regions, and increasing international trade, ALB is now a serious threat to vast areas elsewhere in the world (Straw et al. 2015; Kappel et al. 2017). Beginning a little over 20 years ago, new ALB invasions have been successively reported in the USA, Canada, and Europe (Morewood et al. 2003; Straw et al. 2016). The genetic structure of ALB in both invaded and native ranges suggests that the invasion history of this species consists of multiple introduction events and continuous secondary spread within the invaded area (Javal et al. 2019). In the invaded areas, the damage caused by ALB can be even greater than in its native range potentially due to the lack of natural enemies (Haack et al. 2010). Eradication of established ALB populations and prevention of its further dispersal outside of its native range are the objectives of government agencies tasked with managing the ALB problem.
Traps baited with chemical attractants are a highly valuable and cost-effective tool for detecting low-level insect populations, which is often a distinguishing feature of newly established invasive species (Crook et al. 2014). Chemically mediated communication is critical in the life history of the cerambycids, especially during the adult stage which is the only vagile stage in their life cycle (Allison et al. 2004). Semiochemicals typically mediate host location and oviposition, mate location and recognition of cerambycids (Hanks 1999). Among these semiochemicals, volatile pheromones have great importance in longhorn beetle detection, monitoring, and control, due to their high sensitivity and selectivity (Hanks and Millar 2016).
Several different volatile pheromone components have been reported in ALB. Zhang et al. (2002) identified two male-produced dialkyl ethers 4-(n-heptyloxy)butanal and 4-(n-heptyloxy)butanol as potential aggregation pheromones. Wickham et al. (2012) reported that auto- or photooxidation of female contact sex pheromones results in the formation of an attractive compound (hexadecanal). Later, another male-produced pheromone compound was identified as (3E,6E)-α-farnesene (Crook et al. 2014). The male-produced pheromone components were attractive to both sexes in laboratory assays, but they were weakly attractive in the field (Millar et al. 2009; Nehme et al. 2010; Crook et al. 2014). The attraction of male pheromones can be synergistically enhanced when combined with host volatiles (Nehme et al. 2009, 2010; Meng et al. 2014). Hexadecanal emitted by female ALB is so far the only female-pheromone candidate reported in the subfamily Lamiinae, but the field attraction of this compound was only significant when combined with host volatiles such as cis-3-hexen-1-ol, camphene, linalool, and linalool oxide (Wickham et al. 2012).
The weak attraction of the documented pheromones of ALB is likely attributable to incomplete component blends resulting from a poor understanding of adult behavior (Wickham et al. 2012; Crook et al. 2014). He and Huang (1993) found that males were attracted when live females were used as baits in both wind tunnel and field bioassays. The female body part releasing attractive chemicals was determined to be the ovipositor, because males were observed to approach females whose ovipositor was extruded (Lu et al. 1998) and because the detached female ovipositor was attractive to males (Lu et al. 2000). In our previous experiments, virgin females at various ages were often observed repeatedly extruding their genitalia, beyond the tip of the abdomen for approximately 1.5 min on average with their abdomens raised, whereas males were never observed to perform such behavior. Volatiles emitted from either live female adults or the extracts of female genitalia were attractive to male adults, suggesting that sexual attractants are produced in the female genitalia (Xu et al. 2019). The emission of volatile pheromones is associated with specific calling behaviors performed in various cerambycid species (Hanks and Millar 2016). The female calling behavior in many species in the subfamily Prioninae is very similar to that observed in female ALB. These species release sex pheromones from an eversible gland on the female ovipositor (Barbour et al. 2006, 2011; Rodstein et al. 2009, 2011).
To explore potential volatile pheromones emitted from female ALB genitalia, we investigated volatile chemicals from female ALB genitalia that are detectable by male ALB antenna, and evaluated the attractiveness of these chemicals to both sexes in a series of laboratory and field assays. We also examined whether there is a synergism between the attractants produced in the female genitalia and other ALB pheromone components or host volatiles to assess the potential for developing effective lures and to improve our understanding of intraspecies chemical communication in ALB.
Materials and methods
Insects
ALB adults were supplied by the Sarkaria Arthropod Research Laboratory (SARL) at Cornell University, Ithaca, NY, USA. Males were stored at SARL or transported under permit (USDA-PPQ 526) to the quarantine laboratory at the State University of New York, College of Environmental Science and Forestry (SUNY-ESF) in Syracuse, NY, USA. Females were only permitted to be kept inside the SARL quarantine facility at all times. All of the beetles were individually kept in 500-ml plastic cups in an environmental chamber at 23 °C and on a 16:8 photo-period at SARL after emergence, or at ambient room temperature on a 14:10 (L:D) photoperiod at SUNY-ESF. Three to four fresh striped maple (Acer pensylvanicum) twigs (2–10 mm diam × ~ 8 cm long) were provided as food for each adult and replaced weekly.
Identification of male antennally active compounds in female genital extracts
ALB genital extraction
Female ALBs were frozen at − 20 °C until motionless. The genitalia were then grasped and pulled out from abdomen with clean forceps and immediately excised with clean microdissection scissors. The detached genitalia from 10 to 11 females were immersed as a sampling group in 500 μl dichloromethane (DCM) in a 2.0-ml amber glass vial for 24 h to extract the volatile chemicals. The extracts were then concentrated to 100 μl under a gentle stream of nitrogen. Although male ALBs were not observed extruding the genitalia, male genitalia were also dissected and extracted (ten males as a sampling group) following the same method used in female genital extraction. In total, ten groups of females and six groups of males were sampled. A vial with 500 μl DCM was also concentrated to 100 μl as a solvent control. All extracted beetles were virgin (> 14 days old) and fed striped maple twigs after emergence as described above. Beetles were starved for 24 h before being sampled to reduce the level of host residues. Extracts were stored in a freezer at − 50 °C until further use.
Chemical analysis
The extracts were analyzed by coupled gas chromatography–mass spectrometry (GC–MS; Agilent 7890A GC interfaced with a 5975 mass selective detector in EI mode, 70 eV; Agilent Technologies, Santa Clara, CA, USA), fitted with an HP5-MS capillary column (30 m × 0.25 mm ID × 0.25 μm film thickness; Agilent Technologies, Santa Clara, CA, USA). Injections were made in splitless mode (1 min sampling) with an injector temperature of 280 °C. The oven was programmed from 40 °C for 1 min, ramped at 10 °C/min to 250 °C, and held at 250 °C for 10 min. The carrier gas was helium at a constant flow rate of 1 ml/min. Compounds in the samples were identified by comparison of mass spectra with corresponding compounds in an MS library (NIST 08) and confirmed by Kovats indices (KI; Kovats 1965) or co-injection when authentic standards were available. Co-injection with authentic standards was also conducted in the same GC–MS fitted with a DB-Wax capillary column (30 m × 0.25 mm ID × 0.25 μm film thickness; Agilent Technologies, Santa Clara, CA, USA), and Kovats indices of the compounds that lacked authentic standards were calculated. The concentration of the major antennally active component in samples was calculated by using a calibration curve (0.2, 1, 5, 10, and 50 ng/μl) based on authentic sample of α-longipinene. The other antennally active compounds were not quantified due to extremely low concentration in samples or a lack of authentic standard.
Electrophysiological analysis
The antennal responses of male adults to compounds in female genital extracts were analyzed by using coupled gas chromatography–electroantennographic detection (GC–EAD) on an HP 5890 Series GC (Hewlett-Packard, Sunnyvale, CA, USA) with the injector at 280 °C and an HP5-MS column (30 m × 0.25 mm ID × 0.25 μm film thickness; Agilent Technologies, Santa Clara, CA, USA) in splitless mode. Nitrogen was the carrier gas at a flow rate of 2 ml/min. The oven temperature program was as above. The column effluent was split by a glass Y-connector (Supelco, Bellefonte, PA, USA) into two deactivated capillary columns in equal length with nitrogen added as a makeup gas (8 ml/min) by another Y-connector. The two split streams were, respectively, connected to the flame ionization detector (FID) and to the antennal preparation. The FID signals were recorded on an HP 3396A integrator.
The four most distal flagellomeres were separated from living male adults (virgin, > 14 days old, fed with striped maple twigs after emergence), and the tip (< 0.5 mm length) of the last flagellomere was removed with a razor blade. The prepared antenna was then positioned between two gold wire electrodes which were, respectively, inserted into two micropipettes filled with Beadle–Ephrussi Ringer’s solution (Wickham et al. 2012) on a custom acrylic holder. The GC column and effluent passed through the oven wall in a heated transfer line, and the effluent was delivered to the antenna in charcoal-purified and humidified air (1 l/min) inside a cooling condenser. The distance between the antenna and the tip of GC column at the EAD port was approximately 0.75 cm. The output signal from the antenna was amplified by a custom high input impedance DC amplifier. The EAD signals were recorded on an HP 3390A integrator simultaneously with the FID signals.
Because all of the females used in our study had to be kept in SARL, there is no GC-EAD available there. Therefore, we were not able to assess GC–EAD responses by female antennae. In China, although live female adults could be collected in the field, the GC–EAD in the laboratory of our collaborator had low sensitivity and high noise disturbance, which led to an unsuccessful test.
Chemicals
The purities and sources of the compounds used for chemical identification and laboratory and field assays are listed in Table 1.
Laboratory assays
Behavioral activity of the major antennally active compound α-longipinene at different doses was assessed in Y-tube olfactometer assays. The Y-shaped glass olfactometer [main arm: 28 cm length × 6 cm internal dia (ID); two choice arms: 22 cm length × 6 cm ID, the angle between arms was 70°] was placed on a laboratory bench at a ~ 15° upward slant centered under a 500 W halogen lamp. Two 250-ml glass flasks containing a piece of filter paper (1 × 1 cm2) loaded with odor stimuli or solvent control were connected to the ends of the choice arms with Teflon® tubing. Charcoal-filtered and humidified air was pushed into each choice arm (1000 ml/min/arm) through the connected flasks. Directional visual cues were minimized by surrounding the olfactometer with brown paperboard. For each trial, a 10 μl aliquot of odor stimuli in solvent solution was placed on the filter paper in the flasks. A single virgin beetle (> 14 days old, fed with striped maple twigs after eclosion) was placed into the opening at the base end of the main arm and recorded as choice after it walked to the end of a choice arm within 20 min. Beetles that failed to reach the end of either choice arm within 20 min were recorded as no response. The response time was measured with a stopwatch and recorded. The olfactometer arms and the odor stimuli were physically alternated after every assay. The Y-tube olfactometer was cleaned with acetone and dried at room temperature after every other trial. All tests using males were performed between 09:00 and 17:00 at 25–30 °C and 60–70% relative humidity inside the olfactometer in a quarantine laboratory at SUNY-ESF, and females were tested at SARL under the same environmental conditions. Beetles were only used once in each test.
The following two-choice tests were conducted (the amount of α-longipinene listed for each test is for single trial):
-
1.
α-longipinene (0.012 μg: approximately one female equivalent) versus DCM.
-
2.
α-longipinene (0.12 μg) versus DCM.
-
3.
α-longipinene (1.2 μg) versus DCM.
-
4.
α-longipinene (0.12 μg) + stripe maple versus DCM + stripe maple. Two striped maple twigs (~ 0.5 cm diam × 5 cm length) were placed in each glass flask together with the filter paper. Twigs were replaced daily.
Field assays
Experiment 1: Effect of major and minor pheromone components
The attraction of α-longipinene and a blend of α-longipinene with minor antennally active compounds detected in female genital extracts were tested at a weeping willow (Salix babylonica) plantation with known ALB population along the Guo River in Huaiyuan County, Anhui Province, China (32° 58′ 2.73″ N 117° 11′ 33.13″ E). Authentic standards of three male EAD-active compounds [α-ylangene, (−)-α-copaene, and α-bergamotene] were not commercially available and thus not tested. Additionally, determination of the α-farnesene enantiomer produced in the female genitalia was not completed before the ALB flight season, so it was not included in the experiment. Lures were prepared by heat-sealing polyethylene tubing (~ 7 cm × 4.9 cm, wall thickness 0.05 mm; Uline, Pleasant Prairie, WI, USA) containing authentic compounds diluted in 1 ml isopropanol. Lures were hung in the center of black flight-intercept panel traps (IPM Technologies, Portland, OR, USA) which were coated with Teflon® PTFE DISP 30 (DuPont, Wilmington, DE, USA) before use (Graham and Poland 2012). Traps were randomly hung in willow trees 1–2 m above the ground and ≥ 10 m apart. Collection cups contained approximately 1:2 (v:v) solution of automobile antifreeze and water to kill and preserve trapped insects. The experiment was conducted between June 15 and August 10 in 2016. Trap catches were collected and lures were rotated weekly. Lures were replaced every 2–3 weeks. The traps were baited with: (1) 5 mg α-longipinene in 1 ml isopropanol (N = 10), (2) Blend 1: 5 mg α-longipinene, 5 mg α-cubebene, and 5 mg β-caryophyllene in 1 ml isopropanol (N = 10), and (3) 1 ml isopropanol (N = 10).
Experiment 2: Optimum dose of major pheromone component
The attraction of α-longipinene at different doses was examined in the field. The field site was located in a plantation (Populus nigra × P. simonii, P. beijingensis, S. babylonica, A. mono, Betula platyphylla, and Ulmus pumila) in Shengli Village, Hunchun City, Jilin Province, China (32° 05′ 15.8″ N 118° 44′ 29.2″ E). Lures were prepared by the same methods described in Experiment 1. Traps baited with four doses of α-longipinene were randomly hung in the trees 2–3 m above the ground and ≥ 10 m apart. The experiments were carried out during July 15 to September 2, 2017. Trapped ALBs were collected and lures were rotated weekly. Lures were replaced once on August 12. The treatments included (1) 0.2 mg α-longipinene in 1 ml isopropanol (N = 10), (2) 1 mg α-longipinene in 1 ml isopropanol (N = 10), (3) 5 mg α-longipinene in 1 ml isopropanol (N = 10), (4) 25 mg α-longipinene in 1 ml isopropanol (N = 10), and (5) 1 ml isopropanol (N = 10).
Experiment 3: Effect of previously reported pheromones and host volatiles on attraction to the major component
This experiment was conducted to test whether the attractiveness of α-longipinene at the optimum dose found in Experiment 2 could be enhanced when combined with other previously reported ALB pheromone components or host volatiles. Field site and lure preparation were the same as in Experiment 2. The experiments were carried out during July 29 to September 2, 2017. Trapped insects were collected and lures were rotated weekly. Lures were replaced on August 12. The treatments were (1) 5 mg α-longipinene in 1 ml isopropanol (N = 10), (2) Blend 2: 5 mg α-longipinene combined with host compounds including linalool, linalool oxide, cis-3-hexen-1-ol, camphene, 3-carene, and β-caryophyllene (5 mg each; Wickham et al. 2012) in 1 ml isopropanol (N = 10), (3) Blend 3: 5 mg α-longipinene and 5 mg hexadecanal in 1 ml isopropanol (N = 10), (4) Blend 4: 5 mg α-longipinene combined with 5 mg 4-(n-heptyloxy)butanal and 5 mg 4-(n-heptyloxy)butanol in 1 ml isopropanol (N = 10), and (5) 1 ml isopropanol (N = 10).
Statistical analyses
For laboratory assays, binomial tests (expected value = 0.5) were used to compare the numbers of ALBs, which made choices between two odor stimuli. Nonresponding beetles were removed from the analyses. For field experiments, as the trap catch numbers were not normally distributed, SAS Proc Genmod (2011) with Poisson regression with log link was applied to model the numbers of male, female, and male plus female ALB trapped, followed by pairwise contrast tests for assessing differences between treatment pairs. Multiple comparisons were adjusted using Bonferroni correction to control the experiment-wise error rate. Sex ratios were examined using the Chi-square test. The significance level in this study was α = 0.05.
Results
Identification of male antennally active compounds in female genitalic extracts
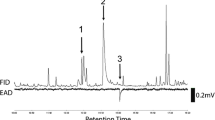
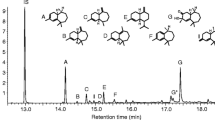
Seven compounds that elicited consistent EAD responses from male ALB were identified from female genital extracts (Fig. 1). The compound with the largest peak area was identified as α-longipinene [retention time (RT) 12.61 min] by GC–MS library match and confirmed by co-injection with authentic standard. α-Longipinene was detected in eight of ten female genital extracts, at approximately 100–300 ng per sample (10–11 female genitalia). In three of six male genital extracts, there was also α-longipinene, however, in much lower quantities (approximately 10–20 ng per sample of ten male genitalia).
Analysis of gas chromatograms (GC) of genital extracts of female and male ALBs and electroantennographic detection (EAD) of a male antenna to a female genital extract showing seven compounds that elicit responses [1. α-cubebene; 2. α-longipinene; 3. α-ylangene; 4. (−)-α-copaene; 5. α-bergamotene; 6. β-caryophyllene; and 7. α-farnesene]
There were another six compounds with much lower concentration compared to α-longipinene in female genital extracts that elicited male antennal responses, including α-cubebene (RT = 12.538 min), α-ylangene (RT = 12.84 min), (−)-α-copaene (RT = 12.921 min), α-bergamotene (RT = 13.389 min), β-caryophyllene (RT = 13.526 min), and α-farnesene (RT = 14.31). The identification of α-cubebene, (−)-α-copaene, β-caryophyllene, and α-farnesene were confirmed with authentic standards, while the identifications of α-ylangene (KI = 1380 in HP5-MS column; KI = 1471 in DB-Wax column) and α-bergamotene (KI = 1435 in HP5-MS column; KI = 1587 in DB-Wax column) were supported by Kovats indices as synthetic standards were not available (Lucero et al. 2003; Juliani et al. 2004; Fanciullino et al. 2005; Sawamura et al. 2006).
The ratio of α-longipinene, α-ylangene, (−)-α-copaene, β-caryophyllene, and α-farnesene was approximately 7:1:1:2:2 in the female genital extracts and 3:1:1:2:4 in the male genital extracts, based on peak area. The relative ratios of α-cubebene and α-bergamotene were not measurable due to extremely low quantities in the extracts.
Laboratory assays
In Y-tube olfactometer bioassays, α-longipinene at all doses except the lowest (0.012 μg) showed significantly stronger attraction to both male and female ALBs than control. The proportion of either males or females choosing α-longipinene increased when the doses were increased tenfold from 0.012 μg. Significantly more males and females were attracted to α-longipinene combined with striped maple twigs compared to the solvent with striped maple twigs. More importantly, the addition of host twigs increased the percentage of both males (79%) and females (67%) responding positively compared to the same dose of α-longipinene alone [males (57%) and females (62%)] (Fig. 2).
Responses of male and female ALBs to different doses of α-longipinene (αL) versus solvent control (DCM) and α-longipinene combined with striped maple twigs (SM) versus solvent plus striped maple twigs. Bars with an asterisk (*) are significantly different from the bars of opposite treatments (P < 0.05)
Field assays
Experiment 1: Effect of major and minor pheromone components
A total of nine male and seven female ALBs were captured in Experiment 1. Traps baited with either α-longipinene or a blend of α-longipinene, α-cubebene and β-caryophyllene (Blend 1) captured significantly more ALBs than those with isopropanol control (Fig. 3; P = 0.016 and < 0.0001, respectively, < 0.05/3 = 0.0167, Bonferroni corrected significance level). Traps with Blend 1 caught numerically more males and females than the ones with only α-longipinene, but the differences were not statistically significant (Fig. 3; male, P = 0.0766; female, P = 0.1469; total of two sexes, P = 0.0657). Both sexes were significantly more attracted to the lures containing Blend 1 compared to solvent control (male, P < 0.0001; female, P = 0.0146), while only the number of males in traps with α-longipinene was significantly larger than that in traps with isopropanol (male, P = 0.0004; female, P = 0.305). There was no significant difference between the sexes of beetles captured in traps baited with α-longipinene (χ2= 0.2, P = 0.6547), Blend 1 (χ2= 0.4, P = 0.5271), or the control (χ2= 1, P = 0.3173).
Number of ALBs captured in traps baited with 5 mg α-longipinene (αL), Blend 1 (5 mg α-longipinene, α-cubebene, and β-caryophyllene), and isopropanol control. White bars represent females and black bars represent males. Each pair of treatments was compared using Poisson regression analysis followed by pairwise contrasts. Bars with the same letter are not significantly different (P > 0.05/3 = 0.0167, Bonferroni adjusted significant level)
Experiment 2: Optimum dose of major pheromone component
There were 24 ALBs caught (14 males and 10 females) in Experiment 2. Traps with 5 mg and 25 mg α-longipinene both captured significantly more ALBs than the isopropanol control (P = 0.0005 in both comparisons, < 0.05/10 = 0.005, Bonferroni adjusted significance level), and those with α-longipinene at the doses of 0.2 mg and 1 mg (P = 0.0005 in all pairwise contrasts) (Fig. 4). No difference was detected between the numbers of ALBs caught in traps with 5 mg and 25 mg α-longipinene (Fig. 4; P = 1). Lures loaded with 0.2 mg and 1 mg α-longipinene did not catch significantly more ALBs than isopropanol controls. When males and females were analyzed separately, only traps baited with 25 mg α-longipinene caught significantly more males than those with the control (P = 0.0009). No females were captured in traps with 0.2 mg and 1 mg α-longipinene. However, the attraction of α-longipinene to females was significantly increased at the 5 mg and 25 mg doses compared to the lower doses (0.2 mg and 1 mg) (P < 0.0001 in all contrasts), and traps baited with α-longipinene at those doses both caught significantly more females than those with solvent control (P = 0.0007 and < 0.0001, respectively). The female catch was reduced when the dose of α-longipinene increased from 5 mg to 25 mg, but the difference was not significant (P = 0.4149). There was no sexual bias in the catch numbers in all treatments or the control (χ2= 2, P = 0.1573, in 0.2 mg and 1 mg α-longipinene; χ2= 0.111, P = 0.7389, in 5 mg and 25 mg α-longipinene; χ2= 0, P = 1, in isopropanol control).
Number of ALBs captured in traps baited with 0.2 mg, 1 mg, 5 mg, and 25 mg α-longipinene (αL) and isopropanol. White bars represent females and black bars represent males. Each pair of treatments was compared using Poisson regression analysis followed by pairwise contrasts. Bars with the same letter are not significantly different (P > 0.05/10 = 0.005, Bonferroni adjusted significant level)
Experiment 3: Effects of previously reported ALB pheromones and host volatiles on attraction to α-longipinene
A total of 14 male and 10 female ALBs were captured in Experiment 3. When combined with host compounds, traps baited with α-longipinene caught significantly more males, females, and the total of both sexes than those captured in traps with the solvent control (Fig. 5; P = 0.0006, < 0.0001, and < 0.0001, respectively, < 0.05/10 = 0.005, Bonferroni adjusted significant level). More males and females were caught in traps with α-longipinene when combined with host compounds (Blend 2) compared to α-longipinene alone, but the differences were not significant (P = 0.0121 and 0.0896, respectively). Traps with a blend of α-longipinene and hexadecanal (Blend 3) caught significantly more females and the total of both sexes than those with the isopropanol control (P < 0.0001 and P = 0.0027, respectively). However, traps baited with α-longipinene and male pheromone components (Blend 4) failed to attract more males or females than the controls (Fig. 5; male, P = 0.312; female, P = 0.0153). Among three blends, Blend 2 was most attractive to both sexes, with significant differences in the number of females caught and the total of both sexes compared to Blend 4 (P = 0.0043 and 0.0027, respectively). No significant difference between the sexes of beetles captured in traps was found in all treatments or the isopropanol control (χ2= 0, P = 1, in α-longipinene alone and Blend 3; χ2= 0.4, P = 0.5271, in Blend 2; χ2= 0.333, P = 0.5637, in Blend 4; χ2= 1, P = 0.3173, in the control). Moreover, other xylophagous species were captured, including Massicus raddei (Cerambycidae) and Paranthrene tabaniformis (Sesiidae). A single M. raddei was caught in a trap baited with isopropanol, and two P. tabaniformis were caught in traps baited with α-longipinene combined with host compounds (Blend 2).
Number of ALBs captured in traps baited with 5 mg α-longipinene (αL), Blend 2 [5 mg α-longipinene, linalool, linalool oxide, cis-3-hexen-1-ol, camphene, β-caryophyllene, and 3-carene], Blend 3 (5 mg α-longipinene and hexadecanal), Blend 4 [5 mg α-longipinene, 4-(n-heptyloxy)butanal, and 4-(n-heptyloxy)butanol], and isopropanol. White bars represent females and black bars represent males. Each pair of treatments was compared using Poisson regression analysis followed by pairwise contrasts. Bars with the same letter are not significantly different (P > 0.05/10 = 0.005, Bonferroni adjusted significant level)
Discussion
Our results show that α-longipinene produced by the genitalia of female ALB is attractive to both sexes. While this compound is also present in male genitalia, the quantity is approximately one-tenth of that in female genitalia. Moreover, α-longipinene in the male genitalia may not function as an attractant, because the quantities are so low and dose–response tests in both the laboratory and field suggest that α-longipinene is not attractive to ALB at low concentrations. The thresholds for males and females may be different as only males were captured in traps baited with lower doses (0.2 mg and 1 mg) of α-longipinene in the field. The influence of mating status on the production of this compound in ALB needs further investigation.
The other antennally active sesquiterpenes found in female genitalia, α-cubebene, α-ylangene, (−)-α-copaene, α-bergamotene, β-caryophyllene, and α-farnesene, were present in much lower quantities compared to α-longipinene. It is noteworthy that the most abundant male antennally active compound in male genital extract is α-farnesene, one of whose isomers, (3E,6E)-α-farnesene, has been reported as a male-produced pheromone component (Crook et al. 2014). Unfortunately, we failed to confirm its isomer due to a lack of synthetic standards in the present study. Although only α-cubebene and β-caryophyllene were available for the field assay, the trap catches of both sexes increased when they were added to α-longipinene. This indicates a potential synergistic function between the major and minor components. These results support the hypothesis that female ALBs release attractive semiochemicals during genital extrusion. However, our results show that α-longipinene is attractive to both sexes, [i.e., an aggregation-sex pheromone sensu Cardé (2014)], rather than a sex pheromone as previously hypothesized (Lu et al. 1998; Xu et al. 2019). This discrepancy is probably because attraction of the female ovipositor to females was not tested in those studies. No significant differences were detected between the numbers of males and females caught in traps, though it is not possible to determine whether the attraction of α-longipinene is equal to males and females without knowing the sex ratios of ALB populations at the field sites (Ray et al. 2009; Silva et al. 2016).
The responses of both sexes to α-longipinene were increased by host volatiles, similar to the synergistic effect of host plant volatiles on pheromone attraction in several cerambycid species including ALB (Nehme et al. 2009, 2010; Meng et al. 2014; Hanks and Millar 2016). Hexadecanal is generated by the oxidation of female-produced cuticular hydrocarbon precursors and is attractive to ALB when combined with host kairomones (Wickham et al. 2012). Our trapping results show that the attraction of α-longipinene was slightly enhanced when combined with hexadecanal. The two ethers reported by Zhang et al. (2002), 4-(n-heptyloxy)butanal and 4-(n-heptyloxy)butanol, are male-produced compounds that are attractive to both sexes in laboratory behavioral assays. Statistically significant attraction of these components was mainly obtained when combined with host kairomones in field assays (Nehme et al. 2010; Meng et al. 2014). We formulated a blend of α-longipinene and male-produced ethers to investigate whether the effectiveness of lures can be enhanced for practical use. The trap catches of both sexes in traps baited with this blend were not higher than those in traps with α-longipinene alone. This result indicates no synergism between these components from different producers. Thus, α-longipinene and male pheromones may function separately in different stages of ALB mate location (Wickham et al. 2012).
The overall trap catches in our field trials were not high, and this may have been due to several factors. First, an insecticide was applied at the field site in Huaiyuan County (Field experiment 1), to suppress an outbreak of the fall webworm (Hyphantria cunea); this also reduced the ALB population as dead beetles were frequently observed on the ground. Second, at the field site in Hunchun City (field experiments 2 and 3), the ALB signs including larval frass, exit holes, and oviposition pits indicated that the population of wood borers was high. However, these signs are typical of several other xylophagous species including Lamiomimus gottschei (Cerambycidae), M. raddei, Mesosa myops (Cerambycidae), Cryptorhynchus lapathi (Curculionidae), and P. tabaniformis which were also collected in traps or observed on trees at the site. Third, due to a lack of authentic standards, only three of the male antennally active compounds found in female genitalia were tested in the field trials. The incomplete composition of lures may have reduced field attraction. Fourth, the authentic standards of α-longipinene used in our experiments were a mixture of isomers, so the “wrong” isomer or enantiomer may have weakened the lure attraction (Hanks and Millar 2013; Meier et al. 2016). Fifth, the ratio of the sesquiterpenes released to the air by females appears to be different from that in the genital extracts and seems vary at different physiological status (i.e., age, mating, and feeding) (data not shown), so the formulated lure blends were possibly not released in an optimal ratio. Thus, the optimum ratio must be determined in future studies to develop more effective lures. Furthermore, ALB mate location may also be regulated by multiple other factors besides volatile pheromones, such as visual cues, host volatiles, female produced trail pheromone, and contact pheromone (Zhang et al. 2003; Lund et al. 2005; Wickham et al. 2012; Hoover et al. 2014).
Many sesquiterpenes play important roles in the interactions among plants, insect herbivores, and natural enemies, e.g., direct attractants or deterrents to herbivores or indirect olfactory cues to attract predators or parasitoids that attack herbivores (Joo et al. 2017). Also, sesquiterpenes can be utilized by insects for intraspecies chemical communication. For instance, (E)-β-farnesene is a principal alarm pheromone component in many aphid species (Hemiptera: Aphididae) (Joachim et al. 2015).
In the Cerambycidae, monoterpenes (e.g., α-pinene) emitted by plants are widely used as kairomones by many species (Allison et al. 2004); some monoterpenes and terpenoids such as (S)-(−)-limonene, (−)-α-terpineol, and nerol are synergistic in combination with the male-produced pheromone blend of Megacyllene caryae (subfamily Cerambycinae) (Lacey et al. 2008). In the subfamily Lamiinae, some male-produced aggregation-sex pheromones are composed of sesquiterpene catabolic products, such as geranylacetone, its corresponding alcohol (fuscumol), and the acetate ester of the alcohol (fuscumol acetate), as well as a newly identified compound (S)-6-methylhept-5-en-2-ol [(S)-sulcatol] (Sweeney et al. 2010; Meier et al. 2019). In the genus Anoplophora, β-elemene, β-caryophyllene, α-humulene, and α-farnesene are released from the elytra of A. chinensis and act as intraspecific attractants. Similar chemical compositions are also found in the bark and leaf extracts of hosts, indicating that A. chinensis may derive these compounds from their hosts (Yasui et al. 2007, 2008). The volatiles from wounded willow twigs even showed a higher attraction to A. chinensis adults than the male-produced pheromone 4-(n-heptyloxy)butanol (also a male produced pheromone component in ALB) (Yasui et al. 2019). The role of these sesquiterpenes in A. chinensis is ambiguous as they are kairomones in the sense that they may be originally produced by the host plants, but they are also pheromones because beetles acquire and release them for intraspecies communication (Wyatt 2003; Yasui et al. 2008). In ALB, Crook et al. (2014) identified (3E,6E)-α-farnesene as a potential third component of the male-produced pheromone blend. These findings indicate that sesquiterpenes and related chemicals may play an unexpectedly important role in intraspecies communication of lamiines in which adults commonly need to feed on host tissues (e.g., bark) before sexual maturation and sequester host compounds, rather than being utilized as host kairomones only.
The semiochemicals produced by both herbivorous insects and herbivore-damaged plants are cues indicating the presence of host or prey for natural enemies (Hatano et al. 2008; Hare 2011). However, information that natural enemies can gain from these chemicals may vary. Large amount of plant volatiles may provide more detectable cues but with much lower accuracy; prey-produced pheromones are often considered as more reliable semiochemicals due to their specialization, but more difficult to detect due to their trace amounts (Vet and Dicke 1992; Wiskerke et al. 1993). Sesquiterpenes are common phytochemicals released by many plants. The employment of these compounds in intraspecies chemical communication by herbivorous insects thus may reduce the exploitation by their natural enemies, lowering the probability that they would attract natural enemies.
Our study suggests that α-longipinene is a candidate for future lure development for monitoring ALB in both its native range in Asia and invaded regions in Europe and North America and detecting new invasions in other parts of the world. More effective lures could be expected by optimizing the lure compositions with synergistic components.
Author contributions
TX and ST conceived and designed research. TX, LH, and DC conducted experiments. DH and LZ offered laboratory facility. TX analyzed data and wrote the manuscript. All authors read and approved the manuscript.
References
Allison JD, Borden JH, Seybold SJ (2004) A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 14:123–150
Barbour JD, Cervantes DE, Lacey ES, Hanks LM (2006) Calling behavior in the primitive longhorned beetle Prionus californicus Mots. J Insect Behav 19:623–629
Cardé RT (2014) Defining attraction and aggregation pheromones: teleological versus functional perspectives. J Chem Ecol 40:519–520
Crook DJ, David RL, Mastro VC (2014) Identification of a potential third component of the male-produced pheromone of Anoplophora glabripennis and its effect on behavior. J Chem Ecol 40:1241–1250
Fanciullino AL, Gancel AL, Froelicher Y, Luro F, Ollitrault P, Brillouet JM (2005) Effects of nucleo-cytoplasmic interactions on leaf volatile compounds from citrus somatic diploid hybrids. J Agric Food Chem 53(11):4517–4523
Gao RT, Li GH (2001) A review of studies on Asian longhorned beetle Anoplophora glabripennis (Coleoptera: Cerambycidae) and development trend. Chin J Appl Entomol 38(4):252–258 (in Chinese)
Graham EE, Poland TM (2012) Efficacy of Fluon for conditioning different designs of intercept traps in capturing cerambycid beetles and the effect of Fluon on panel traps over time. J Econ Entomol 105:395–401
Haack RA, Herard F, Sun JH, Turgeon JJ (2010) Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: a worldwide perspective. Annu Rev Entomol 55:521–546
Hanks LM (1999) Influence of the larval host plant on reproductive strategies of cerambycid beetles. Annu Rev Entomol 44:483–505
Hanks LM, Millar JG (2013) Field bioassays of cerambycid pheromones reveal widespread parsimony of pheromone structures, enhancement by host plant volatiles, and antagonism by components from heterospecifics. Chemoecology 23:21–44
Hanks LM, Millar JG (2016) Sex and aggregation-sex pheromones of cerambycid beetles: basic science and practical applications. J Chem Ecol 42:631–654
Hare JD (2011) Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu Rev Entomol 56(1):161–180
Hatano E, Kunert G, Michaud JP, Weisser WW (2008) Chemical cues mediating aphid location by natural enemies. Eur J Entomol 105(5):797–806
He P, Huang JF (1993) Adult behavior of Anoplophora glabripennis (Coleoptera: Cerambycidae). Acta Entomol Sin 36:51–55 (in Chinese)
Hoover K, Keena M, Nehme M, Wang S, Meng P, Zhang A (2014) Sex-specific trail pheromone mediates complex mate finding behavior in Anoplophora glabripennis. J Chem Ecol 40:169–180
Javal M, Roques A, Haran J, Hérard F, Keena M, Roux G (2019) Complex invasion history of the Asian long-horned beetle: fifteen years after first detection in Europe. J Pest Sci 92:173–187
Joachim C, Vosteen I, Weisser WW (2015) The aphid alarm pheromone (E)-β-farnesene does not act as a cue for predators searching on a plant. Chemoecology 25:105–113
Joo Y, Schuman MC, Goldberg JK, Sang K, Yon F, Brütting C, Baldwin IT (2017) Herbivore-induced volatile blends with both “fast” and “slow” components provide robust indirect defence in nature. Funct Ecol 32:136–149
Juliani HR, Zygadlo JA, Scrivanti R, de la Sota E, Simon JE (2004) The essential oil of Anemia tomentosa (Savigny) Sw. var. anthriscifolia (Schrad.) Mickel. Flavour Fragr J 19:541–543
Kappel AP, Trotter RT, Keena MA, Rogan J, Williams CA (2017) Mapping of the Asian longhorned beetle’s time to maturity and risk to invasion at contiguous United States extent. Biol Invasions 19:1999–2013
Kovats ES (1965) Gas chromatographic characterization of organic substances in the retention index system. In: Giddings JC, Keller RA (eds) Advances in chromatography. Marcel Dekker Inc, New York, pp 229–247
Lacey ES, Moreira JA, Millar JG, Hanks LM (2008) A male-produced aggregation pheromone blend consisting of alkanediols, terpenoids, and an aromatic alcohol from the cerambycid Beetle Megacyllene caryae. J Chem Ecol 34:408–417
Lingafelter SW, Hoebeke ER (2002) Revision of Anoplophora (Coleoptera: Cerambycidae). Entomol Soc Wash, Washington
Lu Q, Zhang YF, Zhang HS (1998) Calling, mating, and ovipositing behaviors of Anoplophora glabripennis (Coleoptera: Cerambycidae). Inner Mong For Sci Technol 3:7–9 (in Chinese)
Lu Q, Zhang YF, Wang WX, Zhang HS (2000) The bioactivity of female ovipositor and gland excretion of Anoplophora glabripennis (Coleoptera: Cerambycidae). Inner Mong For Sci Technol 2:41–42 (in Chinese)
Lucero ME, Estell RE, Fredrickson EL (2003) The essential oil composition of Psorothamnus scoparius (A. Gray) Rydb. J Essent Oil Res 15(2):108–111
Lund J, Francese JA, Teale SA (2005) The effect of placement height, color and release rate on trap catches of the Asian Longhorn Beetle, Anoplophora glabripennis. In: Gottschalk KW (ed) Proceedings, XV U.S. Department of Agriculture interagency research forum on gypsy moth and other invasive species 2004; 13–16 Jan 2004; Annapolis, MD. General Technical reports NE-332. U.S. Department of Agriculture, Forest Service, Northeastern Research Station, Newtown Square
Luo YQ, Huang JF, Li JG (2000) Main achievements, problems and prospects on researches of poplar longhorn beetles in China. Chin J Appl Entomol 37(2):116–121 (in Chinese)
Meier LR, Zou Y, Millar JG, Mongold-Diers JA, Hanks LM (2016) Synergism between enantiomers creates species-specific pheromone blends and minimizes cross-attraction for two species of cerambycid beetles. J Chem Ecol 42:1181–1192
Meier LR, Millar JG, Mongold-Diers JA, Hanks LM (2019) (S)-Sulcatol is a pheromone component for two species of cerambycid beetles in the subfamily Lamiinae. J Chem Ecol. https://doi.org/10.1007/s10886-019-01071-7
Meng PS, Trotter RT, Keena MA, Baker TC, Yan S, Schwartzberg EG, Hoover K (2014) Effects of pheromone and plant volatile release rates and ratios on trapping Anoplophora glabripennis (Coleoptera: Cerambycidae) in China. Environ Entomol 43(5):1379–1388
Millar JG, Hanks LM, Moreira JA, Barbour JD, Lacey ES (2009) Pheromone chemistry of cerambycid beetles. In: Nakamuta K, Millar JG (eds) Chemical ecology of wood-boring insects. Forestry and Forest Products Research Institute, Ibaraki, pp 52–79
Morewood WD, Neiner PR, Mcneil JR, Sellmer JC, Hoover K (2003) Oviposition preference and larval performance of Anoplophora glabripennis (Coleoptera: Cerambycidae) in four eastern North American hardwood tree species. Environ Entomol 32:1028–1034
Nehme ME, Keena MA, Zhang A, Baker TC, Hoover K (2009) Attraction of Anoplophora glabripennis to male-produced pheromone and plant volatiles. Environ Entomol 38(6):1745–1755
Nehme M, Keena MA, Zhang A, Baker TC, Xu Z, Hoover K (2010) Evaluating the use of male-produced pheromone components and plant volatiles in two trap designs to monitor Anoplophora glabripennis. Environ Entomol 39(1):169–176
Ray AM, Millar JG, McElfresh JS, Swift IP, Barbour JD, Hanks LM (2009) Male-produced aggregation pheromone of the cerambycid beetle Rosalia funebris. J Chem Ecol 35:96–103
Rodstein J, Mcelfresh JS, Barbour JD, Ray AM, Hanks LM, Millar JG (2009) Identification and synthesis of a female-produced sex pheromone for the cerambycid beetle Prionus californicus. J Chem Ecol 35:590–600
Rodstein J, Millar JG, Barbour JD, McElfresh JS, Wright IM, Barbour KS, Ray AM, Hanks LM (2011) Determination of the relative and absolute configurations of the female-produced sex pheromone of the cerambycid beetle Prionus californicus. J Chem Ecol 37:114–124
SAS Institute (2011) The SAS system for Windows. Release 9.4. SAS Inst., Cary
Sawamura M, Onishi Y, Ikemoto J, Tu NTM, Phi NTL (2006) Characteristic odour components of bergamot (Citrus bergamia Risso) essential oil. Flavour Fragr J 21(4):609–615
Silva WD, Millar JG, Hanks LM (2016) 10-Methyldodecanal, an attractant pheromone produced by males of the cerambycid species Eburodachrys vittata. PLoS ONE 11(8):e0160727
Straw NA, Tibury C, Fielding NJ, Williams DT, Cull T (2015) Timing and duration of the life cycle of Asian longhorn beetle Anoplophora glabripennis (Coleoptera: Cerambycidae) in southern England. Agric For Entomol 17:400–411
Straw NA, Fielding NJ, Tibury C, Williams DT, Cull T (2016) History and development of an isolated outbreak of Asian longhorn beetle Anoplophora glabripennis (Coleoptera: Cerambycidae) in southern England. Agric For Entomol 18:280–293
Sweeney J, Silk PJ, Gutowski JM, Wu J, Lemay MA, Mayo PD, Magee DI (2010) Effect of chirality, release rate, and host volatiles on response of Tetropium fuscum (F.), Tetropium cinnamopterum Kirby, and Tetropium castaneum (L.) to the aggregation pheromone, fuscumol. J Chem Ecol 36:1309–1321
Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a, tritrophic context. Annu Rev Entomol 37(1):141–172
Wickham JD, Xu Z, Teale SA (2012) Evidence for a female-produced, long range pheromone of Anoplophora glabripennis (Coleoptera: Cerambycidae). Insect Sci 19:355–371
Wiskerke JSC, Dicke M, Vet LEM (1993) Larval parasitoid uses aggregation pheromone of adult hosts in foraging behaviour: a solution to the reliability–detectability problem. Oecologia 93:145–148
Wyatt TD (2003) Pheromones and animal behaviour: communication by smell and taste. Cambridge University Press, Cambridge
Xu T, Hansen L, Teale SA (2019) Female calling behaviour in the Asian longhorn beetle (Coleoptera: Cerambycidae). Can Entomol. https://doi.org/10.4039/tce.2019.37
Yan XF, Li XJ, Luo YQ, Xu ZC, Tian GF, Zhang TL (2008) Taxis response of Anoplophora glabripennis adults to volatiles emanating from their larval host twigs. J Beijing For Univ 30(3):80–84 (in Chinese)
Yasui H, Yasuda T, Fukaya M, Akino T, Wakamura S, Hirai Y, Kawasaki K, Ono H, Narahara M, Kousa K, Fukuda T (2007) Host plant chemicals serve intraspecific communication in the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae). Appl Entomol Zool 42:255–268
Yasui H, Akino T, Fukaya M, Wakamura S, Ono H (2008) Sesquiterpene hydrocarbons: kairomones with a releaser effect in the sexual communication of the white-spotted longicorn beetle, Anoplophora malasiaca (Thomson) (Coleoptera: Cerambycidae). Chemoecology 18:233–242
Yasui H, Fujiwara-Tsujii N, Yasuda T (2019) Detection of volatile pheromone candidates from the white-spotted longicorn beetle, Anoplophora malasiaca (Coleoptera: Cerambycidae). Appl Entomol Zool 54(2):203–211
Zhang A, Oliver JE, Aldrich JR, Wang B, Mastro VC (2002) Stimulatory beetle volatiles for the Asian longhorned beetle, Anoplophora glabripennis (Motschulsky). Z Naturforsch 57:553–558
Zhang A, Oliver JE, Chauhan K, Zhao BG, Xia LQ, Xu ZC (2003) Evidence for contact sex recognition pheromone of the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Naturwissenschaften 90:410–413
Acknowledgements
We thank Ann Hajek of Cornell University for providing ALB from the Sarkaria Arthropod Research Laboratory for our use, Shaoming Hong of Huaiyuan Bureau of Forestry, Huaiyuan, Anhui, China, and Guobin Gu of Hunchun Bureau of Forestry, Hunchun, Jilin, China, for access to field sites and assistance with field work. We also thank Dr. Chunbo Zhang of Yanbian University, Yanji, Jilin, China, for providing laboratory facilities and solvents used in lure preparation. This research was made possible by the generous support of the Alphawood Foundation.
Funding
This study was funded by grants from the Alphawood Foundation to SAT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Research involving human participants and/or animals
This article does not involve any studies with human participants or vertebrate animals.
Additional information
Communicated by J.J. Duan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, T., Hansen, L., Cha, D.H. et al. Identification of a female-produced pheromone in a destructive invasive species: Asian longhorn beetle, Anoplophora glabripennis. J Pest Sci 93, 1321–1332 (2020). https://doi.org/10.1007/s10340-020-01229-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-020-01229-3