Abstract
Aromia bungii is a serious pest of stone fruit trees including cherries, plums, peaches and apricots. It is native to eastern Asia but has recently been introduced into and has established in Japan, Germany and Italy and has been intercepted in cargo entering the USA and Great Britain. We synthesized the naturally produced enantiomer of the major pheromone component, (E)-2-cis-6,7-epoxynonenal, and in field tests, comparing its attractiveness to that of the racemate. We also tested different ratios of a minor pheromone component, (2E,6Z)-nona-2,6-dienal, on attraction to the major component. Lastly, we conducted a dose–response assay to determine the optimal loading rates. Addition of the minor component at a ratio of 0.31 mg minor to 25 mg major component is more than double the trap captures of males but not females. Using this ratio, we found no difference in trap captures with 10 mg, 32 mg or 100 mg of the major component, but the 10 mg load attracted significantly more females than the 3.2 mg load, and only the 32 mg load attracted significantly more males than the 3.2 mg load. The natural enantiomer was significantly more attractive to females than the racemate. However, the multi-step, low-yielding synthesis likely will prohibit the insect-produced enantiomer from being used for operational trapping. The addition of the minor component to the racemate significantly increased trap catches, and the dose–response assay did not indicate significant differences in loads above 10 mg of the racemic major component when formulated with ~ 1.2% of the minor component.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key messages
-
Aromia bungii is a serious pest of stone fruit trees and is becoming globally invasive.
-
A blend of the pheromone components (E)-2-cis-6,7-epoxynonenal and (2E,6Z)-nona-2,6-dienal constitutes an effective lure for detection and management of this pest.
-
The optimum ratio of minor to major pheromone components is 0.31:25, and doses of 10 mg or more should be effective as trap lures.
-
The natural enantiomer attracted more females but the multi-step, low-yielding synthesis would likely prohibit its operational use.
Introduction
Aromia bungii (Falderman) (Coleoptera: Cerambycidae) is a destructive wood-boring pest of trees in the genus Prunus, including important stone fruit trees such as cherries, plums, peaches and apricots (EPPO 2014; Wang 2017). The basic biology of the beetle was described by Wang et al. (2007). Eggs are laid in bark cracks and crevices of host trees, and the developing larvae bore in the phloem, before turning inward to form overwintering/pupation chambers in the wood. The beetles overwinter as larvae and then emerge from June to August, depending on climate. Adults feed on ripe or rotting fruit and live about 2 months, during which time females can mate and oviposit multiple times, laying on average > 300 eggs. The life cycle takes 2–4 years, depending on climatic conditions. Infested trees are characterized by the copious frass which is expelled by the developing larvae.
Aromia bungii is native to eastern Asia from Vietnam to Mongolia and the Russian Far East but has recently become established in Japan, Germany and Italy, and it has been intercepted in cargo entering the USA, Great Britain and Australia (Ma et al. 2007; Anonymous 2013, 2015; Burmeister et al. 2012; Garonna et al. 2013). While there are no known established populations in the latter three countries, it is seen as a serious potential threat and detection and quarantine measures have been initiated in all three countries to prevent establishment. In the short time since its introduction into Japan in 2013, it has already caused serious damage to peach orchards and the renowned cherry blossom trees (Anonymous 2013; Yasui et al. 2018). In Germany and Italy, eradication protocols are in effect, including removal of infested trees, public education campaigns and intensive surveillance around any known sites of infestation. A very recent Center for Agriculture and Bioscience International (CABI) datasheet states that A. bungii “presents a significant risk to all stone fruit-growing countries in Europe and neighboring countries” (CABI 2018).
The life cycle and feeding habits of A. bungii are typical of wood-boring cerambycids. Larvae tunnel in the vascular tissues of the stem and branches and later the heartwood, where they form pupal chambers and overwinter, sometimes for multiple years. Feeding damage weakens the tree’s structure, damages vascular function and creates infection courts, all of which lead to stem decay, breakage, reduced fruit yields and tree death when infestations are extensive (Anonymous 2015; Yu and Gao 2005). As is the case with most subcortical insects, insecticidal control is difficult.
Detection surveys based on visual inspection of trees and wooden materials are inefficient because larvae and pupae may be located deep in the xylem (EPPO 2014). In the later stages of larval development, frass expelled from the galleries conspicuously accumulates around the base of infested trees (Xu et al. 2017). Adult emergence holes are also easily discernable, as are the conspicuous diurnal adults. However, all of these visual signs of infestation are reliable only after beetle population densities have reached damaging levels.
The severity of the threat posed by A. bungii has spurred interest in the development of semiochemically based tools for its detection and management. Fukaya et al. (2017) reported that females were attracted to the odor of males in laboratory bioassays, and Xu et al. (2017) identified the structure of the major male-produced aggregation-sex pheromone as (E)-2-cis-6,7-epoxynonenal. This component elicited electrophysiological responses from antennae of female beetles and, in preliminary field trials, traps baited with racemic (E)-2-cis-6,7-epoxynonenal were significantly attractive to beetles of both sexes (Xu et al. 2017). Here, we report the results of further experiments aimed at optimizing the attractant lure for practical applications. Our specific objectives were:
-
1.
To determine which enantiomer of (E)-2-cis-6,7-epoxynonenal the beetles produce, and to directly compare attraction to the insect-produced enantiomer versus the much cheaper racemic material,
-
2.
To determine whether a minor male-produced component, (2E,6Z)-nona-2,6-dienal, identified by Wei et al. (2013) and Xu et al. (2017), might increase attraction to the major component and
-
3.
To run a dose–response trial, to determine the optimal lure loading rates.
Materials and methods
Sources of chemicals
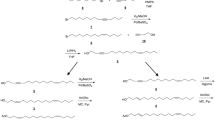
(2E,6Z)-Nona-2,6-dienal was purchased from Bedoukian Research (Danbury CT, USA). Racemic (E)-2-cis-6,7-epoxynonenal was synthesized as described in Xu et al. (2017). The enantiomers of (E)-2-cis-6,7-epoxynonenal were synthesized as shown in Fig. 1, and as described below.
(4R,5S)-4,5-Epoxyhept-2-enoic acid methyl ester (5)
(2R,3S)-Epoxy alcohol 4a was prepared by Sharpless asymmetric epoxidation of cis-2-penten-1-ol 3 with d-(-)-diisopropyl tartrate, titanium isopropoxide and t-butylhydroperoxide, followed by preparation and fractional crystallization of the corresponding 2,4-dinitrobenzoate ester, and subsequent hydrolysis of the ester to give 4a in high enantiomeric purity, as previously described (Gao et al. 1987; Chong 1989). The enantiomeric purity was checked by analysis on a chiral stationary phase Cyclodex-B GC column (30 m × 0.25 mm i.d., 0.25-μm film thickness, J&W Scientific, Folsom, CA, USA), with an injector temp of 150 °C. Injections were made in split mode at 25 psi head pressure, with an isothermal oven temperature (85 °C). Under these conditions, (2R,3S)-4a matched the earlier eluting peak at 13.43 min, and (2S,3R)-epoxyalcohol 4b (see below) matched the later peak at 13.79 min. To the limits of detection, 4a and 4b were enantiomerically pure.
A suspension of (2R,3S)-epoxyalcohol 4a (2.55 g, 25 mmol), MnO2 (21.74 g, 250 mmol) and methyl (triphenylphosphoranylidene)acetate (10.03 g, 30 mmol) in CHCl3 (75 mL) was refluxed for 18 h. After cooling to room temperature, the suspension was filtered through celite and the filter pad was washed with CH2Cl2. The filtrate was concentrated in vacuo, and hexane was added to the resulting yellow solid. The suspension was stirred for 30 min, and the precipitated triphenylphosphine oxide was removed by filtration. After concentration, the crude product was purified by flash chromatography (hexane/EtOAc = 95/5) to give ester 5 (E:Z = 7:3) as a colorless liquid (2.32 g, 59%). E-isomer: 1H NMR (CDCl3, 400 MHz) δ 6.82 (dd, J = 15.6, 6.4 Hz, 1H), 6.13 (dd, J = 15.6, 0.8 Hz, 1H), 3.75 (s, 3H), 3.51–3.54 (m, 1H), 3.13–3.17 (m, 1H), 1.58–1.69 (m, 1H), 1.43–1.54 (m, 1H), 1.01 (t, J = 7.6 Hz, 3H); 13C NMR (CDCl3, 100.5 MHz) δ 166.15, 142.36, 124.92, 60.97, 55.41, 51.88, 21.13, 10.46; MS (m/z, rel abundance) 41 (17), 55 (21), 59 (11), 73 (39), 83 (96), 97 (35), 98 (100), 99 (14). Z-isomer: 1H NMR (CDCl3, 400 MHz) δ 6.04 (d, J = 2.0 Hz, 1H), 6.03 (s, 1H), 4.41–4.44 (m, 1H), 3.75 (s, 3H), 3.17–3.21 (m, 1H), 1.56–1.66 (m, 1H), 1.44–1.54 (m, 1H), 1.02 (t, J = 7.6 Hz, 3H); 13C NMR (CDCl3, 100.5 MHz) δ 166.34, 145.21, 123.83, 60.74, 53.93, 51.66, 22.40, 10.46; MS (m/z, rel abundance) 41 (13), 55 (24), 73 (29), 83 (100), 97 (15), 98 (94), 99 (14). HRMS calcd. for C8H12O3 156.0786, found 156.0784.
(4R,5S)-4,5-Epoxyheptanoic acid methyl ester (6)
Phenylsilane (3.7 mL, 30 mmol) was added to a solution of enoate 5 (3.12 g, 20 mmol) in THF (130 mL). The mixture was cooled in an ice-water bath, and triphenylphosphine-copper(I)-hydride-hexamer (0.78 g, 0.4 mmol) was added in one portion and the inner wall of the flask was rinsed with THF (10 mL). The reaction mixture was warmed to room temperature and stirred 2.5 h, then water (50 mL) was added and the mixture was stirred for 30 min. The mixture was filtered through celite, rinsing with Et2O. The organic layer was separated, washed with saturated aqueous NH4Cl and brine and dried with anhydrous Na2SO4. After concentration, the crude product was purified by Kugelrohr distillation (0.6 Torr, 75–80 °C) to give 6 as a colorless liquid (2.48 g, 78%). 1H NMR (CDCl3, 400 MHz) δ 3.68 (s, 3H), 2.94–2.98 (m, 1H), 2.87–2.92 (m, 1H), 2.43–2.56 (m, 2H), 1.86–1.95 (m, 1H), 1.71–1.81 (m, 1H), 1.45–1.63 (m, 2H), 1.03 (t, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100.5 MHz) δ 173.45, 58.70, 56.25, 51.84, 31.15, 23.41, 21.16, 10.67; MS (m/z, rel abundance) 41 (31), 42 (11), 43 (17), 55 (19), 57 (32), 58 (22), 59 (50), 69 (10), 71 (15), 72 (23), 83 (16), 84 (19), 85 (100), 87 (16), 97 (15), 99 (12), 100 (36), 101 (36), 115 (21); HRMS calcd. for C8H14O3 158.0943, found 158.0934.
(4R,5S)-4,5-Epoxyheptanal (7)
A solution of DIBAL-H in toluene (0.2 M, 30 mL, 6 mmol) was added over 45 min using a syringe pump to a solution of ester 6 (0.79 g, 5 mmol) in toluene (45 mL) at − 78 °C. After the addition was complete, the reaction mixture was immediately quenched by addition of MeOH (6 mL) at − 78 °C followed by 45 mL saturated aqueous sodium potassium tartrate (Rochelle’s salt). The reaction mixture was warmed to room temperature while stirring vigorously over 30 min, then diluted with Et2O and filtered through celite. The organic layer was separated, washed with saturated aqueous NH4Cl and brine and dried over anhydrous Na2SO4. After concentration, the crude product was purified by flash chromatography (hexane/EtOAc = 5/1) to give 7 as a colorless oil (0.41 g, 64%). The neat 7 was unstable; therefore, only GC–MS data were obtained and the compound was used immediately for the next step. MS (m/z, rel abundance) 41 (35), 42 (46), 43 (12), 57 (19), 59 (10), 69 (14), 70 (100), 71 (10), 128 (M+, 1).
(2E,6R,7S)-6,7-Epoxynon-2-enal (1a)
A mixture of aldehyde 7 (0.77 g, 6.0 mmol) and (formylmethylene)triphenylphosphorane (2.19 g, 7.2 mmol) in toluene (30 mL) was refluxed for 18 h. After cooling to room temperature, the solvent was removed in vacuo. Hexane was added to the residue, the suspension was stirred for 30 min, and the precipitated triphenylphosphine oxide was removed by filtration. The crude product was purified by flash chromatography (hexane/EtOAc = 5/1), followed by Kugelrohr distillation (0.1 Torr, 80 °C) to give 1a as a colorless liquid (0.37 g, 40%). 1H NMR (CD2Cl2, 400 MHz) δ 9.50 (d, J = 8.0 Hz, 1H), 6.90 (dt, J = 15.6, 6.8 Hz, 1H), 6.13 (dd, J = 15.6, 8.0 Hz, 1H), 2.84–2.94 (m, 2H), 2.40–2.60 (m, 2H), 1.61–1.81 (m, 2H), 1.44–1.58 (m, 2H), 1.03 (t, J = 7.2 Hz, 3H); 13C NMR (CD2Cl2, 100.5 MHz) δ 194.24, 157.71, 133.78, 58.69, 56.66, 30.42, 26.83, 21.68, 10.89. MS (m/z, rel abundance) 41 (100), 42 (13), 43 (26), 53 (16), 55 (41), 57 (40), 59 (58), 65 (12), 66 (16), 67 (96), 68 (96), 69 (19), 70 (26), 71 (13), 79 (12), 81 (35), 82 (24), 83 (36), 84 (10), 85 (71), 95 (31), 96 (10), 97 (40); HRMS calcd. for C9H14O2 154.0994, found 154.0985.
(2E,6S,7R)-6,7-epoxynon-2-enal (1b) was formed in analogous fashion, starting from the (2S,3R)-epoxyalcohol 4b, which in turn was generated from cis-2-penten-1-ol, using (l)-(+)-diisopropyl tartrate in the Sharpless asymmetric epoxidation step.
Determination of the absolute configuration of the insect-produced (E)-2-cis-6,7-epoxynonenal
The racemate and enantiomers of (E)-2-cis-6,7-epoxynonenal and headspace extracts from male beetles (see Xu et al. 2017) were analyzed on the chiral stationary phase Cyclodex-B GC column, with an injector temp of 150 °C. Injections were made in split mode at 25 psi head pressure, and the oven was programmed from 70 °C, 3 °C/min to 220 °C, hold 20 min. The two enantiomers were not completely resolved to baseline under these conditions, but exhibited two easily distinguishable peaks, with the (6R,7S) enantiomer 1a eluting first. The absolute configuration of the insect-produced compound was confirmed by coinjecting an aliquot of the extract with the synthetic racemate and determining which of the two peaks was enhanced.
Field bioassays
Field bioassays were carried out in Xiao Tao Yuan Park (32°05′05.61″N 118°44′49.85E) in Nanjing, Jiangsu Province, China. The major tree species present were peach, Prunus persica, David’s peach, P. davidiana, various cherries (Prunus spp.) and non-hosts including willow (Salix babylonica) and mixed conifers. Black flight-intercept panel traps (IPM Technologies, Portland, OR, USA) coated with Teflon® PTFE DISP 30 (DuPont Chemical Co., Wilmington, DE, USA) were hung at least 1 m above the ground, with traps spaced > 10 m apart. Traps were baited with treatments or solvent controls in computer-generated random order. Lure solutions were deployed in permeable plastic sachets made from heat-sealable polyethylene tubing (7 cm × 4.9 cm, wall thickness 0.05 mm; cat. no. S-1112 Uline, Pleasant Prairie, WI, USA). Lures were not replaced or switched in position during each experiment, and daily trap catches from the same trap were added together. The trap collection cups contained a 1:1 solution of automobile antifreeze and water to kill and preserve trapped insects.
Experiment 1—Effect of minor component and optimum ratio. The purpose of our first bioassay was to test the effect of the minor component (2E,6Z)-nona-2,6-dienal on lure attraction when combined with the major component racemic (E)-2-cis-6,7-epoxynonenal, and to determine the optimum ratio of the two components. Because the ratio of minor versus major component in the aeration extracts of male A.bungii was 9.8 ± 7.3% (approximately 1:10; range from 0.25:10 to 1.7:10), and only half of racemic (E)-2-cis-6,7-epoxynonenal (25 mg/lure) tested in the field in 2016 was the natural enantiomer, traps were baited with lures containing 0, 0.31, 1.25 or 2.1 mg (2E,6Z)-nona-2,6-dienal combined with 25 mg racemic (E)-2-cis-6,7-epoxynonenal, diluted in 1 mL isopropanol (N = 10). Control traps were baited with sachets containing 1 mL isopropanol. The experiment was deployed from June 11 to 18, 2017, and trap catches were tabulated daily.
Experiment 2—Optimum loading of the major component. The second field assay was conducted to determine the optimum loading of the major component when combined with the minor component at the optimum ratio obtained from the first experiment. The amount of (2E,6Z)-nona-2,6-dienal used was adjusted to account for only half of the racemic (E)-2-cis-6,7-epoxynonenal being the natural enantiomer. Traps were baited with pheromones at four different loads (3.2, 10, 32 or 100 mg racemic (E)-2-cis-6,7-epoxynonenal combined with 40, 125, 400 or 1250 μg (2E, 6Z)-nona-2,6-dienal, respectively, in 1 mL isopropanol), or solvent controls (1 mL isopropanol) (N = 10). Traps were deployed on June 18–July 1, 2017, and A. bungii were collected from traps daily, for a total of 13 temporal replicates.
Experiment 3—Comparison of racemic (E)-2-cis-6,7-epoxynonenal versus the insect-produced (6R,7S)-enantiomer. The effect of the unnatural enantiomer of (E)-2-cis-6,7-epoxynonenal on lure attraction was tested in the third field assay by using the optimum composition ratio and dosages from the first and second experiments. Traps were baited with lures consisting of 16 mg of (2E,6R,7S)-6,7-epoxynon-2-enal and 400 μg (2E,6Z)-nona-2,6-dienal, 32 mg racemic (E)-2-cis-6,7-epoxynonenal and 400 μg (2E,6Z)-nona-2,6-dienal or solvent controls (1 mL isopropanol) (N = 10). Traps were deployed on July 3–15, 2017, and trap catches were counted on July 4, 5, 6, 7, 8, 9, 11, 13 and 15.
Statistical analysis
The numbers of male, female and combined male plus female A. bungii collected were modeled using Poisson regression followed by pairwise contrast tests for assessing differences between treatment pairs. The Bonferroni correction was applied to control the experiment-wise error rate among multiple comparisons. Differences in the sex ratio between a nominal 1:1 ratio were assessed using the chi-square test. All statistical analyses were carried out using SPSS 21.0 (SPSS Inc., Chicago, IL, USA).
Results
Preparation of test compounds
Racemic (E)-2-cis-6,7-epoxynonenal 1 was readily synthesized by regioselective epoxidation of commercially available (2E,6Z)-nona-2,6-dienal 2 as previously described (Xu et al. 2017). The two enantiomers were synthesized using Sharpless asymmetric epoxidation to generate the required chiral epoxide starting materials 4a and 4b (Fig. 1). Thus, (2R,3S)-epoxyalcohol 4a was prepared by Sharpless asymmetric epoxidation of cis-2-penten-1-ol 3 following Chong’s procedure (Gao et al. 1987; Chong 1989). This included forming and recrystallizing the 2,4-dinitrobenzoate ester of the crude product to increase the enantiomeric purity. After hydrolysis of the ester, the resulting enantiomerically pure alcohol was subjected to a one-pot tandem MnO2 oxidation-stabilized phosphorane olefination sequence (Taylor et al. 2008) to prepare α,β-unsaturated ester 5 in one pot. This tandem sequence circumvented possible difficulties in trying to isolate the highly volatile aldehyde intermediate in reasonable yield. Ester 5 was obtained as a mixture of E- and Z-isomers (E:Z = 7:3), which was of no consequence because the double bond was reduced in the next step. Thus, reduction of ester 5 was achieved with phenylsilane in the presence of [(Ph3P)CuH]6 as catalyst to afford epoxyester 6, following the Jamison group’s procedure (Tanuwidjaja et al. 2009; Underwood et al. 2013). Selective reduction of the ester to aldehyde 7, followed by a second Wittig homologation with (formylmethylene)triphenylphosphorane gave the desired product (2E,6R,7S)-6,7-epoxynon-2-enal 1a.
The corresponding enantiomer 1b was prepared from cis-2-penten-1-ol in identical fashion, with the exception that l-(+)-DIPT was used in the initial epoxidation step to generate the (2S,3R)-enantiomer 4b as the key starting material.
Determination of the absolute configuration of the insect-produced epoxide
The synthesized enantiomers of the Aromia bungii pheromone 1 were analyzed on a chiral stationary phase Cyclodex-B GC column, with the (6R,7S) enantiomer 1a eluting first. Coinjection of an aliquot of the headspace volatiles collected from males with the racemate resulted in enhancement of the early eluting peak, confirming that the insect-produced compound had the (6R,7S) configuration.
Field bioassays
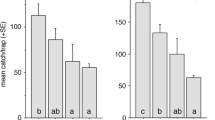
Experiment 1—Effect of minor component and optimum ratio. A total of 180 A. bungii were trapped in the first experiment. Of these, 93 (51.7%) were male and 87 (48.3%) were female. The sex ratios of the overall trap catch and those of the individual treatments did not differ significantly from 1:1 (chi-square tests, P > 0.1). Females did not discriminate among any of the treatment blends containing the major component (Fig. 2), and the solvent controls captured significantly fewer females than any of the test treatments (Poisson regression, P < 0.05 [Bonferroni corrected]). The treatment containing 0.31 mg of the minor component and 25 mg of the racemic major component captured significantly more males than all other treatments (Fig. 2; Poisson regression, P < 0.05 [Bonferroni corrected]). The treatment containing 1.25 mg of the minor component and 25 mg of the racemic major component captured significantly more males than the solvent control, but not significantly more than the treatments containing either 0 mg or 2.1 mg of the minor component. The numbers of males attracted to the treatments containing 0 mg and 2.1 mg of the minor component were not significantly different from the solvent control. When trap captures of males and females were combined, all treatments attracted more A. bungii than the control, and the treatment containing 0.31 mg of the minor component attracted significantly more A. bungii adults than the treatments containing either 0 mg or 2.1 mg of the minor component.
Numbers of A. bungii trapped with differing pheromone component ratios in Nanjing, Jiangsu Province, China, June 11 to 18, 2017. The minor pheromone component is (2E,6Z)-nona-2,6-dienal and the major pheromone component is (E)-2-cis-6,7-epoxynonenal. Bars within each sex with the same letters are not statistically different (Poisson regression with Bonferroni correction)
Experiment 2—Optimum loading rate of the major component. The total number of A. bungii captured in the second experiment was 214, including 88 males and 126 females. This sex ratio was marginally different from 1:1 (chi-square test, P = 0.067) due to a significant excess of females (49F, 18 M) caught in the treatment with 1.25 mg of the minor component and 100 mg of the major component (chi-square test, P < 0.001). The sex ratios of beetles caught in the other treatments did not differ significantly from 1:1 (chi-square test, P > 0.1). The 32 mg treatment captured significantly more males than the 3.2 mg treatment and the solvent control (Fig. 3; Poisson regression, P < 0.05 [Bonferroni corrected]), but not significantly more than the 10 and 100 mg loads (Poisson regression, P > 0.05 [Bonferroni corrected]). The 10 mg, 32 mg and 100 mg loads all attracted significantly more females than the 3.2 mg load and the solvent control (Fig. 3; Poisson regression, P < 0.05 [Bonferroni corrected]).
Numbers of A. bungii trapped with differing major pheromone component loads in Nanjing, Jiangsu Province, China, June 18 to July 1, 2017. The minor pheromone component is (2E,6Z)-nona-2,6-dienal and the major pheromone component is (E)-2-cis-6,7-epoxynonenal. Bars within each sex with the same letters are not statistically different (Poisson regression with Bonferroni correction)
Experiment 3—Comparison of the racemic and natural enantiomer of the major component of the pheromone. The 16 mg load of the naturally produced enantiomer of the major component combined with 0.4 mg of the minor component attracted significantly more females than either 32 mg of the racemic major component combined with the minor component, or the solvent control (Fig. 4, Poisson regression, P = 0.016, P < 0.001, respectively [Bonferroni corrected]). Although the presence of the other enantiomer in the racemate significantly reduced trap catches, when combined with the minor component, this lure still attracted significantly more A. bungii than the solvent control (Poisson regression, P = 0.02 [Bonferroni corrected]). There was no significant difference in attraction of males between the natural enantiomer of the major component combined with the minor component and the racemic major component combined with the minor component (Poisson regression, P = 0.3 [Bonferroni corrected]), and both treatments attracted significantly more males than the solvent control (Poisson regression, P = 0.001 and 0.043, respectively [Bonferroni corrected]). This experiment was conducted near the end of the A. bungii flight season in Jiangsu, and the overall number of beetles caught was only 57. Of those, 40 were captured in traps baited with the natural enantiomer of the major component combined with the minor component and the sex ratio (27F,13M) deviated significantly from 1:1 (chi-square test, P = 0.027).
Numbers of A. bungii trapped with racemic and enantiomerically pure major pheromone component in Nanjing, Jiangsu Province, China, July 3 to 15, 2017. The minor pheromone component is (2E,6Z)-nona-2,6-dienal, the racemate of the major pheromone component is (E)-2-cis-6,7-epoxynonenal and the natural enantiomer of the major component is (2E,6R,7S)-6,7-epoxy-2-nonenal. Bars within each sex with the same letters are not statistically different (Poisson regression with Bonferroni correction)
Discussion
In preliminary experiments, we had reported that both sexes of A. bungii were significantly attracted to the racemate of the major male-produced compound, (E)-2-cis-6,7-epoxynonenal (Xu et al. 2017). The follow-up experiments reported here suggested that the insect-produced enantiomer of this compound was significantly more attractive than the racemate, although traps baited with the racemate still attracted significantly more beetles than controls. Thus, the unnatural enantiomer appears to be slightly inhibitory. However, it is unlikely that the synthesized natural enantiomer can be deployed in monitoring traps because of the multi-step and relatively low-yielding synthesis required to produce the enantiomer, particularly as each lure must be loaded with ~ 10 mg or more to be effective. In contrast, the less active but still significantly attractive racemate can be readily prepared in multigram quantities in one straightforward step from a commercially available precursor. Thus, for practical purposes such as large-scale detection and monitoring programs using pheromone-baited traps, use of the racemic pheromone should provide a reasonable compromise between cost and efficacy.
Our experiments also showed that the attractiveness of the major component apparently could be enhanced by addition of small quantities (~ 2.5%) of the minor male-produced component, (2E,6Z)-nona-2,6-dienal, resulting in ~ twofold increase in trap captures in comparison with the major component alone. Furthermore, the cost of preparing lures containing the two-component blend should be virtually the same as that for the lures containing the major component as a single component, because (2E,6Z)-nona-2,6-dienal is readily available commercially and, in fact, is the starting material for synthesis of the racemate of the major component. This has an added benefit because if the epoxidation of (2E,6Z)-nona-2,6-dienal, during the preparation of the major compound, does not go to completion, it is not necessary to remove the residual unreacted (2E,6Z)-nona-2,6-dienal. This is a substantial advantage because it would likely be difficult to separate the product from unreacted starting material on a large scale.
In the dose–response experiment, there were no significant differences in trap catches for either males or females when lures were loaded with 10, 32 or 100 mg of the racemic major component, though the 100 mg load caught 2.7 times as many females as males. Thus, for detection purposes, the 32 mg load may represent a good balance between efficiency and cost effectiveness, particularly as this dose appeared to be equally attractive to males and females.
While our field bioassays were in progress, we learned that the enantiomers of the major component of the pheromone had been independently synthesized (Mori 2018). Although our and Professor Mori’s syntheses both proceeded via epoxyalcohols 4a and 4b as chiral synthons, the later steps in the syntheses differed, with ours proceeding via two sequential Wittig olefinations to produce the α,β-unsaturated aldehydes 1a and 1b, whereas Mori’s syntheses used an olefin metathesis reaction to introduce the α,β-unsaturated aldehyde. In addition, the results of laboratory and field trials with Mori’s compounds have very recently been reported (Yasui et al. 2018). In electroantennogram assays, these authors found that both enantiomers of (E)-2-cis-6,7-epoxynonenal elicited responses from the antennae of beetles of both sexes. In a field bioassay, Yasui et al. (2018) found that beetles were only significantly attracted to the (6R,7S)-enantiomer, analogous to our results. Unlike our field results, these authors found no difference in attraction to the racemate in comparison with the (6R,7S)-enantiomer. However, the relatively small number of beetles trapped in their field trial (44 total, spread across 4 treatments) may have obscured minor differences between the racemate and the (6R,7S)-enantiomer.
To date, pheromones or likely pheromones have been identified for several hundred cerambycid species (reviewed in Hanks and Millar 2016, Millar and Hanks 2017), and these pheromones include a number of chiral components. In many cases, the racemates of these compounds appear to be satisfactory attractants, and the responses of most species to the naturally produced enantiomer versus the racemate have not yet been tested. However, for at least a subset of species, the stereoisomeric purity of the pheromone components is clearly important. For example, Iwabuchi et al. (1986) found that (R)-2-hydroxyoctan-3-one significantly inhibited attraction of Xylotrechus pyrrhoderus to its pheromone blend, consisting of (S)-2-hydroxyoctan-3-one with (2S,3S)-2,3-octanediol. Along similar lines, the pheromones of Neoclytus acuminatus and N. tenuiscriptus consist of (2S,3S)-2,3-hexanediol, and attraction is inhibited by one or both of the (2R,3S) or (2S,3R) diastereomers (Lacey et al. 2004, Ray et al. 2015). Conversely, synergism between enantiomers has also been reported, with Astylidius parvus requiring both enantiomers of (E)-6,10-dimethyl-5,9-undecadien-2-ol (fuscmol) in its pheromone blend, whereas Lepturges angulatus uses both enantiomers of fuscumol acetate in its pheromone (Meier et al. 2016). Overall, these few examples suggest that, as with pheromone blends of other insect taxa, the use of different stereoisomers is likely to be an important mechanism in the formation of species-specific signals.
In summary, the results reported here should support the development of efficacious and cost-effective lures for detection and monitoring of the dangerous invasive species Aromia bungii as it continues to invade new areas of the world. In particular, both our experiments and the results reported by Yasui et al. (2018) have shown that the racemate of the major component of the pheromone should be adequate for large-scale monitoring, particularly when combined with small amounts of the minor component. Furthermore, we have shown that doses of about 32 mg of the racemate, when dispensed from plastic heat-sealed sachets, offer a reasonable compromise between effectiveness and cost.
Author contributions
JM and ST conceived and designed the research. YZ and JM designed and carried out the enantioselective syntheses. LH and TX conducted the field experiments. TX conducted statistical analyses on the field data. DH located suitable field sites and assisted with the field experiments. YZ, LH, TX, ST and JM wrote the manuscript; all authors read and approved the manuscript.
References
Anonymous (2013) The first longicorn beetle in Japan confirmed in Aichi, damaging cherry and Japanese apricot trees (June 21, 2013). Jpn Agric News. http://english.agrinews.co.jp/?p=482. Accessed 26 Mar 2017
Anonymous (2015) Aromia bungii (Coleoptera: Cerambycidae). Redneck longhorned beetle. Eur Med Plant Prot Org Bull OEPP/EPPO Bull 45(1):4–8. https://gd.eppo.int/download/doc/297_ds_AROMBU_en.pdf. Accessed 9 July 2018
Burmeister EG, Hendrich L, Balke M (2012) Der Asiatische Moschusbock Aromia bungii (Faldermann, 1835)—Erstfund für Deutschland. Nachr bl Bayer Entomol 61:29–31
CABI (2018) Datasheet, Aromia bungii (red necked longicorn). https://www.cabi.org/isc/datasheet/118984#EF51C788-F9D6-4F79-B667-F7DD03B02F65. Accessed 28 Sept 2018
Chong JM (1989) Enantioselective synthesis of sitophilate, the granary weevil aggregation pheromone. Tetrahedron 45:623–628
European and Mediterranean Plant Protection Organization, EPPO (2014) Pest risk analysis for Aromia bungii. EPPO, Paris. https://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRAdocs_insects/15_21043_PRA_record_Aromia_bungii.pdf. Accessed 9 Mar 2017
Fukaya M, Kiriyama S, Yasui H (2017) Mate-location flight of the red-necked longicorn beetle, Aromia bungii (Coleoptera: Cerambycidae): an invasive pest lethal to Rosaceae trees. Appl Entomol Zool 52:559–565
Gao Y, Klunder JM, Hanson RM, Masamune H, Ko SY, Sharpless KB (1987) Catalytic asymmetric epoxidation and kinetic resolution: modified procedures including in situ derivatization. J Am Chem Soc 109:5765–5780
Garonna AP, Nugnes F, Espinosa B et al (2013) Aromia bungii, a new Asian worm found in Campania. Inf Agrar 69:60–62
Hanks LM, Millar JG (2016) Sex and aggregation pheromones of cerambycid beetles: basic science and practical applications. J Chem Ecol 42:631–654
Iwabuchi K, Takahashi J, Nakagawa Y, Sakai T (1986) Behavioral responses of female grape borer Xylotrechus pyrrhoderus Bates (Coleoptera: Cerambycidae) to synthetic male sex pheromone components. Appl Entomol Zool 21:21–27
Lacey ES, Ginzel MD, Millar JG, Hanks LM (2004) Male-produced aggregation pheromone of the cerambycid beetle Neoclytus acuminatus acuminatus. J Chem Ecol 30:1493–1507
Ma W, Sun L, Yu L, Wang J, Chen J (2007) Study on the Occurrence and Life History in Aromia bungii (Faldermann). Acta Agric Bor Sin 22:247–249
Meier LR, Zou Y, Millar JG, Mongold-Diers JA, Hanks LM (2016) Synergism between enantiomers creates species-specific pheromone blends and minimizes cross-attraction for two species of cerambycid beetles. J Chem Ecol 42:1181–1192
Millar JG, Hanks LM (2017) Chemical ecology of cerambycids. In: Wang Q (ed) Cerambycidae of the world: biology and pest management. CRC Press/Taylor and Francis, Boca Raton, pp 161–196
Mori K (2018) Pheromone synthesis. Part 263: synthesis of the racemate and the enantiomers of (E)-cis-6,7-epoxy-2-nonenal, the male-produced pheromone of the red-necked longhorn beetle, Aromia bungii. Tetrahedron 74:1444–1448
Ray AM, Millar JG, Moreira JA, McElfresh JS, Mitchell RF, Barbour JD, Hanks LM (2015) North American species of cerambycid beetles in the genus Neoclytus share a common hydroxyhexanone-hexanediol pheromone structural motif. J Econ Entomol 108:1860–1868
Tanuwidjaja J, Ng S-S, Jamison TF (2009) Total synthesis of ent-dioxepandehydrothyrsiferol via a bromonium-initiated epoxide-opening cascade. J Am Chem Soc 131:12084–12085
Taylor RJ, Campbell L, McAllister GD (2008) (±)-trans-3,3′-(1,2-Cyclopropanediyl)bis-2-(E)-propenoic acid, diethyl ester: tandem oxidation procedure (TOP) using MnO2 oxidation-stabilized phosphorane trapping. Org Synth 85:15–26
Underwood BS, Tanuwidjaja J, Ng S-S, Jamison TF (2013) Total syntheses of the squalene-derived halogenated polyethers ent-dioxepandehydrothyrsiferol and armatol A via bromonium-and Lewis acid-initiated epoxide-opening cascades. Tetrahedron 69:5205–5220
Wang Q (2017) Cerambycid pests in agricultural and horticultural crops. In: Wang Q (ed) Cerambycidae of the world: biology and pest management. CRC Press, Boca Raton
Wang JT, Sun L, Liu T, Zhang L (2007) Research on the occurrence character and control measure of Aromia bungii. J Hebei Ag Sci 11(2):41–43
Wei J, Liu X, Niu Y, Wang J (2013) Identification of volatiles released from the living adult Aromia bungii Faldermann. For Pest Dis 32:8–10 (in Chinese)
Xu T, Yasui H, Teale SA, Fujiwara-Tsujii N, Wickham JD, Fukaya M, Hansen L, Kiriyama S, Hao D, Nakano A, Zhang L, Watanabe T, Tokoru M, Millar JG (2017) Identification of a male-produced sex-aggregation pheromone for a highly invasive cerambycid beetle, Aromia bungii. Sci Rep 7:7330
Yasui H, Fujiwara-Tsujii N, Yasuda T, Fukaya M, Kiriyama S, Nakano A, Watanabe T, Mori K (2018) Electroantennographic responses and field attraction of an emerging invader, the red-necked longicorn beetle Aromia bungii (Coleoptera: Cerambycidae), to the chiral and racemic forms of its male-produced aggregation-sex pheromone. Appl Entomol Zool 1:6. https://doi.org/10.1007/s13355-018-0600-x
Yu G, Gao B (2005) Bionomics of Aromia bungii. For Pest Dis 24:15–16
Acknowledgements
We thank Shuo Tian and Yingyi Cheng of Nanjing Forestry University for assistance with the field experiments, and two anonymous reviewers for their helpful suggestions.
Funding
This study was funded by grants from the United States Department of Agriculture, Animal and Plant Health Inspection Service-Plant Protection and Quarantine program (USDA-APHIS-PPQ) Grants 17-8130-1422-CA and 18-8130-1422-CA) to JGM and SAT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The laboratory research was conducted at the University of California, Riverside. All methods met the ethical requirements of the respective universities and followed guidelines of the Committee of Publication Ethics.
Research involving human participants and/or animals
This article does not involve any studies with human participants or vertebrate animals.
Additional information
Communicated by P.G. Becher.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zou, Y., Hansen, L., Xu, T. et al. Optimizing pheromone-based lures for the invasive red-necked longhorn beetle, Aromia bungii. J Pest Sci 92, 1217–1225 (2019). https://doi.org/10.1007/s10340-019-01108-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-019-01108-6