Abstract
Two components of the Synanthedon bicingulata sex pheromone, (E,Z)-3,13-octadecadienyl acetate (E3,Z13-18:OAc) and (Z,Z)-3,13-octadecadienyl acetate (Z3,Z13-18:OAc), were synthesized to investigate the effect of pheromone blends, trap type and trap color on the capture of S. bicingulata males. The optimal sex pheromone ratio for E3,Z13-18:OAc and Z3,Z13-18:OAc was approximately 4.3:5.7 based on the purity of the two pheromone components in all test areas. A significant difference was observed in the number of S. bicingulata adult males caught in bucket and delta traps. The mean numbers of males caught in bucket and delta traps were 13.2 ± 2.2 and 7.6 ± 2.0, respectively. Trap color affected the number of adult males caught in bucket traps. More adult males were attracted to a yellow bucket trap than to green, white, blue, black and red traps. An analysis of the relationship between trap capture and trap surface-color values (L*a*b*) revealed a positive relationship between trap capture and b* value.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Twenty-five species of clearwing moths (Lepidoptera: Sesiidae) in nine genera are economically important pests of apple, peach, jujube, and persimmon trees and grape vines in Korea (Lee et al. 2004, 2017; Yang et al. 2012; Cho et al. 2016). Among them, Synanthedon bicingulata is one of the most important insect pests in fruit tree orchards (Yang et al. 2012; Cho et al. 2016; Lee et al. 2017).
Cherry trees are popular roadside trees in Korea due to their visually attractive early-spring blossoms. Most local governments in Korea have planted many cherry trees on roadsides, and damage from S. bicingulata has increased in recent years (Kim et al. 2019). Larvae of S. bicingulata bore into the trunk of a host tree and feed on the cambium layer (Kim et al. 2019). Controlling S. bicingulata larval infections with insecticides is difficult, creating a need for alternative control methods. Sex pheromones are promising candidates for this purpose. Traps baited with species-specific sex pheromones provide information on the timing of insecticide application. In addition, sex pheromones can directly control target insects through mass trapping (Naka et al. 2008; Leskey et al. 2009).
Following identification of the first sex pheromones in Synathedon pictipes and Synanthedon exitiosa (Tumlinson et al. 1974), sex pheromones of several other Synanthedon species have been described (The Pherobase, 2020). Two 3,13-octadecadienyl compounds, (E,Z)-3,13-octadecadienyl acetate (E3,Z13-18:OAc) and (Z,Z)-3,13-octadecadienyl acetate (Z3,Z13-18:OAc), were identified as sex pheromones of S. bicingulata by Yang et al. (2011), but follow-up studies focused only on monitoring seasonal occurrence of S. bicingulata using pheromone traps in fruit orchards (Yang et al. 2012; Cho et al. 2016; Lee et al. 2017).
In this study, attraction of S. bicingulata males to various combinations of newly synthesized two sex pheromone components was evaluated to find out the optimal pheromone ratio in field. In addition, effect of trap type and color on the capture of S. bicingulata males was investigated to improve the pheromone-based monitoring of clearwing moth.
Materials and methods
Chemicals
1,8-Dibromooctane (98%), 1-hexyne (97%), n-BuLi (2.5 M in hexane), tetrahydrofuran (THF), palladium on barium sulfate (Pd/BaSO4, 5% Pd basis), methanol (MeOH) (≥ 99.9%), 3-butyn-1-ol (97%), lithium aluminum hydride (LAH) (95%), and hexamethylphosphoramide (HMPA) (98%) were purchased from Sigma-Aldrich (Milwaukee, WI, USA).
Synthesis of (E,Z)-3,13-octadecadienyl acetate and (Z,Z)-3,13-octadecadienyl acetate
Synthetic methods for E3,Z13-18:OAc and Z3,Z13-18:OAc were reported in several previous studies (Armstrong-Chong et al. 2004; Ebata et al. 1979; Naka et al. 2006; Uchida et al. 1978; Uchida et al. 1979; Yamamoto et al. 1989). In this study, we synthesized E3,Z13-18:OAc and Z3,Z13-18:OAc according to the method reported in our patent (Lee et al. 2018), and a synthetic scheme is shown in Fig. 1. Solvents were dried and purified by conventional methods prior to use. 1H NMR (at 400 MHz or 600 MHz) and 13C NMR (at 150 MHz) spectroscopic data were recorded on an Advance 400 MHz and 600 MHz spectrometer (Bruker, Germany) in CDCl3.
Synthetic schemes for two sex pheromone of Synanthedon bicingulata. (3Z,13Z)-octadecadienyl acetate (1); (3E,13Z)-octadecadienyl acetate (2); (Z,Z)-3,13-Octadecadien-1-ol (3); (E,Z)-3,13-Octadecadien-1-ol (4); Z-13-Octadecen-3-yn-1-ol (5); (5Z)-14-Bromotetradeca-5-ene (6); 14-Bromoteradeca-5-yne (7); 1,8-Dibromooctane (8); 1-Hexyne (9); 3-Butyn-1-ol (10)
14-Bromoteradeca-5-yne (7) In a clean and dry 500 mL round-bottom flask, 5.8 g of 70.73 mmol 1-hexyne (9) was dissolved in 150 mL of THF and 20 mL of HMPA and then cooled to − 78 °C. n-BuLi was added to this solution (29.7 mL, 74.27 mmol). The mixed solution was stirred for 1 h, and 1,8-dibromooctane (8) (25.0 g, 91.91 mmol) in 50 mL of THF was added slowly. Stirring was continued for 2 h at − 78 °C. The residue was treated cautiously with saturated 50 mL of NH4Cl and the volatiles were then removed in a rotary evaporator under reduced pressure. The residue was extracted with EtOAc (3 × 150 mL). The combined organic layers were washed with water and dried on anhydrous MgSO4. When crude residue was purified using column chromatography (EtOAc/Hexane, 1/10), 14-bromotetradeca-5-yne (7) (17.9 g, 92% yield) was obtained as a colorless liquid. 1H NMR (400 MHz, CDCl3): δ 3.37 (t, 2H), 2.10 (m, 2H), 1.84- 1.80 (m, 2H), 1.52–1.27 (m, 16H), 0.87 (t, 3H).
(5Z)-14-Bromotetradeca-5-ene (6) Compound (7) (15.0 g, 63.22 mmol), 5% Pd/BaSO4 (1.0 g), and quinoline (25 mg) in MeOH (250 mL) were placed in a 500 mL Parr reaction bottle and shaken in an H2 atmosphere at 40 psi for 2 h using Parr hydrogenation apparatus. The reaction mixture was filtered through celite and volatiles were removed in a rotary evaporator under reduced pressure. The residue was extracted with EtOAc (3 × 150 mL). The combined organic layers were washed with water and dried on anhydrous MgSO4. The crude residue was purified using column chromatography (EtOAc/Hexane, 1/10), and (5Z)-14-bromotetradeca-5-ene (6) (14.5 g, 83% yield) was obtained as a colorless liquid. 1H NMR (400 MHz, CDCl3): δ 5.37–5.31 (m, 2H), 3.38 (t, 2H), 2.00–1.99 (m, 4H), 1.85–1.81 (m, 2H), 1.53 (s, 1H), 1.42–1.28 (m, 13H), 0.87 (m, 3H).
Z-13-Octadecen-3-yn-1-ol (5) A 176 mg sample of 0.44 mmol Fe(NO3)3∙9H2O was added to a 3-necked 150 mL flask attached to a circulating condenser maintained at − 78 °C, and 150 mL of ammonia was condensed in this flask by stirring for 30 min. Lithium metal (1.1 g, 157 mmol) was added slowly to the mixture, which turned from a brown color to white–gray after 30 min of stirring. A solution of 3-butyn-1-ol (10) (3.67 g, 52.31 mmol) in 10 mL of dry THF was then added dropwise to the reaction mixture. The resulting solution was stirred for 1 h, and compound (6) (12.0 g, 43.59 mmol) in THF (25 mL) was added slowly. Stirring was continued for 2 h at − 78 °C, after which the reaction mixture was stirred overnight at ambient temperatures to evaporate the ammonia completely. The residue was cautiously treated with 50 mL of saturated NH4Cl and volatiles were then removed in a rotary evaporator under reduced pressure. The residue was extracted with EtOAc (3 × 150 mL). The combined organic layers were washed with water and dried on anhydrous MgSO4. The crude residue was purified using column chromatography (EtOAc/Hexane, 1/10), and Z-13-octadecen-3-yn-1-ol (5) (8.6 g, 74% yield) was obtained as a colorless liquid. 1H NMR (400 MHz, CDCl3): δ 5.32 (m, 2H), 3.70–3.63 (m, 2H), 2.41 (m, 2H), 2.13 (m, 2H), 2.00- 1.96 (m, 4H), 1.55 (m, 1H), 1.46 (m, 2H), 1.26 (m,14 H), 0.87 (m, 3H).
(E,Z)-3,13-Octadecadien-1-ol (4) Lithium aluminum hydride (LAH) (2.14 g, 56.47 mmol) and diethylene glycol dimethyl ether (diglyme) (120 mL) were added to a 250 mL round-bottom flask, and compound (5) (4.0 g, 15.13 mmol) in 15 mL of dry THF was added slowly to this solution. The mixture was stirred at a reflux condition for 72 h. The reaction mixture was then cooled to 0 °C and cautiously treated with saturated NH4Cl (50 mL). The reaction mixture was extracted with EtOAc (3 × 150 mL) and then washed sequentially with water and brine. The combined organic layers were dried over MgSO4. The crude residue was purified using column chromatography (EtOAc/Hexane, 1/9), and pure (E,Z)-3,13-octadecadien-1-ol (4) (2.9 g, 72% yield) was obtained as a colorless liquid. 1H NMR (400 MHz, CDCl3): δ 5.56–5.51 (m, 1H), 5.33(m, 3H), 3.60 (m, 2H), 2.25–2.23 (m, 2H), 2.00 (m, 6H), 1.55–1.25 (m, 16H), 0.87 (m, 3H).
(E,Z)-3,13-Octadecadienyl acetate (2) Pyridine (1.12 g, 14.20 mmol) was added to compound (4) (2.5 g, 9.38 mmol) in 120 mL of dry dichloromethane at room temperature in a N2 atmosphere. After stirring for 15 min, acetic anhydride (1.45 g, 14.20 mmol) was added dropwise at 0 °C. The reaction temperature was allowed to warm to room temperature and stirred for 15 h, and 100 mL of water was added to the reaction mixture and then extracted with dichloromethane (2 × 100 mL). The combined organic layers were washed with water, dried over MgSO4, filtered, and concentrated under reduced pressure. Crude material was purified by column chromatography (EtOAc/Hexane, 1/9) and (E,Z)-3,3-octadecadienyl acetate (2) (2.49 g, 86% yield) was obtained as a colorless liquid. 1H NMR (600 MHz, CDCl3,): δ 5.50–5.48 (m, 1H), 5.37–5.31 (m, 3H), 4.07–4.04 (t, J = 6.96 Hz, 2H), 2.39–2.35 (m, 2H), 2.07–1.99 (m, 9H), 1.35- 1.27 (m, 16H), 0.90- 0.88 (m, 3H). 13C NMR (150 MHz, CDCl3): δ 171.09, 133.01, 129.87, 124.24, 64.00, 31.97, 29.76, 29.60, 29.51, 29.50, 29.29, 27.31, 27.19, 26.92, 26.83, 22.35, 20.98, 13.99.
(Z,Z)-3,13-Octadecadien-1-ol (3) Compound (5) (4.0 g, 63.22 mmol), 5% Pd/BaSO4 (0.25 g), and quinoline (25 mg) in MeOH (50 mL) were placed in a 250 mL Parr reaction bottle and shaken in an H2 atmosphere at 20 psi for 2 h using a Parr hydrogenation apparatus. The reaction mixture was filtered through celite, and volatiles were removed in a rotary evaporator under reduced pressure. The residue was extracted with EtOAc (3 × 150 mL). The combined organic layers were washed with water and dried on anhydrous MgSO4. The crude residue was purified using column chromatography (EtOAc/Hexane, 1/9). Pure (Z,Z)-3,13-octadecadien-1-ol (3) (3.6 g, 90% yield) was obtained as a colorless liquid. 1H NMR (400 MHz, CDCl3): δ 5.56–5.50 (m, 1H), 5.35–5.30 (m, 3H), 3.61 (m, 2H), 2.31–2.29 (m, 2H), 2.04–1.94 (m, 6H), 1.29–1.22 (m, 16H), 0.85 (t, 3H).
(Z,Z)-3,13-Octadecadienyl acetate (1) Pyridine (1.12 g, 14.20 mmol) was added to 2.5 g of 9.38 mmol compound (3) in 120 mL of dry dichloromethane at room temperature under an N2 atmosphere. After stirring for 15 min, 1.45 g of 14.20 mmol acetic anhydride was added dropwise at 0 °C. The reaction temperature was allowed to warm to room temperature, stirred for 15 h, 100 mL of water was added to mixture, and extraction was performed with dichloromethane (2 × 100 mL). The combined organic layers were washed with water, dried over MgSO4, filtered, and concentrated under reduced pressure. Crude material was purified by column chromatography (EtOAc/Hexane, 1/9). (Z,Z)-3,13-Octadecadienyl acetate (1) (2.53 g, 87% yield) was obtained as a colorless liquid. 1H NMR (600 MHz, CDCl3,): δ 5.52–5.48 (m, 1H), 5.37–5.31 (m, 3H), 4.07–4.04 (t, J = 6.96 Hz, 2H), 2.39–2.35 (m, 2H), 2.06–1.99 (m, 9H), 1.35–1.27 (m, 16H), 0.90–0.88 (m, 3H). 13C NMR (150 MHz, CDCl3): δ 171.09, 133.62, 129.87, 124.96, 64.15, 32.60, 31.97, 31.96, 29.76, 29.50, 29.47, 29.40, 29.30, 29.12, 27.19, 26.92, 22.35, 20.98, 13.99.
Gas chromatography–mass spectrometry of E3,Z13-18:OAc and Z3,Z13-18:OAc
The purity of E3,Z13-18:OAc and Z3,Z13-18:OAc was determined using a gas chromatograph (Agilent 7890A)–mass spectrometer (Agilent 5975C MSD, Agilent Technologies, Santa Clara, CA, USA) (GC–MS) equipped with a HP-5MS column (30 m × 0.25 mm i.d., 0.25 µm film thickness, Agilent, CA, USA). The oven temperature for the HP-5MS column was initially set at 70 °C for 1 min, increased to 180 °C (10 °C/min), and increased to 220 °C (5 °C/min), and finally to 325 °C at a rate of 25 °C/min. Helium was used as a carrier gas at a flow rate of 1.0 mL/min. Electron impact (70 eV, source temperature 230 °C) was used to obtain ionization, with a scan range of 41–400 amu.
Field experiment
Field trapping experiments were conducted on roadside cherry trees in several areas of South Korea from 2017 to 2019 (Fig. 2). Bucket [Korea Institute of Insect Pheromone (KIP), Daejeon, Repblic of Korea] and delta traps (Green Agro Tech, Gyeongsan, Republic of Korea) were prepared using sleeve-stopper septa with a bottom internal diameter of 2.4 mm and an outer diameter of 5.3 mm (Sigma-Aldrich, Milwaukee, WI, USA) loaded with a hexane solution containing 1 mg of synthetic pheromones and an equivlent amount of 99.0% pure 2,6-di-tert-butyl-4-methylphenol (BHT) (Samchun Chemicals, Pyeongtaek, Republic of Korea). Traps baited with synthetic pheromones were hung on a cherry tree branch approximately 3 m above the ground. The distance between the traps within the same block was at least 15 m. Each block was at least 50 m from surrounding blocks. The number of males caught in traps was counted and the position of each trap was re-randomized within the block every two weeks to minimize the effect of trap position. Pheromone lures were changed monthly.
Experiment 1 Attraction of male S. bicingulata to 1:9, 4:6, 6:4 and 9:1 (w:w) mixture of E3,Z13-18:OAc and Z3,Z13-18:OAc was tested in Gongju (N36°21′29.9″, E127°14′52.0″) from April 28 to June 13, 2017. Control traps received only hexane. In 2018, 1:9, 2:8, 3:7, 4:6 and 5:5 (w:w) mixture of E3,Z13-18:OAc and Z3,Z13-18:OAc was tested in Jinju (N35°11′58.7″, E128°10′214″), Gongju (N36°21′29.9″, E127°14′52.0″), and Gapyeong (N37°46′57.2″, E127°28′07.3″) from 27 August to 4 October. Green and yellow bucket traps were used in 2017 and 2018, respectively, and traps were supplied by the KIP. Traps were installed in a randomized complete-block design with four replicates.
Experiment 2 The efficacy of the two different types of traps (delta and bucket) was investigated in Chuncheon (N37°55′42.4″, E127°46′27.3″) from August 9 to September 25, 2017. Green bucket and white delta traps were purchased from KIP and Green Agro Tech, respectively. The ratio of E3,Z13-18:OAc to Z3,Z13-18:OAc was 4:6. Traps were installed in a randomized complete-block design with five replicates.
Experiment 3 The effect of two different colors of bucket traps on the capture of S. bicingulata males was investigted in Gongju (N36°21′29.9″, E127°14′52.0″) and Seoul (N37°31′57.8″, E126°55′10.2″) from April 26 to June 7, 2018. Green and yellow bucket traps (Fig. 3) were supplied by KIP. The ratio of E3,Z13-18:OAc to Z3,Z13-18:OAc was 4:6. Traps were installed in a randomized complete-block design with four replicates.
Experiment 4 The effect of six different bucket trap colors (black, blue, green, red, yellow, and white) (Fig. 3) on the capture of S. bicingulata males was investigated in Gapyeong (N37°46′57.2″, E127°28′07.3″) and Gongju (N36°21′29.9″, E127°14′52.0″) from August 27 to September 28, 2018, and in Yanggu (N38°05′33.1″, E127°59′32.5″) from August 19 to October 6, 2019. The ratio of E3,Z13-18:OAc to Z3,Z13-18:OAc was 4:6. Traps were installed in a randomized complete-block design with three replicates at Gongju and Gapyeong and four replicates at Yanggu.
Color spectrometry analysis
Bucket-trap surface-color values (L*a*b) and reflectance spectra at wavelengths between 360 and 750 nm were measured with a color spectrophotometer (ColorMate, SCINCO, Seoul, Republic of Korea). L* indicates a measure of “lightness”; black (0) to white (100). Symbol a* indicates a red shade when greater than zero (+) and a green shade when lower than zero (−). Symbol b* indicates a yellow shade when greater than zero (+) and a blue shade when lower than zero (−).
Statistical analysis
The mean numbers of male S. bicingulata caught in two different trap types (Experiment 2) and two different color bucket traps (Experiments 3) were compared using a t test. The results of experiments 1 and 3 were analyzed using one-way analysis of variance (ANOVA), followed by Tukey’s HSD test (IBM SPSS Statistics 23.0). Mean (± SE) values for untransformed data were reported. R (ver. 3.5.1. R Foundation for Statistical Computing, Austria) and R studio (ver. 1.1.456, R Studio Inc. MA, USA) were used for linear regression analysis of the effect of trap surface-color values on trap capture (Mendiburu 2020).
Results
GC–MS data analysis of E3,Z13-18:OAc and Z3,Z13-18:OAc
Total ion chromatograms of synthetic E3,Z13-18:OAc and Z3,Z13-18:OAc are shown in Fig. 4. The purities of E3,Z13-18:OAc and Z3,Z13-18:OAc were 94.18% and 82.8%, respectively, as shown in Fig. 4a, b. EI-MS m/z (RT 13.670): 308 (1), 248 (8), 233 (1), 219 (3), 205 (3), 191 (3), 177 (3), 163 (5), 149 (10), 135 (18), 121 (20), 109 (25), 95 (47), 81 (69), 67 (86), 55 (78), 43 (100). EI-MS m/z (RT 13.635): 308 (1), 248 (8), 233 (1), 219 (2), 205 (2), 191 (2), 177 (3), 163 (5), 149 (10), 135 (18), 121 (20), 109 (25), 95 (47), 81 (70), 67 (84), 55 (79), 43 (100). Gas chromatography–mass spectrometry (GC–MS analysis) of synthetic E3,Z13-18:OAc and Z3,Z13-18:OAc indicated that the two pheromone compounds did not contain isomeric compounds (Fig. 4).
Effect of phermone blends on capture of S. bicingulata males
The number of the adult male moths caught in traps baited with ratios of 1:9, 4:6, 6:4, and 9:1 for E3,Z13-18:OAc to Z3,Z13-18:OAc is shown in Fig. 5a. Among test ratios of 1:9, 4:6, 6:4, and 9:1, a 4:6 mixture was the most attractive, followed by 1:9 and 6:4. No adult males were caught in traps baited with 9:1 or control traps. The number of the adult male moths caught in traps baited with ratios of 1:9, 2:8, 3:7, 4:6, and 5:5 for E3,Z13-18:OAc to Z3,Z13-18:OAc in Jinju, Gongju, and Gapyeong are shown in Fig. 5b–d. No regional differences in the response of S. bicingulata males to sex pheromones were observed. A significant difference in the number of male adults caught in traps baited with 4:6 and 2:8 ratios was observed at all test areas in 2018. Significantly more males were attracted to traps baited with a 4:6 ratio of E3,Z13-18:OAc and Z3,Z13-18:OAc compared with traps baited with a 3:7 ratio of E3,Z13-18:OAc and Z3,Z13-18:OAc at Gonju in 2018.
Number of S. bicingulata adult males caught in traps baited with different ratios of E3,Z13-18:OAc and Z3,Z13-18:OAc in 2017 [Gongju (a), Tukey’s HSD, F4,15 = 38.567, p < 0.001)] and in 2018 [Jinju (b), F4,15 = 14.656, p < 0.001); Gongju (c), F4,15 = 5.962, p = 0.004; Gapyeong (d), F4,15 = 4.787, p = 0.011]
Effect of trap type on capture of S. bicingulata males
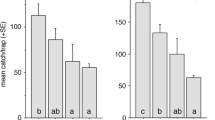
The effect of the two trap types on the capture of S. bicingulata males is shown in Fig. 6. A significant difference was seen in the number of S. bicingulata males caught in bucket and delta traps (t = 4.174, p < 0.01, df = 8).
Color spectrometry analysis
Trap surface-color values (L*a*b*) are shown in Table 1. The highest L* value was obtained from a white bucket trap, followed by yellow, red, green, blue, and black bucket traps. Red and green bucket traps showed the highest and the lowest a* values, respectively. The highest b* values was obtained from a yellow bucket trap, followed by red, green, white, black and blue bucket traps. The reflectance rates of the six different colors of bucket traps in wavelength between 360 and 750 nm are provided in Fig. 7 and Table S1 (Online Resource 1). White bucket traps exhibited > 80% reflectance from 410 to 730 nm. Red and yellow bucket traps showed > 30% reflectance from 605 to 750 nm, and from 545 to 705 nm, respectively. Green, blue, and black bucket traps showed less than 14% reflectance at all test wavelengths.
Effect of bucket trap color on capture of S. bicingulata males
The effect of yellow and green bucket traps on the capture of male S. bicingulata is shown in Fig. 8. A significant difference was evident in the number of male S. biingulata caught in yellow and green bucket traps in Gongju (t = 6.983, p < 0.001, df = 6) and Seoul (t = 3.174, p < 0.05, df = 6). The effect of the six colors of bucket traps on the capture of male S. bicingulata are shown in Fig. 9. A significant difference was observed in the number of males caught in the six different colors of bucket traps. Yellow bucket traps caught the largest number of male, and no adult male was caught in a blue bucket trap at Gongju. There was a significant difference in the number of S. bicingulata males caught in yellow bucket traps compared with the other colors of bucket traps at Yanggu. Linaer regression analysis of the effect of trap surface-color value (L*a*b*) on the capture of males is shown in Fig. 9d–f, respectively. A positive relationship was evident between b* value and trap capture (R2 = 0.389, p < 0.001). A weak relationship was observed between L* value and trap capture (R2 = 0.085, p = 0.023). No relationship was found between a* value and trap capture (R2 = 0.005, p = 0.567).
Number of S. bicingulata adult males caught in bucket traps of six different colors [Gapyeong (a), Tukey’s HSD, F5,12 = 4.668, p = 0.0134; Gongju (b), F5,12 = 5.305, p = 0.0084; Yanggu (c), F5,18 = 5.470, p = 0.0031] and linear regression analysis of the effect of trap surface-color value [L* (d); a* (e); b* (f)] on trap capture
Discussion
In this study, we synthesized two sex phermone components of S. bicingulata, E3,Z13-18:OAc and Z3,Z13-18:OAc, and investigated the optimal sex pheromone ratio and the effect of trap type and color on the capture of S. bicingulata males. The largest number of S. bicingulata males was caught in tarps baited with a 4:6 mixture of E3,Z13-18:OAc and Z3,Z13-18:OAc in this and previous study (Yang et al. 2011). However, a difference was evident in the interest shown by S. bicingulata males to other E3,Z13-18:OAc-to-Z3,Z13-18:OAc ratios between this study and the Yang et al. (2011) study. Yang et al. (2011) reported no significant difference in the numbers of male adults caught in traps baited with 6:4, 5:5, 4:6, 3:7, and 2:8 ratios for E3,Z13-18:OAc to Z3,Z13-18:OAc. The largest number of S. bicingulata males was caught in traps baited with a 4:6 ratio followed by 2:8, 3:7, 6:4, and 5:5 ratios. However, the largest number of S. bicingulata males was caught in traps baited with a 4:6 ratio, followed by 5:5, 3:7, 2:8, and 1:9 in this study. In addition, there was a significant difference in the numbers of male adults caught in traps baited with 4:6 and 2:8 ratio of E3,Z13-18:OAc to Z3,Z13-18:OAc in our study. This may be a product of differences in the purity of pheromone components. The previous study by Yang et al. (2011) used E3,Z13-18:OAc and Z3,Z13-18:OAc containing an isomeric compound (a mixture of E,Z and Z,Z) for field experiments, and this may have caused the similar attractiveness of S. bicingulata to several ratios of E3,Z13-18:OAc and Z3,Z13-18:OAc. However, the authors did not supply information on the isomeric ratio of E3,Z13-18:OAc and Z3,Z13-18:OAc, and the most attractive ratio could not be determined, whereas we synthesized and purified two pure components without a mixture of isomers in the laboratory. Based on the purity of the two pheromone components, we concluded that the optimal ratio of E3,Z13-18:OAc to Z3,Z13-18:OAc for S. bicingulata adult males was approximately 4.3:5.7.
Trap capture are greatly affected by pheromone trap design for many insect species (Malo et al. 2001; Athnaassiou et al. 2004, 2007; Strong et al. 2008; Kim and Park 2013). Various pheromone trap designs, such as delta traps, Jackson traps, wing-style, and bucket traps have been used for Sesiidae moths (Bakowski 2001; Adler 1983; Pfeiffer et al. 1999; Weihman and Liburd 2007). Zhang et al. (2013) compared the efficacy of delta, wing-style, and bucket traps for the dogwood borer Synanthedon scitula but could not identify a clearly superior single trap design. No reports have been published on the effect of trap design on the capture of S. bicingulata males, and only delta traps have been used for field experiments with the S. bicingulata sex pheromone (Yang et al. 2011, 2012; Cho et al. 2016; Lee et al. 2017). This study showed that bucket traps were superior to delta traps for capturing S. bicingulata males.
Colored traps baited with sex pheromones affected the capture of S. bicingulata males in this study. Previous studies have reported an effect of the color of traps baited with sex pheromones on the capture of clearwing moths (Suckling et al. 2005; Judd and Eby 2013; Karlius and Būda 2007) and other insect species (Athnaassiou et al. 2004, 2007; Gadi and Reddy 2014; Abuagla and Al-Deeb 2012). Athanassiou et al. (2004) reported that more Palpita unionalis males were caught in white bucket trap with strong light reflectance at 370–450 nm compared with brown bucket traps with weak light reflectance. Suckling et al. (2005) also reported that more Synanthedon tipuliformis males were caught in yellow and green delta traps compared with red, white, black, and blue delta traps. Judd and Eby (2013) investigated the effect of various solid and multicolored bucket traps on the capture of Synanthedon myopaeformis males; more were attracted to bucket traps with a yellow lid. Judd and Eby argued that color with reflectance in the green region of the light specturm (500–550 nm) was an important factor affecting trap capture. However, white bucket traps with a high intensity of reflectance in the green region were associated with low capture of male S. myopaeformis. This suggests that S. myopaeformis, like S. tipuliformis (Karlius and Būda 2007), has blue-sensitive photoreceptors that enable discrimination of white and yellow bucket traps using differnces in their blue-green spectral compositions. Similar results were observed in this study. Yellow bucket traps with reflectance in the green region (Fig. 7, Table S1 in online Resource 1) were the most attractive, but capture with white bucket traps were lower than those with yellow bucket traps. S. bicingulata may be able to discriminate yellow and white bucket traps using differnces in their blue–green spectral compositions, as is the case with S. myopaeformis (Judd and Eby 2013) and S. tipuliformis (Karlius and Būda 2007). Bucket traps of other colors with low reflectance in the green region were associated with low trap capture in this study. Analysis of trap surface-color values indicated that S.bicingulata males were most affected by b* values rather than L* and a* values (Fig. 9). The results of this study suggest that male S. bicingulata uses both olfactory and visual cues to locate female adults. However, it is unclear why some clearwing moths are strongly attracted to patches of yellow. Based on the day-flying activity of S. myopaeformis, Judd and Eby (2013) hypothesized that a yellow bucket trap mimics patches of sunlit foliage of host trees and that males preferentially head toward to a yellow trap in search of females when baited with pheromones. This study showed that a yellow bucket trap baited with 4.3:5.7 ratio of E3,Z13-18:OAc to Z3,Z13-18:OAc could improve the efficacy of monitoring of S. bicingulata.
References
Abuagla AM, Al-Deeb MA (2012) Effect of bait quantity and trap color on the efficacy of the pheromone trap for the red palm weevil Rhynchophorusferrugineus. J Insect Sci 12:120
Adler VE (1983) Adult Sesiidae of Maryland collected in traps baited with isomer of 3,13-octdecadien-1-ol acetate. J Environ Sci Health Part A 18:611–619
Armstrong-Chong RJ, Matthews K, Chong JM (2004) Sequential alkynylation of ω-bromoalkyl triflates: facile access to unsymmetrical non-conjugated diynes including precursors to diene pheromones. Tetrahedron 60:10239–10244
Athanassiou CG, Kavallieratos NG, Mazomenos BE (2004) Effect of trap type, trap color, trapping location, and pheromone dispenser on captures of male Palpitaunionalis (Lepidoptera: Pyralidae). J Econ Entomol 97:321–329
Athanassiou CG, Kavallieratos NG, Gakis SF, Kyrtsa LA, Mazomenos BE, Gravanis FT (2007) Influence of trap type, trap colour, and trapping location on the capture of the pine moth, Thaumetopoeapityocampa. Entomol Exp Appl 122:117–123
Bakowski M (2001) The use of sex pheromone in faunistic studies on clearwing moths (Lpidoptera: Sesiidae). Wiad Entomol 20:165–170
Cho YS, Kim J, Jang SA, Park CG (2016) Seasonal occurrence patterns of Synathedontenuis and S.bicingulata (Lepidoptrea: Sesiidae) in sweet persimmon orchards in the southern part of Korea. Korean J Appl Entomol 55:297–301
Ebata T, Mori K (1979) A convenient synthesis of a mixture of (Z, Z)-3,13-octadecadienyl acetate and its (EsZ)-isomer, the attractant for the cherry borer. Agric Biol Chem 43(7):1567–1570
Gadi N, Reddy GVP (2014) Are sweetpotato weevils (Coleoptera: Brentidae) differentially attracted to certain color? Ann Entomol Soc Am 107:274–278
Judd GJR, Eby C (2013) Spectral discrimination by Synanthedonmyopaeformis (Lepidoptera: Sesiidae) when orienting to traps baited with sex pheromone or feeding attractants. Can Entomol 146:8–25
Karlius V, Būda V (2007) Colour vision in currant clearwing moth (Synanthedontipuliformis) (Lepidoptera: Sesiidae). Acta Zool Lit 17:198–202
Kim J, Park IK (2013) Female sex pheromone components of the box tree pyralid, Glyphodesperspectalis, in Korea: Field test and development of film-type lure. J Asia-Pac Entomol 16:473–477
Kim JK, Koh SH, Koo CD, Kim KW, Kim JK, Kim JJ, Park GS, Park YC, Park IK, Byun BK, Chae HM, Han SS (2019) Forest Protection. Haengmoonsa, Seoul, Republic of Korea, pp 326–337
Lee CM, Bae YS, Arita Y (2004) Morphological description of Synanthedonbicingulata (Staudinger, 1987) in life stages (Lepidoptera, Sesiidae). J Asia-Pac Entomol 7:177–185
Lee S, Kim C, Lee KH, Lee JW, Oh HK, Han J, Kim SH, Kim Y (2017) Seasonal occurrence of three pest moths in jujube orchards in Boeun, Korea. Korean J Appl Entomol 56:261–265
Lee DH, Ku YC, Jung WJ, Lee CW, Lee SY, Park IK, Lee SC (2018) Method for preparation of (3E,13Z)- octadecadienyl acetate, (3Z,13Z)-octadecadienyl acetate as major sex pheromone of cherry tree borer, Synanthedon bicingulata. Korea Patent, 1019018080000
Leskey TC, Bergh JC, Walgenbach JF, Zhang A (2009) Evaluation of pheromone-based management strategies for dogwood borer (Lepidoptera: Sesiidae) in commercial apple orchard. J Econ Entmol 102:1085–1093
Malo EA, Cruz-Lopez L, Valle-Mora J, Virgen A, Sanchez JA, Rojas JC (2001) Evaluation of commercial pheromone lures and traps for monitoring male fall armyworm (Lepidoptera: Noctuidae) in the coastal region of Chiapas, Mexico. Fla Entomol 84:659–664
Mendiburu FD (2020) Agricolae: Statistical Procedures for Agricultural Research Version 1.2–8. http://tarwi.lamolina.edu.pe/~fmendiburu. Accessed 12 Apr 2020
Naka H, Nakazawa T, Sugie M, Yamamoto M, Horie Y, Wakasugi R, Arita Y, Sugie H, Tsuchida K, Ando T (2006) Synthesis and characterization of 3,13- and 2,13-octadecadienyl compounds for identification of the sex pheromone secreted by a Clearwing Moth Nokonapernix. Biosci Biotechnol Biochem 70(2):508–516
Naka H, Horie Y, Mochizuki F, Vang LV, Yamamoto M, Saito T, Watarai T, Tsuchida K, Arita Y, Ando T (2008) Identification of the sex pheromone secreted by Synanthedonhector (Lepidoptera: Sesiidae). App Entomol Zool 43:467–474
Pfeiffer DG, Killian JC (1999) Dogwood borer (Lepidoptera: Sesiidae) flight activity and an attempt to control damage in ‘Gala’ apples using mating disruption. J Entomol Sci 34:210–218
The Pherobase (2020) https://www.pherobase.com/database/genus/genus-Synanthedon.php. Accessed on 31 Mar 2020
Strong WB, Millar JG, Grant GG, Moreira JA, Michael Chong J, Rudolph C (2008) Optimization of pheromone lure and trap design for monitoring the fir coneworm, Dioryctriaabietivorella. Entomol Exp Appl 126:67–77
Suckling DM, Gibb AR, Burnip GM, Snelling C, De Ruiter J, Langford G, El-Sayed AM (2005) Optimization of pheromone lure and trap characteristics for currant clearwing, Synanthedontipuliformis. J Chem Ecol 31:393–406
Tumlinson JH, Yonce CE, Doolittle RE, Heath RR, Gentry CR, Mitchell ER (1974) Sex pheromones and reproductive isolation of the lesser peach tree borer and the peach tree borer. Science 185:614–616
Uchida M, Mori K, Matsui M (1978) Synthesis of (Z, Z)-3,13-octadecadienyl acetate and its (E, Z)-isomer, the attractant for the cherrytree borer. Agric Biol Chem 42:1067–1070
Uchida M, Nakagawa K, Mori K (1979) Stereoselective synthesis of (Z, Z)-3,13-octadecadienyl acetate, the attractant for smaller clear wing moth. Agric Biol Chem 43:1919–1922
Weihman SW, Liburd OE (2007) Seasonal distribution and evaluation of two trap types for monitoring grape root borer Vitaceapolistiformis (Lepidoptera: Sesiidae) in Florida vineyards. Fla Entomol 90:480–487
Yamamoto A, Ishihara T, Fukumoto T (1989) Improved synthesis of (3Z,13Z)-and (3E,13Z)-3,13-octadecadinyl acetate, sex pheromone of the Synanthedon species. Agric Biol Chem 53:285–287
Yang CY, Kim SJ, Lee SG (2011) Identification and field evaluation of the sex pheromone of Synanthedonbicingulata (Staudinger). J Chem Ecol 37:398–402
Yang CY, Kim SJ, Yang SJ, Cho MR (2012) Seasonal adult occurrence of four clearwing moths in Suwon orchards. Korean J Appl Entomol 51:443–447
Zhang A, Leskey TC, Bergh JC, Walgenbach JF (2013) Sex pheromone dispenser type and trap design affect capture of dogwood borer. J Chem Ecol 39:390–397
Funding
This study was carried out with the support of ‘R&D Program for Forest Science Technology (Project No. “2020185B10-2022-AA02”)’ provided by Korea Forest Service (Korea Forestry Promotion Institute).
Author information
Authors and Affiliations
Contributions
JHK and IKP designed the research and wrote the first draft of manuscript. JHK conducted field experiments. DHL conducted the chemical syntheses. JHK and MJH analyzed data. SMS conducted chemical analysis. All authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare we have no competing interests.
Additional information
Communicated by Günther Raspotnig.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kwon, JH., Huh, MJ., Lee, DH. et al. Effect of pheromone blends, trap type and color on the capture of male clearwing moths, Synanthedon bicingulata (Lepidoptera: Sesiidae). Chemoecology 31, 289–299 (2021). https://doi.org/10.1007/s00049-021-00352-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-021-00352-6