Abstract
Plantation forestry with non-native trees is steadily increasing in the southern hemisphere and alien pest problems are also accumulating, as a consequence of the growing international movement of people and goods. Here, we present an overview of studies on the ecology and on the control practices deployed against the European woodboring wasp Sirex noctilio, in South America. Management actions have been largely adopted from other southern hemisphere countries, although generally with very little local adaptation or critical evaluation of successes and failures. The knowledge acquired in South America allows us to look retrospectively at critical issues that explain woodwasp invasion success and damage levels, and to identify specific research areas that warrant further work. We emphasize the need of population ecology studies in both the invaded and native ranges, the development of specific sampling protocols, and detailed studies aimed at evaluating the role played by natural enemies in preventing large-scale population outbreaks. These demands may be generalized to the management of other invasive pests in plantation forestry with non-native trees in the southern hemisphere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
The woodwasp Sirex noctilio is currently a widespread invasive forest insect pest of pine plantations throughout the southern hemisphere.
-
In South America, and unlike what happens in its native range, populations show a pulse-like eruptive behavior that may cause massive tree killings.
-
Several management actions have been deployed in the region, largely adopted from other southern hemisphere countries, lacking local adaptation and a critical evaluation of local successes and failures.
-
Our review identifies several specific research areas for further work. These highlight the need for studies on the population dynamics of S. noctilio, with protocols that allow comparison among regions.
Introduction
Plantation forestry with non-native trees is substantially increasing in many developing countries of the southern hemisphere (Brockerhoff et al. 2008; Wingfield et al. 2015). In South America, more than 12 million hectares are planted with fast-growing exotic species, essentially pine (Pinus spp.) and eucalypt (Eucalyptus spp.) (Payn et al. 2015). Plantations are established for the production of timber, pulp and fuel, and largely consist of intensively managed, even aged, and regularly spaced stands of single species, usually with low levels of genetic variation (FAO 2015).
Disease and insect outbreaks affect a significant proportion of forest worldwide, especially natural and planted native tree forests in the temperate northern hemisphere (Payn et al. 2015; van Lierop et al. 2015). In contrast, plantations with non-native species in the Southern Hemisphere are generally considered to be less impacted (Keane and Crawley 2002). This is mostly because they are being kept free of coevolved pests and diseases, which are usually associates in their native range, until they are eventually reunited (the “enemy releases hypothesis”). This enemy-free period, during which substantial stand losses can be avoided, can be variable, but is expected to be reduced in the current context of increasing accidental transportation of alien forest organisms (Brockerhoff and Liebhold 2017). Global change, and in particular the unprecedented movement of goods and people, can therefore arguably be considered the most important threat to plantation forestry with non-native species (Walther et al. 2009; Wingfield et al. 2015).
In forest plantations in South America, the detection of established non-native pests has increased in recent years. In Argentina, Botto (2008) has shown an exponential increase in the annual detection rates of non-native insect pests on Eucalyptus spp. between 1991 and 2005. A recent report jointly produced by FAO and the quarantine agencies of several South American countries (Argentina, Bolivia, Brazil, Chile, Paraguay and Uruguay) states that by 2008 there were at least 58 non-native insects established (FAO 2008). Argentina reported the largest number of species (37), followed by Brazil (24) and Chile (14), and Eucalyptus spp. were the most affected tree species. About a quarter of the alien pests and pathogens in this list were found in more than one country (Corley and Villacide 2014).
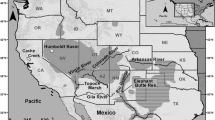
Among the non-native forest insects shared by many countries and regions of South America in pines, the woodwasp Sirex noctilio F. (Hymenoptera: Siricidae) is probably the most notorious, because of its widespread distribution and impact (Fig. 1; Villacide and Corley 2012; Corley and Villacide 2014; Lantschner et al. 2014). The species is native to Eurasia and northern Africa and has successfully invaded several countries of the southern hemisphere in the last century. It was first reported in New Zealand around 1900 (Miller and Clark 1935), in Australia in 1951, and arrived in South America by 1980 and in South Africa in 1994 (Gilbert and Miller 1952; Tribe 1995; Maderni 1998; Boissin et al. 2012).
Sirex noctilio is a woodboring wasp that has a solitary, cryptic lifestyle. Females lay eggs directly into the wood by drilling holes in stems of stressed pines. The successful egg hatching and development of S. noctilio larvae relies on the disruption of tree physiology caused by the combined effect of a symbiotic fungus (Amylostereum areolatum) and a phytotoxic venom introduced into the tree at the time of drilling by the female (Madden 1988; Slippers et al. 2015). After 1–3 years development period inside the wood (depending on the climatic region), adults emerge, mate and attack new trees (Morgan and Stewart 1966; Madden and Coutts 1979). A list of specific life history traits of S. noctilio recorded in South America is provided in Table 1.
Our aim is to provide an overview of the ecology of Sirex noctilio in South America. We also devote attention on the successes and failures of the management strategies for the pest in the continent, to identify future research needs. We expect this synopsis will support further understanding of the risks and impacts posed by alien insects to plantation forest in South America and elsewhere in the world.
Distribution in South America
The historical spread of S. noctilio across South America is shown in Fig. 2, with emphasis on the two most important regions in terms of pine plantations surface: the south of Brazil, Uruguay and northeastern Argentina (about 2.4 million ha); and Chile and southwestern Argentina (about 2 million ha; for details see Table 2).
Sirex noctilio was recorded for the first time in South America in Cerro Largo Department, Uruguay in 1980 (Maderni 1998). The wasp then rapidly colonized all regions with pines in this country, reaching Argentina in 1985 and Brazil in 1988 (Iede et al. 1998). Within Brazil, it was first detected in the state of Rio Grande do Sul, from where it spread northwards, rapidly colonizing the states of Santa Catarina in 1989, Paraná in 1996, São Paulo in 2004 and Minas Gerais in 2005 (Iede and Zanetti 2007). In Argentina, the first report in 1985 was in the province of Entre Rios, and over a ten-year period, it spread toward the north, reaching the provinces of Corrientes and Misiones, which border with Brazil. Later, accidental introductions into the other parts of Argentina took place: the province of Buenos Aires in 1988, the province of Río Negro (Patagonia) in 1993, the north-western province of Jujuy in 1994 and the central region of the country (Cordoba province) in 1995 (Klasmer and Botto 2012). In 2001, a few female wasps were caught in funnel traps located in the Valparaiso and distant Los Lagos regions of Chile (SAG 2004). There were no captures of S. noctilio in Valparaiso after 2004, suggesting local extinction (Beèche 2012). In contrast, in Los Lagos, the species became established and spread during the following years, toward the north and south of that country. Establishments were first recorded in 2002 in Los Ríos and La Araucanía and then in 2009 in Bio–Bio, in 2011 in Maule, in 2012 in Aysén and in 2016 in O’Higgins and Valparaiso (Beèche et al. 2012; SAG 2017).
Over the last 50 years, S. noctilio has shown an extraordinary capacity to reach most areas where pines are grown in South America. The northernmost location known to date is the Minas Gerais province in Brazil (21° S; Iede and Zanetti 2007), and the southernmost location is The Aysén region in Chile (46° S; SAG 2017). This broad distribution within South America includes contrasting eco-climatic regions with mean annual temperatures that range from 5.1 to 20.5 °C and rainfall regimes from 450 to 2300 mm annually (Hijmans et al. 2005). Likewise, S. noctilio has adapted and succeeded in locations with different forest composition, growth rates and management practices.
Populations of S. noctilio in South America appear to originate from two major sources or lineages; one most likely from Europe, and the other of an unknown origin (Boissin et al. 2012). Despite some national, regional and local efforts deployed to prevent the geographical spread of S. noctilio in South America (e.g., quarantine programs; Iede et al. 1998; Villacide and Corley 2012; SAG 2017), spread rates shown by this insect were high along all invaded districts (Table 2; Lantschner et al. 2014), being even higher than most of those observed for other invasive forest insect species in different parts of the world (see examples in Evans 2016). For S. noctilio, it has been shown that spread rates increase with increasing mean annual temperature and isothermality, ranging from 17 km/year in SW Argentina, to 32.6 km/year in Chile, and 46.3 km/year in Brazil (Table 1; Lantschner et al. 2014). Climatic models have predicted that S. noctilio could establish further north from its current distribution—i.e., northern Brazil, Bolivia, Peru, Ecuador, Colombia and Venezuela (Carnegie et al. 2006; Ireland et al. 2018). However, there is no evidence to date that the species has established in any of these areas (see Fig. 1). Furthermore, the northern edge of current distribution, at latitude 21°S (Minas Gerais, Brazil), does not correspond to an eco-climatic rupture (Lantschner et al. 2014; Ireland et al. 2018).
Population dynamics and impact
In most regions within the invaded range, the S. noctilio displays a pulse-like eruptive population behavior (Berryman 1987). The species population dynamics is characterized by long periods during which populations remain at relatively low densities and rapidly increase to outbreak levels in an unpredictable fashion (Berryman 1987; Villacide and Corley 2012; Lantschner et al. under review). The occurrence of S. noctilio population outbreaks in South America and the resulting damage has been described for most areas where the woodwasp has established, such as Uruguay (Maderni 1998), southern Brazil (Rodigheri et al. 2006), southern Argentina (Lantschner and Corley 2015) and central Chile (Poisson et al. 2016) (Table 2).
Sirex noctilio outbreaks in South America, as well as in other parts of the southern hemisphere, have been found to be commonly triggered by increased disposal of stressed trees, suggesting that resource availability plays a key role in regulating local population abundance. Outbreaks have been found to be more likely in pine stands that are overstocked, damaged and unmanaged (Beèche et al. 2012; Iede et al. 2012; Lantschner and Corley 2015), as well as in plantations set on slopes at locations with drier conditions (Lantschner and Corley 2015). For southern Argentina, Lantschner and Corley (2015) suggest that the forest composition may also be an important factor determining outbreak probability: lodgepole pine (P. contorta) is remarkably more attacked than other pines planted there (i.e., P. ponderosa). This has also been noted in Uruguay and Brazil where P. taeda is reportedly more susceptible than P. elliottii (Iede et al. 2012).
The “intermittent drought hypothesis” proposed by Madden (1988) for Australia proposes that tree stress factors, usually rapid changes in the physiological status of trees associated with drought stresses, are predisposing particular stands or occasionally entire forest regions to S. noctilio attacks. Once a population has initially established in a susceptible area, the spatial aggregation of attacks becomes stronger as the woodwasp population grows. This indicates positive density-dependent processes are certainly a key factor for the onset of outbreaks at the landscape scale (Corley et al. 2007; Aparicio et al. 2013). At this scale, another feature associated with the probability of wasp populations reaching outbreak levels is their proximity to stands bearing outbreaks concurrently or recently (Lantschner and Corley 2015). Female dispersal may have a significant effect both on the onset and in expanding outbreaks throughout the landscape (Aparicio et al. 2013; Lantschner and Corley 2015; Lantschner et al. under review). Consequently, under the influence of an abrupt drought, S. noctilio populations rise and disperse from epicenters to create more widespread epidemics (Table 1; Fig. 3).
The termination of the epidemics appears to result from intra-specific competition due to widespread food depletion caused by the massive tree killings, or because of increased mortality due to natural enemies, as it has been suggested for other parts of the world (i.e., Australia and New Zealand; Madden 1988). Although in South America extensive biocontrol programs have been deployed (see below “Management strategies”), the role played by natural enemies in preventing and/or terminating outbreaks remains an open question. Information about the success of natural enemies at different phases of S. noctilio population dynamics is still very limited.
Monitoring Sirex noctilio populations in South America
Damage due to established S. noctilio populations may be reduced through adequate silvicultural practices and biological control. However, damage levels can be important upon the arrival of woodwasps into new afforested areas, related to the absence of natural enemies and possibly a local high abundance of susceptible trees. This has resulted in the need of detection methods for new establishments and to monitor established populations.
Historically, S. noctilio early detection has been carried out in many countries through indirect methods (i.e., looking for attack symptoms, rather than the insect itself). Such methods rely on the identification of attacked pines (i.e., crown chlorosis and resin droplets on the main stem, produced after wasp drilling during oviposition), which are carried out through ground surveys (e.g., sequential sampling) or aerial surveys. However, these methods lack sensitivity during the early phases of establishment due to the small number of affected trees (Carnegie et al. 2005).
An additional method widely deployed throughout affected areas it the “trap-tree” technique. This method is based on the attraction shown by female S. noctilio wasps toward stressed trees (Madden and Irvine 1971; Bordeaux and Dean 2012) and consists of treating a small group of trees (e.g., 4–10 trees) with herbicide or through other methods such as girdling, a few months prior to the wasp flight season. In South America, after the flight season ends, treated trees are felled and cut into billets that are caged to check for adult emergence. The density of trap-tree plots is variable, ranging from 1 plot/25 ha in areas with known presence of S. noctilio, to 1 plot/10.000 ha in border areas or sites located more than 50 km away from established S. noctilio populations (COSAVE 2002). Trap trees also can be used in biological control practices, by using the felled trees as substrate for the release of the nematode D. siricidicola, and to estimate the presence and parasitism rates achieved by nematode and parasitoid releases.
More recently, developments have been made toward cost-effective methods based on the direct detection of adult wasps. This is generally implemented by means of static trapping systems baited with semiochemicals (Bashford 2008; Crook et al. 2008). A typical capture unit consists of an intercept panel trap or Lindgren multiple funnel trap in combination with a blend of kairomones, usually terpenes (different combinations of α and β pinene). These volatiles are those released by stressed trees (Simpson 1976; Simpson and Mc Quilkin 1976; Bashford and Madden 2012). Kairomone traps have already been implemented by nationwide programs in many countries affected by S. noctilio (i.e., Australia, South Africa, Canada and the United States), and their use seems to be slowly taking off in South America (Table 2). Despite the fact that the benefits of kairomone-baited traps methods are clear, their effectiveness has been questioned when S. noctilio populations are low. This is because kairomones from traps compete with volatiles released by trees that are stressed naturally (i.e., in drought conditions) or artificially (i.e., trap trees) (Bashford and Madden 2012).
The challenge presented for effective monitoring and hence management is the limited ability of cost-effective methods in detecting low-density populations in forests (Bashford and Madden 2012; Hurley et al. 2015) or in high-risk areas such as ports and sawmills. In this sense, pheromone-baited traps may prove an important addition, since this type of traps can be highly specific and work at low population densities (Witzgall et al. 2010). Sirex noctilio chemical communication, especially the compounds involved in mate location, has been generally understudied, probably because of the elusive mating behavior displayed by this insect (i.e., copulation generally occurs at the top of tall trees). Some recent work shows that a male produced pheromone attracts both females and males in laboratory assays but not in the field (Böröczky et al. 2009; Cooperband et al. 2012; Hurley et al. 2015). It has also been shown that S. noctilio females as well as its parasitoids can discriminate previously attacked trees from other, by specific blends of chemicals they release (Martínez et al. 2006; Fischbein et al. 2012; Fernández Ajó et al. 2015; Jofré et al. 2016). Understanding the orientation response of foraging S. noctilio females, notably the volatiles that are elicited by the development of the fungal symbiont A. areolatum, represents another prospect for early detection.
Management strategies
The management of S. noctilio in South America is primarily set on reducing tree mortality associated with outbreaks. It is mainly comprised by two components: silviculture and the introduction of natural enemies for biological control (Klasmer and Botto 2012; Beèche et al. 2012; Iede et al. 2012).
Silvicultural management can be of preventive nature when it is aimed at reducing tree susceptibility to S. noctilio new attacks, essentially through pruning and thinning of stands. Sanitary thinning is also a common practice to reduce woodwasp populations. In this case, stands are carefully ground surveyed, and all trees found with symptoms of woodwasp attacks are felled and either chipped, burned or buried in trenches (Klasmer and Botto 2012; Beèche et al. 2012; Iede et al. 2012).
The parasitic nematode D. siricidicola is considered the primary biological control agent for S. noctilio in the southern hemisphere (Slippers et al. 2015). In South America, the release of nematodes was performed from 1989 in Brazil and Northern Argentina (Iede et al. 2012), from 1999 in Southern Argentina (Klasmer and Botto 2012), and from 2006 in Chile (Beèche et al. 2012). The released nematodes were mass produced in laboratories in Chile, Brazil, and Argentina, from original Kamona strains from Australia, and occasionally supplemented by nematodes re-isolated from initial release sites in Brazil and Chile (SAG 2004; Beèche et al. 2012; Iede et al. 2012). In 2004, a new strain obtained from infested S. noctilio adults brought to Chile from New Zealand was isolated for mass production (the “Tangoio strain”; SAG 2004; Beèche et al. 2012). Nematode populations have become well established in most release sites, although parasitism levels reported are highly variable across areas and with time, ranging from 14 to 90% in some locations (Table 2).
Variability in parasitism success by D. siricidcola in different regions has been proposed to be associated with the wood moisture of pine trees, the development of local resistance by S. noctilio, loss of nematode virulence and/or the presence of competing micro-organisms or fungi (Hurley et al. 2008; Slippers et al. 2012). A study carried out in Southern Argentina suggested that the spatial arrangement of the releases can also affect the local abundance of parasitism achieved at the landscape scale, during the first years (Corley et al. 2014). However, the reduction of diversity in nematode populations due to the selection process and inbreeding as a consequence of intensive laboratory rearing, may be the key factor affecting parasite success (Bedding and Iede 2005; Mlonyeni et al. 2011). Likely, the lack of diversity in nematode populations may reduce their capacity to adapt and establish in climatically different environments, on different Pinus spp., and to parasitize different populations of S. noctilio (Slippers et al. 2012).
In addition, three species of parasitoid wasps, native to Europe and North America have been released into the invaded range of S. noctilio in South America (Cameron 2012). The hymenopteran species Ibalia leucospoides (Hochenwarth), Megarhyssa nortoni (Cresson) and Rhyssa persuasoria (Linneaus) were introduced and established successfully in many, though not all, Sirex-infested pine-growing areas of South America (Iede et al. 2012; Beèche et al. 2012; Fischbein and Corley 2015). Interestingly, and in contrast with other regions of the Southern Hemisphere where similar biological control programs have been carried out (e.g., South Africa), I. leucospoides arrived in Argentina, Brazil and Uruguay accidentally, presumably in pest-infested wood from unknown origins [refer to Boissin et al. (2012) for description of possible origins of S. noctilio in South America]. This parasitoid has effectively established viable populations in these countries (Fischbein and Corley 2015). In other countries, notably Chile, females of I. leucospoides were collected in the field, to rear and locally augment populations (Beèche et al. 2012). M. nortoni from Tasmania were released for the first time in 1996 in Brazil (Iede et al. 2012), and since 2005 individuals from New Zealand and Tasmania were repeatedly imported in southern Argentina and Chile (Beèche et al. 2012). However, these releases have been associated with variable establishment success (see Table 2 for details). For instance, this parasitoid has never been recovered from the field in Brazil (Iede et al. 2012). R. persuasoria was introduced in Brazil from Tasmania and to Chile and Argentina (via Chile) from New Zealand. This species has apparently not established in Argentina [F. Azzaro (SENASA), personal communication and personal observation], nor in Chile (Beèche et al. 2012) or Brazil (Iede et al. 2012). A general observation is that while I. leucospoides has established well and may perform better in warmer and drier climatic regimes (Spradbery and Kirk 1978; Taylor 1978; Corley and Bruzzone 2009), M. nortoni may do better in temperate to cold climates (Collett and Elms 2009; Cameron 2012).
The mortality on S. noctilio populations in South America assigned to Ibalia leucospoides, Megarhyssa nortoni and Rhyssa persuasoria is largely variable, ranging from an estimated 13–90% (Table 2). Although, in broad terms, parasitism rates are not negligible allowing us to consider these species as effective mortality agents of S. noctilio in a number of regions (Cameron 2012; Fischbein and Corley 2015), they may not contribute to prevent woodwasp outbreaks (Lantschner et al. under review). Their impact as non-regulating mortality factors depends on the environmental conditions in which they have established (Taylor 1978; Corley and Bruzzone 2009), their different life history traits such as reproductive mode, host stage attacked, attack rate, host searching strategy and dispersal abilities (Spradbery 1970; Fischbein et al. 2011, 2018; Fischbein and Corley 2015). Some data on host-parasitoid population interactions among these parasitoids collected in other Southern Hemisphere regions, suggesting that the populations of M. nortoni and R. persuasoria respond to delayed density-dependent factors, whereas I. leucospoides populations primarily respond to density-independent factors (e.g., Taylor 1978).
Conclusions and future prospects
Sirex noctilio is currently one of the most important threats to plantation forestry throughout South America (Villacide and Corley 2012). The pest is present in most Pinus spp. plantations in the region, and in some localities, may cause remarkably high levels of tree mortality. Such impact has led to national efforts in the pine-growing countries, to prevent pest spread and manage populations where these have established. These practices have been largely adopted from management programs developed in other regions of the world, with limited local adaptation and lacking comparative evaluation of success and failures. Nonetheless, recent studies of S. noctilio in South America provide further insight of the general ecology and behavior in that species. These may contribute more widely to improved management of damaging populations throughout the invaded range.
In South America, S. noctilio has reached most areas where pine are grown above the 21° latitude south, largely favored by human activities such as the movement of infested wood (Fig. 1; Carnegie et al. 2006; Boissin et al. 2012). However, inside each area, the spread has also been suggested to be strongly determined by the wasps’ natural dispersal capacity (Table 2; Bruzzone et al. 2009; Villacide et al. 2014; Villacide 2015), and spread rates have been found to be particularly influenced by temperature (i.e., higher spread rates in warmer climates; Lantschner et al. 2014).
Sirex noctilio population across South America, unlike what possibly happens in its native range, have been found consistently to show pulse-like eruptive population behavior, although there are some particular areas where outbreaks seem to be more sustained (e.g., Southern Argentina). The onset of S. noctilio outbreaks in South America appears to be triggered by density-independent factors, such as abrupt increase in the drought severity, which in inappropriately managed stands (e.g., overcrowded), increase the availability of susceptible host trees. Dispersal, on the other hand, may also have a significant effect in expanding outbreaks at more local scales, while the role of biological control in regulating populations is still unclear (see Fig. 3 for observed outbreak patterns and predisposing factors as affected by spatial scale). Long-term studies to understand the drivers of these population eruptions have been very scarce in South America (Corley et al. 2007; Aparicio et al. 2013; Lantschner and Corley 2015).
Classical biocontrol has been a key component of integrated pest management practices deployed against invasive S. noctilio populations in the southern hemisphere. In South America, three insect parasitoids and a parasitic nematode have been introduced in all affected countries. The historical data available about their releases suggests generally a ‘haphazard approach’, lacking quantitative follow-up surveys. Few if any quantitative recommendations or lessons may be drawn from the available data, to improve current and future biocontrol practices with parasitic insects in the region. For instance, we are unable to determine appropriate release strategies that consider the spatial and temporal organization of releases, and the optimal number of individuals to release. It is known that the number of individuals released serves to overcome demographic stochasticity and the negative effects of inverse density-dependent processes (Grevstad 1999a; Fauvergue and Hopper 2009) and the number of release events helps to reduce the influence of environmental stochasticity (Grevstad 1999b).
Given that bottom-up effects are important drivers of woodwasp population dynamics, management practices that contribute to tree vigor will help prevent outbreaks. Thus, efforts aimed at reducing the availability of susceptible host trees, such as silviculture and landscape design, should be prioritized, especially during years in which a rapid increase in drought severity is expected. Alternative proposed control measures, such as breeding programs to select varieties of Pinus spp. resistant to S. noctilio attacks, will also help (Slippers et al. 2015).
Early detection is a key factor contributing to the success of non-native pest management programs (Liebhold and Tobin 2008). For forest insects in general, the use of monitoring methods that employ semiochemicals has increased in the last decades, becoming a key component of many Integrates pest management (IPM) programs (Nadel et al. 2012). In South America, kairomone traps currently available for S. noctilio have a limited availability to detect small populations, and for this reason early detection still in many cases still rely on labor-intensive survey types such as visual surveys or trap trees.
The knowledge acquired on the ecology and management for the Sirex woodwasp in South America identifies five specific research areas for further work on this forest pest worldwide:
-
1.
The establishment of effective and standardized sampling protocols that allow for the collection of quantitative S. noctilio population data, including early detection in sites with high risk of initial introductions such as ports and urban areas. These include efficient trap and lure designs and statistically valid sampling efforts, which should provide data for populations during endemic phases and in diverse environmental conditions. Such protocols are needed to replace current population size estimates based on tree damage or designed for other purposes such as the implementation of control measures (i.e., trap trees, sequential sampling protocols).
-
2.
The collection of cross-border comparative data on woodwasp phenology, distribution and damage levels. This information is important to establish common management practices and to map pest incidence. This is also baseline information needed to interpret the success of the control practices deployed.
-
3.
Long-term studies of focal populations and field experiments based on tree/stand droughting for a better understanding of S. noctilio population dynamics in the invaded range, especially focused on outbreak behavior. Population studies developed to date where a set of predictive variables are analyzed against observed past population eruptions, and no mechanisms are put to test. Moreover, much of this research has been carried out in one region, in the presence of biocontrol practices, and there is little information for other regions of South America or for other countries in which S. noctilio is present.
-
4.
Spatio-temporal and life-table studies of host-parasitoid and host–pathogen interactions of S. noctilio in its natural range, with the aim of completing past work (e.g., Taylor 1978) to fully understand the role played by the natural enemies in woodwasp population regulation. Such studies, enriched by modern niche modeling techniques and genetic tools, could also allow for the identification of new, better climatically matched or more efficient biological control agents.
-
5.
Treatment–control studies on the efficacy of given IPM methods in preventing populations outbreaks, slowing geographical spread or even driving local populations to extinction. These should include comparisons of different silvicultural regimes (timing and intensity of pruning and thinning), and comparisons of methods to release biocontrol agents (timing, number and spatial distribution of releases, number of individuals).
Many of the ideas listed above, may be generally relevant for the management of invasive alien species in plantation forestry with non-native tree species.
Author contributions
JC conceived and wrote the manuscript. All other authors discussed ideas and wrote, read and approved the manuscript. VL and JV prepared the figures.
References
Aparicio JP, Corley JC, Rabinovich JE (2013) Life history traits of Sirex noctilio F. (Hymenoptera: Siricidae) can explain outbreaks independently of environmental factors. Math Biosci Eng 10:1265–1279
Bashford R (2008) The development of static trapping systems to monitor for wood-boring insects in forestry plantations. Aust For 71:236–241
Bashford R, Madden JL (2012) The use of kairomone lures for the detection of Sirex noctilio in susceptible Pinus radiata plantations in Australia. In: Slippers B, de Groot P, Wingfield MJ (eds) The Sirex Woodwasp and its fungal symbiont. Springer, Dordrecht, pp 159–166
Bedding RA, Iede E (2005) Application of Beddingia siricidicola for Sirex woodwasp control. In: Grewal PS, Ehlers RU, Shapiro-Ilan DI (eds) Nematodes as biocontrol agents. CABI Publishing, Wallingford, pp 385–399
Beèche M (2012) Sirex noctilio (Hym.: Siricidae): situación y marco regulatorio en Chile. http://www.corma.cl/_file/seminarios/documento/silvoteca-2012.pdf. Accessed 20 Dec 2017
Beèche M, Lanfranco D, Zapata M, Ruiz C (2012) Surveillance and control of the Sirex Woodwasp: the Chilean experience. In: Slippers B, de Groot P, Wingfield MJ (eds) The Sirex Woodwasp and its fungal symbiont. Springer, Dordrecht, pp 229–245
Berryman AA (1987) The theory and classification of outbreaks. In: Barbosa P, Schults J (eds) Insect outbreaks. Academic Press, Cambridge, pp 3–30
Boissin E, Hurley B, Wingfield MJ et al (2012) Retracing the routes of introduction of invasive species: the case of the Sirex noctilio woodwasp. Mol Ecol 21:5728–5744
Bordeaux JM, Dean JF (2012) Susceptibility and response of pines to Sirex noctilio. In: Slippers B, de Groot P, Wingfield MJ (eds) The Sirex Woodwasp and its fungal symbiont. Springer, Dordrecht, pp 31–50
Böröczky K, Crook DJ, Jones TH, Kenny JC, Zylstra KE, Mastro VC, Tumlinson JH (2009) Monoalkenes as contact sex pheromone components of the woodwasp Sirex noctilio. J Chem Ecol 35(10):1202–1211
Botto (2008) Plagas forestales introducidas en la argentina. Análisis de su situación actual. Disertation, Seminario Internacional de Plagas Forestales Cuarentenarias, Guyra-Paraguay
Brockerhoff EG, Liebhold AM (2017) Ecology of forest insect invasions. Biol Invasions 19:3141–3159
Brockerhoff EG, Jactel H, Parrotta JA, Quine CP, Sayer J (2008) Plantation forests and biodiversity: oxymoron or opportunity? Biodivers Conserv 17(5):925–951
Bruzzone OA, Villacide JM, Bernstein C, Corley JC (2009) Flight polymorphism in the woodwasp Sirex noctilio (Hymenoptera: Siricidae): an analysis of tethered flight data using wavelets. J Exp Biol 212:731–737
Caetano I, Hajek AE (2017) Mating behavior and sexual receptivity of Sirex noctilio (Hymenoptera: Siricidae). Ann Entomol Soc Am 110(3):276–280
Cameron EA (2012) Parasitoids in the management of Sirex noctilio: looking back and looking ahead. In: Slippers B, de Groot P, Wingfield MJ (eds) The Sirex Woodwasp and its fungal symbiont. Springer, Dordrecht, pp 103–117
Carnegie AJ, Elderidge RH, Waterson DG (2005) History and management of Sirex wood wasp in pine plantations in New South Wales, Australia. N Z J For Sci 35:3–24
Carnegie AJ, Matsuki M, Haugen DA et al (2006) Predicting the potential distribution of Sirex noctilio (Hymenoptera: Siricidae), a significant exotic pest of Pinus plantations. Ann For Sci 63:119–128
Carvalho AG, Pedrosa-Macedo JH, Santos HR (1993) Bioecologia de Sirex noctilio F. 1793 (Hymenoptera: Siricidae) em povoamentos de Pinus taeda L. In: Conferência Regional da Vespa-da-Madeira, Sirex noctilio, na América do Sul, Florianópolis, 1992. Anais. Embrapa-CNPF/FAO-ONU/USDA—Forest Service, Colombo
Collett NG, Elms S (2009) The control of Sirex wood wasp using biological control agents in Victoria, Australia. Agric For Entomol 11(3):283–294
Cooperband MF, Boroczky K, Hartness A, Jones TH, Zylstra KE, Tumlinson JH, Mastro VC (2012) Male-produced pheromone in the European woodwasp, Sirex noctilio. J Chem Ecol 38(1):52–62
Corley JC, Bruzzone OA (2009) Delayed emergence and the success of parasitoids in biological control. Biol Control 51(3):471–474
Corley JC, Villacide JM (2012) Population dynamics of Sirex noctilio: influence of diapause, spatial aggregation and flight potential on outbreaks and spread. In: Slippers B, de Groot P, Wingfield MJ (eds) The Sirex Woodwasp and its fungal symbiont. Springer, Dordrecht, pp 51–64
Corley JC, Villacide JM (2014) Sistema regional de sanidad forestal en los países del cono sur y Bolivia. Componente 3: plataforma tecnológica de detección y alerta temprana. Final report
Corley JC, Villacide JM, Bruzzone OA (2007) Spatial dynamics of a Sirex noctilio woodwasp population within a pine plantation in Patagonia, Argentina. Entomol Exp Appl 125(3):231–236
Corley JC, Villacide JM, Liebhold AM (2014) Can entomophagous nematodes slow the spread of invasive pest populations? The case study of Beddingia siricidicola released for the management of Sirex noctilio. J Pest Sci 84(4):551–557
COSAVE (2002) Lineamientos vigilancia de Sirex noctilio F. http://www.cosave.org. Accessed 1 Nov 2017
Crook DJ, Kerr LM, Mastro VC (2008) Sensilla on the antennal flagellum of Sirex noctilio (Hymenoptera: Siricidae). Ann Entomol Soc Am 101(6):1094–1102
Eskiviski E, Nuñez Cresto M, Olmedo D, de Coll O (2004) Biological aspects of Sirex noctilio F. and Ibalia leucospoides H. parasitism in forest plantations of Pinus sp. in Santo Tome, Corrientes. Report: Jornadas Técnicas Forestales y Ambientales - FCF, UNaM - EEA Monetecarlo
Evans AM (2016) The speed of invasion: rates of spread for thirteen exotic forest insects and diseases. Forests 7(5):99. https://doi.org/10.3390/f7050099
FAO (2008) Informe de la Reunión de Expertos para el establecimiento de la “Red de los Países del Cono Sur sobre Especies Forestales Invasoras”
FAO (2015) Global forest resources assessment 2015: how have the world’s forests changed? Rome
Fauvergue X, Hopper KR (2009) French wasps in the new world: experimental biological control introductions reveal a demographic Allee effect. Popul Ecol 51:385–397
Fernández Ajó A, Martínez AS, Villacide JM, Corley JC (2015) Behavioural response of the woodwasp Sirex noctilio to volatile emissions of its fungal symbiont. J Appl Entomol 139(9):654–659
Fischbein D, Corley JC (2015) Classical biological control of an invasive forest pest: a world perspective of the management of Sirex noctilio using the parasitoid Ibalia leucospoides (Hymenoptera: Ibaliidae). Bull Entomol Res 105(1):1–12
Fischbein D, Corley JC, Villacide JM, Bernstein C (2011) The influence of food and con-specifics on the flight potential of the parasitoid Ibalia leucospoides. J Insect Behav 24:456–467
Fischbein D, Bettinelli J, Bernstein C, Corley JC (2012) Patch choice from a distance and use of habitat information during foraging by the parasitoid Ibalia leucospoides. Ecol Entomol 37:161–168
Fischbein D, Villacide JM, De la Vega G, Corley JC (2018) Sex, life history and morphology drive individual variation in flight performance of an insect parasitoid. Ecol Entomol 43:60–68
Gilbert JM, Miller LW (1952) An outbreak of Sirex noctilio F. in Tasmania. Aust For 16:63–69
Grevstad FS (1999a) Experimental invasions using biological control introductions: the influence of release size on the chance of population establishment. Biol Invasions 1(4):313–323
Grevstad FS (1999b) Factors influencing the chance of population establishment: implications for release strategies in biocontrol. Ecol Appl 9(4):1439–1447
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hurley BP, Slippers B, Croft PK, Hatting HJ, van der Linde M, Morris AR, Dyer C, Wingfield MJ (2008) Factors influencing parasitism of Sirex noctilio (Hymenoptera: Siricidae) by the nematode Deladenus siricidicola (Nematoda: Neotylenchidae) in summer rainfall areas of South Africa. Biol Control 45(3):450–459
Hurley BP, Garnas J, Cooperband MF (2015) Assessing trap and lure effectiveness for the monitoring of Sirex noctilio. Agric For Entomol 17(1):64–70
Iede E, Zanetti R (2007) Ocorrência e recomendações para o manejo de Sirex noctilio Fabricius (Hymenoptera, Siricidae) em plantios de Pinus patula (Pinaceae) em Minas Gerais, Brasil. Rev Bras Entomol 51:529–531
Iede ET, Penteado SR, Bisol JL (1988) Primeiro registro de ataque de Sirex noctilio em Pinus taeda no Brasil. EMBRAPA-CNPF, Circular tecnica 20:1–12
Iede E, Penteado SRC, Schaitza EG (1998) Sirex noctilio problem in Brazil: detection, evaluation, and control. In: Iede E, Schaitza EG, Penteado S et al. (eds) Proceedings of a conference: training in the control of Sirex noctilio by use of natural enemies. Colombo, pp 45–52
Iede E, Penteado SC, Filho W (2012) The Woodwasp Sirex noctilio in Brazil: monitoring and control. In: Slippers B, de Groot P, Wingfield MJ (eds) The Sirex Woodwasp and its fungal symbiont: research and management of a worldwide invasive pest. Springer, Dordrecht, pp 217–228
Ireland KB, Bulman L, Hoskins AJ et al (2018) Estimating the potential geographical range of Sirex noctilio: comparison with an existing model and relationship with field severity. Biol Invasions. https://doi.org/10.1007/s10530-018-1721-4
Jofré N, Pildain MB, Cirigliano AM, Cabrera GM, Martínez AS, Corley JC (2016) Host selection by Ibalia leucospoides based on temporal variations of volatiles from the hosts’ fungal symbiont. J Appl Entomol 140(10):736–743
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17(4):164–170
Klasmer P, Botto E (2012) The ecology and biological control of the woodwasp Sirex noctilio in Patagonia, Argentina. In: Slippers B, de Groot P, Wingfield MJ (eds) The Sirex Woodwasp and its fungal symbiont: research and management of a worldwide invasive pest. Springer, Dordrecht, pp 203–215
Lantschner MV, Corley JC (2015) Spatial pattern of attacks of the invasive woodwasp Sirex noctilio, at landscape and stand scales. PLoS One 10:e0127099. https://doi.org/10.1371/journal.pone.0127099
Lantschner MV, Villacide JM, Garnas JR, Croft P, Carnegie AJ, Liebhold AM, Corley JC (2014) Temperature explains variable spread rates of the invasive woodwasp Sirex noctilio in the Southern Hemisphere. Biol Invasions 16:329–339
Lantschner MV, Aukema B, Corley JC (under review) Droughts drive outbreak dynamics of an invasive forest insect on an exotic host. Forest Ecol Manag
Liebhold AM, Tobin PC (2008) Population ecology of insect invasions and their management. Annu Rev Entomol 53:387–408
Lopez A, Demaestri M, Zupan E, Barotto O (2002) Antecedentes del Sirex noctilio (Hymenoptera: Siricidae) en el Valle de Calamuchita, Córdoba, Argentina. Bosque 23(1):111–114
Madden JL (1988) Sirex in Australasia. In: Berryman AA (ed) Dynamics of forest insect populations. Plenum Press, New York, pp 407–429
Madden JL, Coutts MP (1979) The role of fungi in the biology and ecology of woodwasps (Hymenoptera: Siricidae). In: Insect fungus symbiosis, pp 165–174
Madden JL, Irvine CJ (1971) The use of lure trees for the detection of Sirex noctilio in the field. Aust For 35:164–166
Maderni JFP (1998) Sirex noctilio present status in Uruguay. In: Iede E, Shaitza E, Penteado S et al (eds) Training in the control of Sirex noctilio by use of natural enemies. USDA Forest Services, Morgantown, pp 81–82
Martinez AS, Fernandez-Arhex V, Corley JC (2006) Chemical information from the fungus Amylostereum areolatum and host-foraging behaviour in the parasitoid Ibalia leucospoides. Physiol Entomol 31(4):336–340
Miller D, Clark AF (1935) Sirex noctilio (Hym.) and its parasite in New Zealand. Bull Entomol Res 26:149–154
Mlonyeni XO, Wingfield BD, Wingfield MJ, Ahumada R, Klasmer P, Leal I, De Groot P, Slippers B (2011) Extreme homozygosity in Southern Hemisphere populations of Deladenus siricidicola, a biological control agent of Sirex noctilio. Biol Control 59:348–353
Morgan FD, Stewart NC (1966) The biology and behavior of the woodwasp Sirex noctilio F. in New Zealand. Trans R Soc N Z Zool 7:195–204
Nadel RL, Wingfield MJ, Scholes MC, Lawson SA, Slippers B (2012) The potential for monitoring and control of insect pests in Southern Hemisphere forestry plantations using semiochemicals. Ann For Sci 69:757–767
Payn T, Kollert W, Liu S, Rodriguez L, Neves Silva L, Wingfield M, Michel-Carnus J, Orazio C, Freer-Smith P (2015) Changes in planted forests and future global implications. For Ecol Manag 352:57–67
Poisson MA, Ahumada R, Angulo AO, Muñoz F, Sanfuentes E (2016) Mega-trap-plots: a novel method of Sirex woodwasp management on Pinus radiata plantations in Chile. South For 78:289–297
Rodigheri HR, Iede ET, Penteado S, Reis Filho W (2006) Avaliação dos impactos do programa de manejo integrado de pragas para o controle da vespa-da-madeira em plantios de Pinus no Sul do Brasil. Embrapa Florestas-Comunicado Técnico (INFOTECA-E). https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/307317/1/comtec158.pdf. Accessed 25 Jan 2017
Rojas E, Beèche M (2010) Detección y control de Sirex noctilio F. en Chile (Hymenoptera: Siricidae). In: Lanfranco D, Ruiz C (eds) Entomología forestal en Chile. Universidad Austral de Chile, Valdivia, pp 303–323
Ruiz C (2006) Razón sexual de Sirex noctilio Fabr. y detección de sus potenciales enemigos mediante el estudio de parcelas cebo implementadas por el Servicio Agrícola y Ganadero entre los años 2002–2003 en la X Región de Chile. Tesis Licenciado en Ciencias Biológicas, Facultad de Ciencias. Universidad Austral de Chile, Valdivia
SAG (2004) Informe anual del Sub-departamento de Vigilancia y Control de Plagas Forestales y Exóticas Invasoras, año 2004. http://www.sag.cl. Accessed 25 Jan 2017
SAG (2017) Áreas bajo cuarentena de Sirex noctilio calificadas según riesgo de dispersión en madera hospedante. Programa de control oficial Sirex noctilio en Chile. http://historico.sag.gob.cl/common/asp/pagAtachadorVisualizador.asp. Accessed 1 Dec 2017
Simpson RF (1976) Bioassay of pine oil components as attractants for Sirex noctilio (Hymenoptera: Siricidae) using electroantennogram techniques. Entomol Exp Appl 19:11–18
Simpson RF, Mc Quilkin RM (1976) Identification of volatiles from felled Pinus radiata and the electroantennograms they elicit from Sirex noctilio. Entomol Exp Appl 19:205–213
Slippers B, de Groot P, Wingfield MJ (2012) The Sirex woodwasp and its fungal symbiont: research and management of a worldwide invasive pest. Springer, Dordrecht
Slippers B, Hurley BP, Wingfield MJ (2015) Sirex Woodwasp: a model for evolving management paradigms of invasive forest pests. Ann Rev Entomol 60:601–619
Spradbery JP (1970) Host finding by Rhyssa persuasoria (L.), an ichneumonid parasite of siricid woodwasps. Anim Behav 18:103–114
Spradbery JP, Kirk AA (1978) Aspects of the ecology of siricid woodwasps (Hymenoptera: Siricidae) in Europe, North Africa and Turkey with special reference to the biological control of Sirex noctilio F. in Australia. Bull Entomol Res 68:341–359
Taylor KL (1978) Evaluation of the insect parasitoids of Sirex noctilio (Hymenoptera: Siricidae) in Tasmania. Oecologica 32:1–10
Tribe GD (1995) The woodwasp Sirex noctilio Fabricius (Hymenoptera: Siricidae), a pest of Pinus species, now established in South Africa. Afr Entomol 3:215–217
van Lierop P, Lindquist E, Sathyapala S, Franceschini G (2015) Global forest area disturbance from fire, insect pests, diseases and severe weather events. For Ecol Manag 352:78–88
Villacide JM (2015) Ecología de la dispersión de Sirex noctilio: implicancias sobre el éxito de invasión de una avispa plaga. Tesis de licenciatura, Universidad Nacional del Comahue, Río Negro Argentina
Villacide JM, Corley JC (2008) Parasitism and dispersal potential of Sirex noctilio: implications for biological control. Agric For Entomol 10(4):341–345
Villacide JM, Corley JC (2012) Ecology of the woodwasp Sirex noctilio: tackling the challenge of successful pest management. Int J Pest Manag 58:249–256
Villacide JM, Masciocchi M, Corley JC (2014) Avispas exóticas en la Patagonia: la importancia de la ecología de invasiones en el manejo de plagas. Ecol Austral 24(2):154–161
Walther GR, Roques A, Hulme PE, Sykes MT et al (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24(12):686–693
Wingfield MJ, Brockerhoff EG, Wingfield BD, Slippers B (2015) Planted forest health: the need for a global strategy. Science 349(6250):832–836
Witzgall P, Kirsch P, Cork A (2010) Sex pheromones and their impact on pest management. J Chem Ecol 36:80–100
Acknowledgements
This work was partially supported by Grants: PICT 2013-557, PICT-2014-419, PICT 2014-527, PICT StarUp-2015-3864 (Agencia Nacional para la Promoción Científica y Tecnológica, Argentina) INTA PNFOR-1104072 (Instituto Nacional de Tecnología Agropecuaria, Argentina) and IcoS, FS- USDA International Programmes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by N. Meurisse.
Special Issue on Invasive Pests of Forests and Urban Trees.
Rights and permissions
About this article
Cite this article
Corley, J.C., Lantschner, M.V., Martínez, A.S. et al. Management of Sirex noctilio populations in exotic pine plantations: critical issues explaining invasion success and damage levels in South America. J Pest Sci 92, 131–142 (2019). https://doi.org/10.1007/s10340-018-1060-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-018-1060-3