Abstract
The white grub Holotrichia oblita Faldermann (Coleoptera: Scarabaeidae) causes serious damage in peanut fields in China. The development of an environmentally friendly control method for this pest is urgently needed. The efficacy of entomopathogenic nematodes against this pest in the laboratory and peanut fields was evaluated in this study. Both Steinernema longicaudum Shen and Wang and Heterorhabditis bacteriophora Poinar (Rhabditida: Steinernematidae and Heterorhabditidae) had promising control efficacy against H. oblita larvae in the laboratory. S. longicaudum X-7 and H. bacteriophora H06 at 1.0 × 104 and 5.0 × 103 IJs per plant caused a similar percentage reduction of the grub larvae and percentage of the injured legumes to chlorpyrifos. The peanut yields from the nematode-treated plots at 5.0 × 103 IJs per plant were at least 55 and 15 % higher than those from the water control and the chlorpyrifos-treated plots. No significant differences were found in the percentage reductions of the grub larvae at different larval stage applications. The peanut yields in plots treated by S. longicaudum X-7 and H. bacteriophora H06 were also not significantly different. The cost-benefit analysis showed that S. longicaudum X-7 and H. bacteriophora H06 are promising agents for H. oblita larvae control in peanut fields. Our findings indicate that entomopathogenic nematodes have good potential for safe management of H. oblita in peanut production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Key message
-
White grubs Holotrichia oblita cause serious damage to peanuts in China
-
Control of H. oblita in China needs environmentally friendly control methods
-
Steinernema longicaudum and Heterorhabditis bacteriophora had promising control efficacy against H. oblita larvae
-
Peanut yields were higher with nematode treatment than water and chlorpyrifos treatment
Introduction
White grubs (Coleoptera: Scarabaeidae) are root-feeding larvae of scarab beetles. They are serious soil-borne pests of many agricultural and horticultural plants worldwide (Koppenhöfer and Fuzy 2008; Liu et al. 2009a; Guo et al. 2013). White grubs have a wide distribution in China, with the greatest population among soil-borne pests and causing the most serious damage (Li et al. 2008). Holotrichia oblita Faldermann is one of the dominant species in China, causing damage to wheat, peanuts, potato, soybean and other economically important plants (Zhang et al. 2006). The control of H. oblita in China using environmentally friendly control methods is urgently needed with more attention paid to environmental and human safety (Gaugler 1998; Choo et al. 2002; Liu et al. 2009b).
Holotrichia oblita appears in one generation every 2 years, and adults or last instar larvae spend the winter in peanut fields in North China (Wang 2004). H. oblita outbreaks are consistent with the growth of peanuts, which leads to serious damage to peanut production (Wang 2004). When peanuts grow to the flowering period (usually from late June to early July), H. oblita occur as young larvae. Then, when peanuts are in the fruit period (usually in early August), the larvae grow to the third-instar stage and feed on the fresh peanut legumes. From middle August, the third-instar larvae eat more, leading to easier infection by other soil pathogens and the decay of the injured legumes, eventually causing the death of the whole plants (Wang and Wang 2006). The reduction in peanut yield caused by white grubs is estimated to be 20–30 %. If there is no effective control, the losses are even over 50 % (Liu et al. 2009b).
Entomopathogenic nematodes (EPNs) of the genera Steinernema and Heterorhabditis (Rhabditida: Steinernematidae and Heterorhabditidae) are potential agents for the control of soil-dwelling pests (Toepfer et al. 2010) because of their ability to actively search for their hosts (Grewal et al. 2005; Georgis et al. 2006; Yan et al. 2012) and to be easily mass-produced (Ehlers 2001; Grewal 2002; Mukuka et al. 2010). In previous studies, EPN species such as S. scarabaei Stock & Koppenhöfer, H. zealandica Poinar, H. bacteriophora Poinar, Rhabditis spp., S. longicaudum Shen and Wang, and S. lanmjungense Khatri-Chhetri, Waeyenberge, Spiridonov, Manandhar & Moens (Li et al. 1993; Koppenhöfer et al. 2000a, 2002; Koppenhöfer and Fuzy 2003a, b; Grewal et al. 2004; Li et al. 2007; Du et al. 2009; Khatri-Chhetri et al. 2011) were found to have potential control effects for different species of white grub. Previous research found that H. bacteriophora H06 and S. longicaudum X-7 are effective against H. parallela Motschulsky in north China (Guo et al. 2013), showing promising effects when using EPN for white grub control. H. oblita caused similar damage to peanut production as H. parallela, but with different life cycles. Usually one peanut field is infested with one species of white grub at one time, but sometimes different species of white grub co-occur (Li et al. 2003). To effectively protect peanut production, it is necessary to screen suitable EPN isolates for controlling H. oblita.
In order to select more effective nematode isolates for the biological control of H. oblita in peanut fields, the efficacy and application timing of S. longicaudum X-7, H. bacteriophora H06 and S. carpocapsae All for the control of H. oblita in the laboratory and peanut fields were determined in the present study.
Materials and methods
Nematodes
Steinernema longicaudum X-7, H. bacteriophora H06 and S. carpocapsae All used in this study were taken from the collection of Guangdong Entomological Institute, Guangzhou, China. These isolates were identified by morphological and molecular methods (Yan et al. 2010) and cultured in in vitro solid sponge media according to the method of Bedding (1981) with modifications (Han 1996). The infective juveniles (IJs) were harvested and stored at 15 °C for no more than 1 week before use in the experiments. Prior to the experiment, the IJs were checked under a microscope and considered alive when actively moving or showing a response after probing with a needle. The IJ suspensions could be used for the experiments when more than 95 % of living IJs were present (Guo et al. 2013; Yan et al. 2013).
Insects
The second-instar larvae of H. oblita were provided by Cangzhou Academy of Agriculture and Forestry Science, China. The size and weight of each instar larvae of H. oblita are listed in Table 1. The white grubs were reared and fed on dry potato pieces (0.5 × 0.5 × 0.5 cm). The white grubs were individually placed in plastic cups (4.3 cm diameter, 7 cm height, with a 2-mm-diameter hole in the lid) filled with 50 g of sandy loam soil collected from the experimental field (78 % sand, 14 % silt, 5 % clay, 0.4 % organic matter; pH 6.8; 15 %, w/w, soil moisture). Six wheat seeds were added to each cup as food for the white grubs. The cups were kept at 25 °C for 24 h, and only actively feeding grubs were selected for laboratory experiments (Koppenhöfer and Fuzy 2008).
Yellow mealworms, Tenebrio molitor L., were purchased from the Shandong Taian Wuma market. The mealworms were reared at 25 ± 2 °C and 50 % relative humidity (RH) and fed on wheat bran and fresh vegetable leaves. Similarly sized 9th- to 11th-instar larvae were chosen to evaluate nematode persistence in the soil by burying the larvae in the field during field experiments (Yan et al. 2013).
Laboratory experiment
One ml of IJ suspensions containing 200, 100 and 50 IJs of H. bacteriophora H06, S. longicaudum X-7 or S. carpocapsae All was applied in each cup (equal to 1.5 × 109, 7.5 × 108 or 3.8 × 108 IJs ha−1), respectively. Chlorpyrifos (EC 48 %, Shandong United Pesticide Industry Co., Ltd., Jinan, China) was used as a positive control at a rate of 2.160 kg ha−1 (0.0003 g per larvae). Water without nematodes or insecticides was used as a negative control. A similar volume was used for the chlorpyrifos and water control. Each replicate contained 15 individual larvae, and 3 replicates were established for each treatment. The cups were placed in the dark at 25 °C. Grub mortality was recorded after 4, 7 and 14 days, and the cadavers were placed on moist filter paper and dissected 3 days later to estimate IJ invasion (Yan et al. 2013).
Field experiments
Two experiments were conducted in peanut fields naturally infested with H. oblita at two different locations in Taian, China. Before the experiment, the natural EPN population was detected by baiting soil samples with yellow mealworms as described by Liu et al. (2009a). Briefly, 20 soil samples (20 cm long and wide and 10 cm deep) were collected from the experimental field. Each sample was transferred to a box (20 × 20 cm × 10 cm) with four holes (diameter = 2 cm) in the cover. Ten 9th- to 11th-instar larvae of Tenebrio were buried in the soil. The boxes were stored at 25 °C in the dark, and the mortality of Tenebrio larvae was examined 72 h later. No EPN populations were recovered from the experimental fields. The larval population of the white grub was estimated as described by Du et al. (2009) and Liu et al. (2007). Thirty peanut plants were randomly selected, and the plants together with the soil around the roots (diameter = 20 cm, depth = 10 cm) were taken out. The number of white grub larvae was determined, and the density of the white grub larvae was calculated. The experimental plot was approximately 15 m2 (10 m long and 1.5 m wide) with a 0.75-m buffer space between plots. Each plot contained 200 “Large Baisha” peanut plants with 20 cm space between the plants within a row.
The first experiment was conducted in Zhaozhuang on 2 August 2012 (sandy soil with water content of 14.76 ± 0.38 %; applications carried out at 4:30 p.m.; cloudy, air temperature 29 °C, soil temperature 26 °C at −5 cm) when the peanut plants were 90 days old and 25–35 cm in height. The population density of white grubs was 13.17 ± 1.56 larvae per m2, and the larvae were mainly in the second instar at the time of the experiment. Steinernema longicaudum X-7, H. bacteriophora H06 and S. carpocapsae All were applied at 1.0 × 104, 5.0 × 103 and 2.5 × 103 IJs per plant (equal to 1.5 × 109, 7.5 × 108 or 3.8 × 108 IJs ha−1). Chlorpyrifos was used as a positive control at a rate of 2.160 kg ha−1, and water without nematodes or insecticides was used as a negative control. During the experimental period, the temperature in the plots at a −5 cm soil depth ranged from 18 to 26 °C.
The second experiment was conducted in Fanzhen to examine the efficacy of S. longicaudum X-7 and H. bacteriophora H06 against H. oblita at different application times in the same field. The first application time was on 15 July 2013 (sandy soil with water content of 16.16 ± 0.16 %; applications done at 2:00 p.m.; light rain; air temperature 25 °C; soil temperature 22 °C at −5 cm), when the H. oblita larvae were mainly in the first-instar stage (first-instar larval application). At the first-instar larval application time, the peanut plants were 73 days old and 15–25 cm in height. The population density of H. oblita was about 8.92 ± 1.85 larvae per m2. The second application time was on 5 August 2013 (sandy soil with water content of 18.42 ± 0.33 %; applications done at 4:00 p.m.; sunny; air temperature 31 °C; soil temperature 26 °C at −5 cm) when the H. oblita larvae were mainly in the second-instar stage (second-instar larval application). At the second-instar larval application time, the peanut plants were 93 days old and 25–35 cm in height. The population density of H. oblita was about 11.90 ± 2.11 larvae per m2. Different experimental plots were used for the two application times. At both application times, S. longicaudum X-7 and H. bacteriophora H06 were applied at 5.0 × 103 IJs per plant (equal to 7.5 × 108 IJs ha−1). Chlorpyrifos and water were used as controls as described above. During the experimental period, the temperature in the plots at −5 cm soil depth ranged from 17 to 27 °C.
In both experiments, 50 ml IJ suspensions in a measuring spoon containing different concentrations of IJs were applied to the soil around each peanut root. A similar volume was used for chlorpyrifos treatment and water control. No additional irrigation or other insecticides were supplied. There were five replicates (plots) for each treatment, and all the plots were arranged in a randomized complete block design.
Populations of the grub larvae were monitored 7, 14, 21 and 41 (harvesting day) days after the application in the first experiment and after the second-instar larval application time in the second experiment. The number of injured legumes and the fresh peanut yield from each plot were recorded immediately on harvesting day. In each plot for each sample time, 16 peanut plants were randomly taken.
In order to assess nematode persistence in the soil, the mortality of yellow mealworm larvae buried in the field was evaluated 7, 14, 28, 42, 56, and 70 days after EPN application in the first experiment and after the second-instar larval application time in the second experiment. Ten larvae individually caged in wire meshes (2 cm × 2 cm) were buried 5 cm deep in the soil in each plot. Four days later, they were retrieved and taken back to the laboratory, and the live and dead T. molitor larvae were counted. Dead larvae were incubated in clean petri dishes with moist filter paper and dissected 3 days later to estimate IJ invasion (Yan et al. 2013).
Statistical analysis
Corrected mortality of H. oblita in the laboratory experiment was calculated for each treatment using the following equation: M A (%) = (P t − P c )/(1 − P c), where M A is the corrected mortality of the white grub; P t and P c indicate the mortality of larvae in the treatment and in the (water) control, respectively (Abbott 1925). P t (c) (%) = N d/N a × 100, where P t (c) is the percentage mortality of the white grub; N d and N a indicate the number of dead larvae and the number of all larvae, respectively. Percentage reduction of the white grub larvae was calculated for each treatment using the following equation: R A (%) = (A c − A t)/A c × 100, where R A is the percentage reduction of the white grub in the treatment; A c and A t indicate the number of larvae in the water control and the treatment, respectively. The percentage of the injured legumes was calculated using the following equation: P w (%) = W i/W a × 100, where P w is the percentage of the injured legume; W i and W a indicate the number of injured legumes and the number of all peanut legumes, respectively (Liu et al. 2007; Guo et al. 2013).
The cost and benefit of the white grub control in peanut fields were estimated based on an IJ application rate of 7.5 × 108 IJ ha−1 (5 × 103 IJs per plant) in the first experiment in Zhaozhuang. Usually, there were 1.5 × 105 plants ha−1. Average peanut yield was calculated as kg ha−1. The cost for the treatments was estimated based on the retail prices of the nematodes and the chlorpyrifos in September 2012. Net profit was estimated based only on the income from peanuts (USD 1.68/kg on 23 September 2012) and the cost per hectare from different treatments (Guo et al. 2013).
M A, R A, P w and mortality rates of the yellow mealworm larvae were arcsine transformed before analysis by one-way ANOVA, followed by Tukey’s multiple means comparison procedure at P = 0.05 (SPSS 16.0, SPSS Inc., Chicago, IL, USA).
Results
Laboratory efficacy of nematodes
Corrected mortality rates of H. oblita varied significantly among different treatments (F = 16.269, 36.933 and 27.184 for 4, 7 and 14 days after treatment, respectively; df = 9, 20; P < 0.001) (Fig. 1). Heterorhabditis bacteriophora H06 and S. longicaudum X-7 at 200 IJs per larva caused similar control effects on H. oblita to those of chlorpyrifos 4 and 7 days after treatment. Fourteen days after treatment, corrected mortalities of H. oblita caused by the above two isolates at 200 and 100 IJs per larva and the chlorpyrifos were all higher than 69 %. Corrected mortality rates of H. oblita caused by S. carpocapsae All were the lowest, ranging from 2.2 to 17.8 %, and were not significantly different among different days after treatment (F = 1.020, 4.423 and 2.915,df = 2, 6; P = 0.415, 0.066 and 0.130 for 4, 7 and 14 days after treatment, respectively).
Corrected mortality of third-instar larvae of Holotrichia oblita Faldermann treated with different species and concentrations of EPNs and chlorpyrifos in laboratory experiments evaluated at 4, 7 and 14 days after treatment. Sl: Steinernema longicaudum X-7; Hb: Heterorhabditis bacteriophora H06; Sc: S. carpocapsae All; 200 = 200 IJs per larva, 100 = 100 IJs per larva, 50 = 50 IJs per larva. Chlorpyrifos (Chl) was applied at 0.0003 g per larva (2.160 kg ha−1). Bars with different uppercase letter(s) indicate significant differences among the same treatment on different days after applications and with different lowercase letter(s) indicate significant differences among different treatments on the same day after application (P < 0.05, Tukey’s test)
Field experiments
Percentage reduction of H. oblita
Percentage reductions of H. oblita in EPN- and chlorpyrifos-treated plots at different sample times in both experiments are shown in Table 2.
In the first experiment, percentage reductions of H. oblita in plots treated with S. longicaudum X-7, H. bacteriophora H06 and chlorpyrifos were significantly higher than those in plots treated with S. carpocapsae All (F = 47.076, 62.498, 56.675 and 97.106 for 7, 14, 21, 28 days after application, respectively; df = 9, 40; P < 0.001). As the extension of sample time, S. longicaudum X-7 and H. bacteriophora H06 at 5.0 × 103 and 2.5 × 103 IJs per plant had increasingly high control efficacy of H. oblita (F = 9.883, 25.778, 17.852 and 35.957 for S. longicaudum X-7 at 5.0 × 103 and 2.5 × 103 IJs per plant, H. bacteriophora H06 at 5.0 × 103 and 2.5 × 103 IJs per plant, respectively; df = 3, 16; P ≤ 0.001), and on the contrary, chlorpyrifos had increasingly less control efficacy (F = 4.309; df = 3, 16; P = 0.021).
In the second experiment, S. longicaudum X-7 and H. bacteriophora H06 at 5.0 × 103 IJs per plant at the first-instar larval application time caused a significantly lower percentage reduction of the grub larvae than that at the second-instar larval application time (F = 10.907; df = 5, 24; P < 0.001) and chlorpyrifos at both application times at 7 days after application, but no significant differences were found at 14 days (F = 2.455; df = 5, 24; P = 0.062) and 21 days (F = 2.625; df = 5, 24; P = 0.05) after application. At 41 days after application, both nematode species caused >94 % percentage reduction of the grub larvae, which were significantly higher than the reduction rated of the grub larvae in plots treated with chlorpyrifos at the first-instar larval application time (F = 4.353; df = 5, 24; P = 0.006). As the extension of sample time, S. longicaudum X-7 and H. bacteriophora H06 at 5.0 × 103 IJs per plant at the first-instar larval application time and H. bacteriophora H06 at 5.0 × 103 IJs per plant at the second-instar larval application time caused increasingly high control efficacy of H. oblita (F = 17.136, 11.224 and 5.809, respectively; df = 3, 16; P ≤ 0.007). No differences were found for S. longicaudum X-7 at 5.0 × 103 IJs per plant and chlorpyrifos at the second-instar larval application time (F = 2.101; df = 3, 16; P = 0.140 and F = 3.226; df = 3, 16; P = 0.510) as time extended, while for chlorpyrifos at the first-instar larval application time, increasingly less control efficacy was found (F = 5.282; df = 3, 16; P = 0.010).
Nematode persistence
Mortality rates of T. molitor bait larvae were calculated. The three tested EPNs were able to persist in the soil 70 days after application in both experiments. Mortality rates of the T. molitor larvae ranged from 16.0 ± 2.4 to 42.0 ± 5.8 %, and the differences were not significant among treatments on different sample days (experiment 1, F ≤ 1.317; df = 8, 36; P ≥ 0.266; experiment 2, F ≤ 2.485; df = 3, 16; P ≥ 0.098).
Percentage of the injured legumes
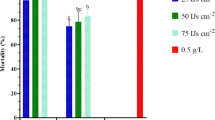
Percentages of the injured legumes on the harvesting day are shown in Fig. 2. In the first experiment, the percentages of the injured legumes in peanut plots treated with S. longicaudum X-7, H. bacteriophora H06 and chlorpyrifos were significantly lower than those in the plots treated with S. carpocapsae All and the water control (F = 88.437; df = 10, 44; P < 0.001). Heterorhabditis bacteriophora H06 at 1.0 × 104 IJs per plant caused the lowest percentage of the injured legumes of 1.67 ± 0.32 %. The percentage of the injured legumes in plots treated with S. longicaudum X-7 at three rates, H. bacteriophora H06 at 5.0 × 104 and 2.5 × 103 IJs per plant, and chlorpyrifos ranged from 2.33 ± 0.37 to 4.97 ± 0.39 %, while the percentages of the injured legumes in plots treated with S. carpocapsae All at different rates were similar to the water control, which ranged from 16.32 ± 1.57 to 20.45 ± 1.06 %.
Percentages of the injured legumes in peanut fields with different treatments 41 days after application in the first experiment (Zhaozhuang) and after the second-instar larval application time in the second experiment (Fanzhen) in Shandong, China. Sl: Steinernema longicaudum X-7; Hb: Heterorhabditis bacteriophora H06; Sc: S. carpocapsae All; 10 = 1.0 × 104 IJs per plant, 5 = 5.0 × 103 IJs per plant, 2.5 = 2.5 × 103 IJs per plant. Chlorpyrifos (Chl) was applied at 2.160 kg ha−1; 1 and 2 indicate the first-instar larval (15 July 2013) and the second-instar larval (5 August 2013) application time, respectively, when EPNs were applied at 5.0 × 103 IJs per plant and chlorpyrifos at 2.160 kg ha−1 in the second experiment. Bars with different letter(s) indicate significant differences among treatments in each experiment (P < 0.05, Tukey’s test)
In the second experiment, EPN and chlorpyrifos treatments significantly reduced the percentages of injured legumes compared to the water control (F = 105.560; df = 7, 32; P < 0.001). No significant differences were found among the EPN and chlorpyrifos treatment at both first- and second-instar larval application times, with the percentages of the injured legumes ranging from 2.82 ± 0.19 % to 6.41 ± 0.37 %.
Peanut yield
Fresh weights of peanuts in plots with different treatments 41 days after application are shown in Fig. 3. In the first experiment, the peanut yields (kilograms per sample) varied significantly among different treatments (F = 23.979; df = 10, 44; P < 0.001). In plots treated with S. longicaudum X-7 and H. bacteriophora H06 at the three rates and chlorpyrifos, the peanut yield ranged from 0.69 ± 0.02 to 0.85 ± 0.02 kg/16 plants. While in plots treated with S. carpocapsae All and water, the peanut yields were less than 0.60 kg/16 plants, which were significantly lower than those in plots with the above treatments. In the second experiment, the peanut yields in plots treated with EPN and chlorpyrifos were significantly higher than those in the water control plots, but were not significantly different among each other (F = 20.891; df = 7, 32; P < 0.001). Compared to the increased peanut yield rate in the chlorpyrifos-treated plots (34.6–47.9 %), an increased rate of 32.7–64.6 % of the peanut yield was obtained in the plots treated with S. longicaudum X-7 and H. bacteriophora H06 at different rates.
Fresh weight of peanuts in peanut fields with different treatments 41 days after application in the first experiment (Zhaozhuang) and after the second-instar larval application time in the second experiment (Fanzhen) in Shandong, China. Sl: Steinernema longicaudum X-7; Hb: Heterorhabditis bacteriophora H06; Sc: S. carpocapsae All; 10 = 1.0 × 104 IJs per plant, 5 = 5.0 × 103 IJs per plant, 2.5 = 2.5 × 103 IJs per plant. Chlorpyrifos (Chl) was applied at 2.160 kg ha−1; 1 and 2 indicate the first-instar larval (15 July 2013) and the second-instar larval (5 August 2013) application time, respectively, when EPNs were applied at 5.0 × 103 IJs per plant and chlorpyrifos at 2.160 kg ha−1 in the second experiment. Bars with different letter(s) indicate significant differences among treatments in each experiment (P < 0.05, Tukey’s test)
Cost estimation of the EPN application
The costs for using S. longicaudum X-7 and H. bacteriophora H06 at the rate of 7.5 × 108 IJ ha−1 to control the H. oblita in one hectare were USD 352.66 and 176.33, respectively, while the costs for using chlorpyrifos for the same area was USD 70.50. Peanut yields were 5,695.4, 5,837.3, 4,921.8 and 3,671.1 kg ha−1 and net profits were USD 9,215.61, 9,630.33, 8,198.12 and 6,168.46 ha−1 in fields treated with S. longicaudum X-7, H. bacteriophora H06, chlorpyrifos and water.
Discussion
Entomopathogenic nematodes have been used to control different species of white grubs but with various control efficacies, i.e., S. scarabaei (100 %), H. bacteriophora (34–97 %), H. bacteriophora (65–92 %) and H. zealandica (73–98 %), against Popillia japonica Newman (Koppenhöfer et al. 2000a, b, 2002; Koppenhöfer and Fuzy 2003a, b; Grewal et al. 2004). To be effective, choosing the appropriate nematode to be matched with the particular target pest is most important (Shapiro-Ilan et al. 2012). No previous studies have reported on the efficacy of EPNs against H. oblita. In the present study, both S. longicaudum X-7 and H. bacteriophora H06 showed promising control efficacy against the white grub H. oblita in the laboratory and peanut fields. In the field experiment conducted in 2012, the peanut yields harvested from the plots treated with S. longicaudum X-7 and H. bacteriophora H06 at high application rates (≥5.0 × 103 IJs per plant) were at least 55 % higher than those from the water control, which was similar to their control efficacy against the white grub H. parallela in peanut fields (Guo et al. 2013). However, the peanut yield rate increased in the plots treated with the two EPNs at high application rates (≥5.0 × 103 IJs per plant), which were 15.71–21.43 % higher than those in the plots treated with chemical insecticide (chlorpyrifos), which were less than those in the former study (Guo et al. 2013). This was possibly due to the lower density of the white grub larvae in our study.
Steinernema carpocapsae has been reported to perform well against some white grub species (Forschler and Gardner 1991). However, the mortality of H. oblita treated with S. carpocapsae All was <20 % in the laboratory, and the control efficacy against H. oblita was also not satisfied in the peanut fields in our study, which was similar to the result when using S. carpocapsae All for the control of H. parallela in peanut fields (Guo et al. 2013). Georgis and Gaugler (1991) summarized 82 nematode field trials against Japanese beetle larvae, and they concluded that most test failures could be explained on the basis of unsuitable nematode species or environmental conditions. In particular, they stated that S. carpocapsae was not effective because it was ill adapted to infecting Japanese beetle larvae. Our results indicated that S. carpocapsae All might not be suitable for the effective control of H. oblita larvae.
Virulence of EPNs was also strongly affected by different larval stages (Ma et al. 2013). For white grubs, there was no clear trend for which larval stage was the optimal one for EPNs; it varied with different EPN species and different white grub species (Glazer and Gol’berg 1989, 1993; Lee et al. 2002; Koppenhöfer and Fuzy 2003b, 2004; Grewal et al. 2004). Considering the third-instar larvae of H. oblita in the peanut fields caused the most serious damage to the legume, EPNs or other control agents should be applied before the third-instar larvae appear for effective control. Observations in our study on the different application times (the first- and second-instar larval application) indicated that EPNs applied in the second-instar larval time had better control efficacy as compared with the first-instar larval time, maybe because the second-instar larvae were more susceptible to S. longicaudum X-7 and H. bacteriophora H06 than the first-instar larvae.
If the environmental conditions are favorable, EPNs can produce long-term effects on pest populations (Susurluk and Ehlers 2008; Susurluk et al. 2011). The control effect of chlorpyrifos was not always persistent during the experimental period indicated in the present study. This may be due to the degradation of chlorpyrifos in the soil after 45 days, since the degradation half-life ranged from 10 to 30 days in the leaves, fruits and soil (Zhang et al. 2009). S. longicaudum X-7 and H. bacteriophora H06 performed much better against H. oblita larvae in peanut fields with good persistence. This was possibly due to the favorable factors such as the white grub species, soil texture, moisture and temperature, which were suitable for the establishment of these two EPNs in the peanut fields under the experimental conditions. The H. oblita larvae were susceptible to the tested nematode species. The sandy soil in the field allowed the IJs to disperse in the soil and contact the insect host easily. The soil moisture of approximately 17 % and the soil temperature ranging from 17–27 °C during the control period were all favorable for IJ survival and persistence.
The average profits estimated based on the price of the peanuts and the cost per ha for different treatments in the first field experiment were on the order of water control < chlorpyrifos < S. longicaudum < H. bacteriophora. The costs of using S. longicaudum X-7 and H. bacteriophora H06 at the rate of 7.5 × 108 IJ ha−1 were USD 282.16 and USD 105.83 more than chlorpyrifos, while the net profits were USD 1,017.49 and USD 1,432.21 more than chlorpyrifos, respectively. The control efficacy, net profit and safety by using EPNs were superior to those using chlorpyrifos. Therefore, management of white grubs using EPNs in peanut fields in China has good prospects.
In our study, both S. longicaudum X-7 and H. bacteriophora H06 showed good control efficacy against H. oblita larvae, but H. bacteriophora H06 was more recommended as a promising agent for white grub control in practice, as stated by Guo et al. (2013). The production cost of H. bacteriophora H06 using a solid culture system was much lower than that of S. longicaudum X-7. Especially, H. bacteriophora H06 was more stable than S. longicaudum X-7 under unfavorable conditions (Yan et al. 2010). Our results showed that 5.0 × 103 IJs per plant (equal to 7.5 × 108 IJ ha−1) would be an optimal rate when applying them in peanut fields. Higher rates may increase the cost of using EPNs, but lower rates will increase the risk of low efficacy against white grubs.
In summary, our study indicates the potential of EPNs for the control of H. oblita larvae in the laboratory and peanut fields. More studies are needed on improvements of control rates, joint application of nematode species and chemical insecticides, and formulations that will increase the efficacy of the EPNs.
Author contributions
WG, XY and RH conceived and designed the research. WG and GZ conducted experiments. WG analyzed the data. WG and XY wrote the manuscript. RH, XY and JC obtained the funding. All authors read and approved the manuscript.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Bedding RA (1981) Low cost in vitro mass production of Neoaplectana and Heterorhabditis species (Nematoda) for field control of insect pests. Nematologica 27(1):109–114
Choo HY, Kaya HK, Huh J, Lee DW, Kim HH, Lee SM, Choo YM (2002) Entomopathogenic nematodes (Steinernema spp. and Heterorhabditis bacteriophora) and a fungus Beauveria brongniartii for biological control of the white grubs, Ectinohoplia rufipes and Exomala orientalis, in Korean golf courses. Biocontrol 47:177–192
Du X, Liu Q, Zhang L, Liang L, Xie N, Zhang S (2009) Application technology of Steinernema longicaudum BPS strain in peanut fields for chafer grub control. Agrochemicals 48(5):379–388
Ehlers R-U (2001) Mass production of entomopathogenic nematodes for plant protection. Appl Microbiol Biotechnol 56:623–633
Forschler BT, Gardner WA (1991) Field efficacy and persistence of entomogenous nematodes in the management of white grubs (Coleoptera: Scarabaeidae). J Econ Entomol 84:1454–1459
Gaugler R (1998) Ecological consideration in the biological control using entomopathogenic nematodes. Agr Ecosyst Environ 24:351
Georgis R, Gaugler R (1991) Predictability in biological control using entomopathogenic nematodes. J Econ Entomol 84(3):713–720
Georgis R, Koppenhöfer AM, Lacey LA, Be´lair G, Duncan LW, Grewal PS, Samish M, Tan L, Torr P, van Tol RWHM (2006) Successes and failures in the use of parasitic nematodes for pest control. Biol Control 38:103–123
Glazer I, Gol’berg A (1989) Laboratory evaluation of steinernematid and heterorhabditid nematodes for control of the beetle Maladera matrida Argaman (Coleoptera: Scarabaeidae). Phytoparasitica 17:3–11
Glazer I, Gol’berg A (1993) Field efficacy of entomopathogenic nematodes against the beetle Maladera matrida (Coleoptera: Scarabaeidae). Bioc Sci Technol 3:367–376
Grewal PS (2002) Formulation and application technology. In: Gaugler R (ed) Entomopathogenic nematology. CABI Publishing, Wallingford, pp 265–288
Grewal PS, Power KT, Grewal SK, Suggars A, Haupricht S (2004) Enhanced consistency in biological control of white grubs (Coleoptera: Scarabaeidae) with new strains of entomopathogenic nematodes. Biol Control 30:73–82
Grewal PS, Koppenhöfer AM, Choo HY (2005) Lawn, turfgrass and pasture applications. In: Grewal PS, Ehlers R-U, Shapiro-Ilan DI (eds) Nematodes as biocontrol agents. CABI Publishing, Wallingford, pp 115–146
Guo WX, Yan X, Zhao GY, Han RC (2013) Efficacy of entomopathogenic Steinernema and Heterorhabditis nematodes against white grubs (Coleoptera: Scarabaeidae) in peanut fields. J Econ Entomol 106(3):1112–1117
Han RC (1996) The effects of inoculum size on yield of Steinernema carpocapsae and Heterorhabditis bacteriophora in liquid culture. Nematologica 42:546–553
Khatri-Chhetri HB, Timsina GP, Manandhar HK, Moens M (2011) Potential of Nepalese entomopathogenic nematodes as biocontrol agents against Holotrichia longipennis Blanch. (Coleoptera: Scarabaeidae). J Pest Sci 84:457–469
Koppenhöfer AM, Fuzy EM (2003a) Steinernema scarabaei for the control of white grubs. Biol Control 28:47–59
Koppenhöfer AM, Fuzy EM (2003b) Effects of turfgrass endophytes (Clavicipitaceae: Ascomycetes) on white grub (Coleoptera: Scarabaeidae) control by the entomopathogenic nematode Heterorhabditis bacteriophora (Rhabditida: Heterorhabditidae). Environ Entomo 32:392–396
Koppenhöfer AM, Fuzy EM (2004) Effect of white grub developmental stage on susceptibility to entomopathogenic nematodes. J Econ Entomol 97:1842–1849
Koppenhöfer AM, Fuzy EM (2008) Early timing and new combinations to increase the efficacy of neonicotinoid–entomopathogenic nematode (Rhabditida: Heterorhabditidae) combinations against white grubs (Coleoptera: Scarabaeidae). Pest Manag Sci 64:725–735
Koppenhöfer AM, Brown IM, Gaugler R, Grewal PS, Kaya HK, Klein MG (2000a) Synergism of entomopathogenic nematodes and imidacloprid against white grubs: greenhouse and field evaluation. Biol Control 19:245–252
Koppenhöfer AM, Brown IM, Kaya HK, Gaugler R (2000b) Synergism of entomopathogenic nematodes and imidacloprid against white grubs: greenhouse and field evaluation. Biol Control 19:245–251
Koppenhöfer AM, Cowles RS, Cowles EA, Fuzy EM, Baumgartner L (2002) Comparison of neonicotinoid insecticides as synergists for entomopathogenic nematodes. Biol Control 24:90–97
Lee W, Choo HY, Kaya HK, Lee SM, Smitley DR, Shin HK, Park CG (2002) Laboratory and Weld evaluation of Korean entomopathogenic nematode isolates against the oriental beetle Exomala orientalis (Coleoptera: Scarabaeidae). J Econ Entomol 95:918–926
Li S, Ding W, Han F (1993) Application of entomophagous nematode Taishan No. 1 against peanut grubs. Acta Phytophylacica Sin 26(1):55–60
Li S, Liu A, Wu Y, Luo J, Yin H (2003) Species, distribution, damage and integrated management methods of dominant white grubs in Henan province. J Henan Agric Sci 4:16–18
Li J, Sun C, Kang Y, Ma J (2007) Control effect of entomopathogenic nematodes to peanuts grubs. Agrochemica 46(1):62–63
Li YF, Gao ZL, Dang ZH, Pan WL, Tao LM (2008) Comparison on the toxicities of 18 insecticides to Holotrichia oblita Faldermann and Anomala corpulenta Motschulsky. Chin Agric Sci Bull 24(3):296–299
Liu Q, Li J, Xu X, Sun C, Kang Y, Zhou H, Hu D, Ma J, Li S (2007) The preliminary study on grub control with Rhabditis (Oscheius) spp in peanut fields. Acta Agric Boreal Sin 22:250–253
Liu Q, Du X, Zhang L, Zhang S, Xie N, Liang L (2009a) Effectiveness of Steinernema longicaudum BPS for chafer grub control in peanut plot. Plant Prot 35(6):150–153
Liu S, Li K, Liu C, Wang Q, Yin J, Cao Y (2009b) Identification of a strain of Heterorhabditis (Nematoda: Heterorhabditidae) from Hebei and its virulence to white grubs. Acta Entomo Sin 52(9):959–966
Ma J, Chen SL, Moens M, Han RC, De Clercq P (2013) Efficacy of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) against the chive gnat, Bradysia odoriphaga. J Pest Sci 86:551–561
Mukuka J, Strauch O, Hoppe C, Ehlers R-U (2010) Fitness of heat and desiccation tolerant hybrid strains of Heterorhabditis bacteriophora (Rhabditidomorpha: Heterorhabditidae). J Pest Sci 83:281–287
Shapiro-Ilan DI, Han RC, Dolinksi C (2012) Entomopathogenic nematode production and application technology. J Nematol 44(2):206–217
Susurluk A, Ehlers R-U (2008) Field persistence of the entomopathogenic nematode Heterorhabditis bacteriophora in different crops. Biocontrol 53:627–641
Susurluk IA, Kumral NA, Bilgili U, Açıkgöz E (2011) Control of a new turf pest, Dorcadion pseudopreissi (Coleoptera: Cerambycidae), with the entomopathogenic nematode Heterorhabditis bacteriophora. J Pest Sci 84:321–326
Toepfer S, Kurtz B, Kuhlmann U (2010) Influence of soil on the efficacy of entomopathogenic nematodes in reducing Diabrotica virgifera virgifera in maize. J Pest Sci 83:257–264
Wang XL (2004) The regularity and reason of outbreak and control resources against white grubs in peanut field. J Anhui Agric Sci 32(2):287–303
Wang BL, Wang HL (2006) The outbreak and integrated management of white grubs in peanut fields. J Henan Agric Sci 7:66–68
Yan X, Liu XJ, Han RC, Chen SR, De Clercq P, Moens M (2010) Osmotic induction of anhydrobiosis in entomopathogenic nematodes of the genera Heterorhabditis and Steinernema. Biol Control 53:325–330
Yan X, Moens M, Han RC, Chen SL, De Clercq P (2012) Effects of selected insecticides on osmotically treated entomopathogenic nematodes. J Plant Dis Protect 119(4):152–158
Yan X, Han RC, Moens M, Chen SL, De Clercq P (2013) Field evaluation of entomopathogenic nematodes for biological control of striped flea beetle, Phyllotreta striolata (Coleoptera: Chrysomelidae). Biocontrol 58:247–256
Zhang YL, Yuan YH, Yuan GH, Guo XR, Luo MH (2006) A study on the attraction of Holotrichia oblita (Faldermann) to castor leaves. J Henan Agric Univ 40(1):53–57
Zhang J, Zhao Z, Liang J, Zheng X, Wu W, Lin J (2009) Residue determination of chlorpyrifos in apple fruit, leaf and orchard soil. Chin Agric Sci Bull 25(01):185–189
Acknowledgments
This study was supported by the National High-Technology Research and Development Project (863 Project; 2011AA10A201), Nonprofit Sector Project (201003025), National Natural Science Foundation of China (31101494), Guangdong Provincial Science & Technology Project (2012B050700008), Guangzhou Science & Technology Project (2013J2200090) and funding from Guangdong Academy of Sciences for Excellent Young Scientists (rcjj201201).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.-U. Ehlers.
Rights and permissions
About this article
Cite this article
Guo, W., Yan, X., Zhao, G. et al. Efficacy of entomopathogenic Steinernema and Heterorhabditis nematodes against Holotrichia oblita . J Pest Sci 88, 359–368 (2015). https://doi.org/10.1007/s10340-014-0626-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-014-0626-y