Abstract
Holotrichia longipennis Blanch. (Coleoptera: Scarabaeidae) is a serious pest of commercial crops in Siduwa, Dhankuta, Nepal. Seven indigenous isolates of entomopathogenic nematodes (Steinernema lamjungense LMT5, S. lamjungense SS4, S. everestense DKP4, S. abbasi CS1, S. sp. KL1, Heterorhabditis indica CK2 and H. indica CK6) were used in a series of bioassays against the insect. All isolates showed an increased dispersal in response to H. longipennis. Nematodes were more attracted towards third instar larvae than to second instars. Differences in penetration and multiplication in the insect were observed amongst the seven isolates. Steinernema lamjungense LMT5, S. everestense DKP4 and S. abbasi CS1 caused greater mortality than other isolates to different developmental stages. Pupae and second instar larvae were more susceptible than third instar larvae. Significant differences were observed in LT50 values of the isolates against different stages of H. longipennis. Three isolates (S. lamjungense LMT5, S. everestense DKP4 and S. abbasi CS1) along with a commonly used insecticide (chlorpyrifos) were tested against this insect in pot and field experiments. In pot experiments using maize and cabbage as a host crop, S. lamjungense LMT5 and S. everestense DKP4 performed better than S. abbasi CS1 and yielded a mortality comparable with chlorpyrifos. Similar results were observed in field experiments 3 weeks after nematode application. These experiments overall suggest S. lamjungense LMT5 to be a promising biocontrol agent against H. longipennis followed by S. everestense DKP4 and S. abbasi CS1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
White grubs are the soil inhabiting root-feeding larvae of scarab beetles (Coleoptera: Scarabaeidae). They cause significant damage to many agricultural and horticultural crops, ornamentals, plantation crops, lawn, turf, pasture and forest trees around the world (Jackson 1992; Grewal et al. 2005). In Nepal, white grubs are important pests of many crops including cereals (maize, millet and upland rice), spices (ginger and large cardamom), potato, vegetables and tea. White grubs of the genera Phyllophaga Harris, Holotrichia Hope, Anomala Samouelle, Maladera Mulsant and Rey and Popillia Serville are reported as major pests (GhartyChhetry et al. 2009). Within the genus Holotrichia, H. longipennis Blanch. is one of the major species damaging maize, potato and vegetable crops in Sidhuwa, Dhankuta (2,200 m asl). Holotrichia longipennis is also one of the dominant species in the hilly region of north western India, causing severe damage to various crops like finger millet, upland paddy, maize, soybean, potato, chilli, tomato, cabbage, aubergine and black gram (Singh et al. 2004). The species has a 1-year life cycle (Mishra and Singh 1993). The total larval period ranges from 214 to 262 days. Grubs overwinter in the third instar stage.
Farmers in Sidhuwa are using cultural (summer ploughing and exposing larvae to outer environment), mechanical (collecting and killing of grubs) and chemical (e.g. carbofuran and chlorpyrifos) strategies to reduce the damage caused by H. longipennis. However, none of these approaches are successful. Therefore, farmers are looking for effective, ecologically and economically viable alternatives for the control of this pest.
Entomopathogenic nematodes (EPN) of the genera Steinernema Travassos and Heterorhabditis Poinar are extensively studied biological control agents and commercially used around the world (Ehlers 2001). These nematodes are highly virulent, kill their hosts quickly and can be cultured in vivo or in vitro (Ehlers 1996). They persist long time in soil (Susurluk and Ehlers 2008) and offer further control of target pests in the field. Nematode induced mortality in the field differs with the soil temperature (Susurluk 2008) and soil type (Toepfer et al. 2010). The biocontrol potentiality of any EPN strain/species also differs with different host species (Herrera and Gutiérrez 2009). Many EPN species have been tested against different species of white grubs. Laboratory experiments showed that EPN species differ in their virulence to different white grub species ranging from 0 to 100% (Grewal et al. 2005).
A recent survey on native EPN in Nepal recovered 29 isolates of steinernematids and heterorhabditids (Khatri-Chhetri et al. 2010); two new species have been described, viz. S. everestense (Khatri-Chhetri et al. 2011a) and S. lamjungense (Khatri-Chhetri et al. 2011b). In view of the problem caused by H. longipennis in Sidhuwa, we aimed to screen the collected isolates for their potential as biocontrol agents against this insect. We examined different phases of the interaction between the EPN and their host: viz. the degree of attraction towards the host, the ability to penetrate the host, the virulence in different experimental arenas and the extent of nematode propagation in the host. Thus, a series of laboratory bioassays, pot and field experiments were conducted to clear-up these phenomena and to find the most suitable EPNs candidates.
Materials and methods
The insects
Second and third instars of H. longipennis were collected from Sidhuwa, Dhankuta during November 2008, and February and August 2009. Grubs were kept individually in 60-ml plastic containers filled with soil to which carrot slices were added. The containers were stored at room temperature (22 ± 2°C) before use in the experiments.
The nematodes
Based on a preliminary screening considering the virulence of 1,000 IJs of the 29 isolates to third instar larvae (L3) of H. longipennis, seven isolates (Table 1) were selected for all bioassays, except for the third assay on migration in response of H. longipennis in which only five isolates (Table 1) were used. In the pot and field experiments, three isolates were compared (Table 1). The nematode isolates were cultured in Galleria mellonella L., collected from the cadavers in modified White traps (Woodring and Kaya 1988) and stored at 12–15°C. The nematodes used in the experiments were 15–30 days old.
Nematode dispersal
Vertical or horizontal migration in response to H. longipennis
Three bioassays were conducted to observe the response of the isolates to the presence of H. longipennis larvae. Two of them were to measure the vertical or horizontal migration of nematodes in response to third instar larvae. In these bioassays, three pieces (each of 3 cm length) of PVC pipes (2.5 cm diameter) were taped together. The pipes were filled with autoclaved sand (moisture: 10–12%, particle size: 125–500 μm) that was washed with tap water to remove extra fine particles before autoclaving. A single L3 was caged within an aluminium mesh stapled carefully around the grub (Koppenhöfer and Fuzy 2008) at the last section at one end; pipes were closed with adhesive tape at both ends. Twenty-four hours after set-up of the experiment, 1,000 IJs of one of the selected isolates suspended in 1 ml of water was administered at the end opposite to the insect. The treatments were replicated 15 times; five additional pipes were kept without insects and used as control in each treatment. Pipes were kept vertically or horizontally at 20°C.
Influence of host stage on nematode migration
This bioassay was conducted to measure the response of nematodes to a simultaneous exposure of different developmental stages, viz. second instar larvae (L2, 370 ± 48 mg) and third instar larvae (L3, 1,002 ± 85 mg) of H. longipennis. The experimental set-up was similar to the one used in the assay on horizontal migration. Six pieces of PVC pipes were tied together. One L3 and L2 were placed at the last section of opposite ends of the pipes. A total of 1,500 IJs (the number was increased in this larger arena in order to secure enough nematodes during observation and extraction) suspended in 1 ml of water were administered at the centre. Each treatment was replicated 12 times.
In each of the three bioassays, the pieces of PVC pipes were untied 24 h after nematode application; sand from each piece was collected in individual beakers. Grubs were also rinsed and rinsate was added to the sand of the end section. By agitation and decantation, the supernatant was collected and all nematodes present were counted. Grubs were kept 3–4 days in isolation in 60-ml plastic containers filled with autoclaved sand before checking whether they were dead. As none of the grubs were dead after isolation, the grubs were not dissected.
Virulence in bioassays
Two bioassays were conducted to estimate the virulence of the isolates to different developmental stages of H. longipennis. The bioassays were done in 60-ml plastic containers filled with 25 g autoclaved sand (moisture: 10–12%, particle size: 125–500 μm) and kept at 22 ± 2°C. In the first bioassay, L2 (370 ± 48 mg) and L3 (1,002 ± 85 mg) were used whereas in the second one, L3 and pupae were considered. One millilitre of distilled water containing 1,000 IJs (equivalent of 5 × 109 IJ/ha) was administered per insect/container. Control treatments received 1 ml of distilled water only. The treatments were replicated 20 times, and each assay was repeated two more times. Observations on number of dead and living insects were taken at weekly interval during four (first assay) or 3 weeks (second assay). The cadavers were dissected to confirm nematode infection.
Host penetration and multiplication
At the first observation (1 week after inoculation) of the first virulence bioassay (see above), 10 L3 cadavers caused by each isolate were selected from the first experiment and its repetitions and dissected in distilled water, and nematodes were counted. From the later observations of the first bioassay, 10 L3 cadavers caused by each isolate were selected randomly and kept in individual modified White traps (Woodring and Kaya 1988) for IJs emergence. The emerging juveniles were collected and stored in an incubator; nematodes present in five sub samples of each replication were counted.
Pot experiments
Maize
Plastic 5-l pots were filled with 6,500 g autoclaved sand (75.9% sand, 16.2% silt, 7.9% clay; pH: 5.1; OM: 3.1%; N: 0.15%, P2O5: 259 kg/ha and K2O 134 kg/ha; 12% moisture content). Maize (cv. Hill Pool) was seeded and allowed to grow (8 plants/pot) for 4 weeks before eight L3 (255/m2) were introduced per pot. Grubs that did not enter the soil before the two following hours were replaced.
Seventy-two hours after the introduction of the grubs, approximately 7,000 IJs were inoculated as a 10-ml suspension per pot (2.5 × 109 IJ/ha) in a circular furrow around the plants. Chorpyriphos (Deviban 1.5 DP, Devidayal Agro Chemicals, India) was applied in a similar way at 1.2 mg a.i. (25 kg/ha) as a chemical reference. The untreated control pots received only the same quantity of water. Pots were kept in a completely randomized design in a screen-house (25 ± 2°C) of Plant Pathology Division of Nepal Agricultural Research Council (NARC) at Khumaltar, Lalitpur, Nepal. Soil moisture was maintained. Each treatment was replicated 16 times. After 7, 14, 21 and 28 days of EPN application, four pots of each treatment were observed destructively and the number of live grubs counted. Whenever dead grubs were found, their death caused by nematodes was confirmed after dissection. Live grubs collected were kept for incubation for 1 week more, and the number of dead grubs was added up to the previous data.
Cabbage
A second series of pot experiments was conducted with cabbage as host for the insect. The experimental set-up was the same as for maize. In this experiment, the 5-l pots were filled with 5,500 g EPN-free sandy soil; two cabbage seedlings (cv. Green coronet) at four leaves stage were planted per pot. The plants were allowed to grow for 4 weeks before eight L3 (255/m2) were introduced per pot. The five treatments were replicated 15 times. The insect larvae were observed at 7, 14 and 21 days after EPN application. For each treatment, five pots were observed at a time.
Field experiments
A white grubs-infested field with loamy soil (47.9% sand, 36.2% silt, 15.95% clay; OM: 6.20%, pH: 4.8; N: 0.3%, P2O5: 156 kg/ha and K2O: 672 kg/ha) was selected in the vegetable and potato production area of Sidhuwa, Dhankuta, Nepal. The absence of EPN was confirmed before planting by baiting soil samples with G. mellonella. The cropping pattern on the field was cabbage–mix of maize and potato–cabbage. Twenty ton farm yard manure and 70:50:40 kg NPK/ha were applied per hectare before planting cabbage (cv. Green coronet) at a density of 50 cm × 30 cm.
The experiment was organised as a randomised complete block design with five treatments and five replicates. The plots (2 m × 2 m) were separated by a 50-cm border. The treatments consisted of: the application of 106 IJs (2.5 × 109 IJ/ha) of one of the selected nematode isolates at 21 days after transplanting (DAT) with a watering can (2.5 l/plot); chorpyrifos at 0.15 g a.i. (Deviban 1.5 DP at 25 kg/ha as recommended by the company) suspended in the same volume of water and applied with the watering can; and the untreated control that received only 2.5 l water. The treatments took place at 5:00 pm. At that time, the air and soil temperature (−5 cm) were 19°C and 17°C, respectively. The application was followed immediately by a light sprinkler irrigation that was repeated the following day.
The start population of grubs was estimated in each plot with 3 sampling units of 50 cm × 50 cm each (30 cm deep). The field population in different plots (35–50 grubs/sq m) was composed of 85–90% H. longipennis (75% L3 and 25% L2); the remaining grubs could not be identified. The whole population was considered for further observations. The reduction of the grub population was estimated 3 and 19 weeks after nematode application using the same procedure.
Statistical analyses
In the assays examining the migration of nematodes in sand column, the total number of nematodes found in all sections was calculated and taken as 100%. Subsequently, the percentage of nematodes found in each section was calculated. Data were normalised by arcsine square root transformation. Data obtained in other bioassays were square root (% data) or log (number) transformed whenever necessary for normalisation. In all experiments measuring the mortality of the grubs, mortality was corrected according to Abbott (1925) prior to statistical analysis. Data from repeated virulence bioassays were combined for analysis with repetition being a factor. Cumulative data on mortality of grubs along with time were used for analysis. Data were subjected to analysis of variance or Kruskal–Wallis test whenever assumptions for ANOVA were not met. When differences were found, means were separated with Duncan’s test or Tamhane’s test. In the bioassays comparing the nematode migration towards L2 or L3, data were subjected to a non-parametric Wilcoxon signed ranked test. Similarly, in the experiments comparing the mortality of two stages, data were subjected to independent sample t test. LT50-values of isolates against different developmental stages of H. longipennis were calculated using Probit analysis. Data of time series were analysed using a regression analysis. Differences amongst means in all experiments were considered significant at P < 0.05. All analyses were conducted using SPSS. Mean ± SE are presented.
Results
Nematode dispersal
Vertical migration
In general, the majority of nematodes remained in the section of application; gradually smaller numbers of nematodes were found in subsequent sections. In the control pipes without insect, none of the isolates moved up to the last section thereby covering a 6 cm distance (Fig. 1a). Significant differences in movement were found amongst different isolates in both first and second sections (0–3 cm: F = 3.6, df = 6, 28, P < 0.01; 3–6 cm: F = 3.70, df = 6, 28, P < 0.01, respectively). In the first section (0–3 cm), the lowest numbers of nematodes was found for isolates S. lamjungense SS4 (86.6%) and S. lamjungense LMT5 (88.8%), whereas the highest number was obtained for isolates H. indica CK6 (94.9%) and H. indica CK2 (94.8%). In the middle section (3–6 cm), the lowest number of nematodes was found for isolates H. indica CK6 (5.1%), H. indica CK2 (5.2%) and S. sp. KL1 (5.6%), whereas the highest number was found for isolates S. lamjungense SS4 (13.4%) and S. lamjungense LMT5 (11.1%).
Vertical migration in absence a and in presence b of third instar larvae of Holotrichia longipennis in 24 h at 20°C to three different sections of sand columns of 3 cm each in PVC pipes (2.5 cm diam) (mean % ± SE) of seven isolates of entomopathogenic nematodes. To the opposite end of the caged grub, 1,000 IJs were applied. Bars headed (different case and fonts used for different distances from the point of application) by the same letter are not significantly different (P > 0.05). LMT5 and SS4 = Steinernema lamjungense, CS1 = S. abbasi, CK2 and CK6 = Heterorhabditis indica, KL1 = S. sp. E and DKP4 = S. everestense
When exposed to H. longipennis, significant differences in migration (Fig. 1b) were observed amongst the isolates in each section (0–3 cm: F = 12.42, df = 6, 98, P < 0.001; 3–6 cm: F = 9.56, df = 6, 98, P < 0.001; 6–9 cm: F = 16.5, df = 6, 98, P < 0.001). In general, S. lamjungense LMT5, S. lamjungense SS4 and S. everestense DKP4 migrated in greater numbers than other isolates towards the grub (Fig. 1b).
Horizontal migration
As observed in the vertical migration experiment, the majority of nematodes remained in the section of application; gradually smaller numbers of nematodes were found in subsequent sections. In tubes without insect, none of the isolates moved up to the last section thereby covering a 6 cm distance (Fig. 2a). Significant differences were found amongst different isolates in both first sections (0–3 cm: F = 4.74, df = 6, 28, P < 0.01; 3–6 cm: F = 6.58, df = 6, 28, P < 0.001). In the first section (0–3 cm), the smallest numbers were found for all isolates (82.5–91.5%), except for S. abbasi CS1 (95.7%). In the middle section (3–6 cm), the smallest numbers were found for S. abbasi CS1 (4.3%) and S. sp. KL1 (8.5%); the greatest number was observed for all other isolates (11.2–17.5%).
Horizontal migration in absence a and in presence b of third instar larvae of Holotrichia longipennis in 24 h at 20°C to three different sections of sand columns of 3 cm each in PVC pipes (2.5 cm diam) (mean % ± SE) of seven isolates of entomopathogenic nematodes. To the opposite end of the caged grub, 1,000 IJs were applied. Bars headed (different case and fonts used for different distances from the point of application) by the same letter are not significantly different (P > 0.05). LMT5 and SS4 = Steinernema lamjungense, CS1 = S. abbasi, CK2 and CK6 = Heterorhabditis indica, KL1 = S. sp. E and DKP4 = S. everestense
When exposed to H. longipennis, significant differences in migration (Fig. 2b) were observed amongst the isolates in each sections (0–3 cm: F = 13.16, df = 6, 98, P < 0.001; 3–6 cm: F = 8.7, df = 6, 98, P < 0.001; 6–9 cm: χ² = 64.963, df = 6, P < 0.001). In general, five isolates (S. lamjungense LMT5, S. lamjungense SS4, S. everestense DKP4, H. indica CK2 and H. indica CK6) migrated in greater numbers simultaneously towards the grub whereas S. abbasi CS1 and S. sp. KL1 migrated in lower numbers (Fig. 2b).
Influence of host stages on horizontal nematode dispersal
Significantly greater numbers of nematodes were observed in the sections leading to the L3 than to the opposite sections (leading to L2) for all steinernematid isolates (S. lamjungense LMT5: z = 2.90, P < 0.01; S. abbasi CS1: z = 2.74, P < 0.01; S. everestense DKP4: z = 2.98, P < 0.01 and S. sp. KL1: z = 2.82, P < 0.001); no differences were observed for H. indica CK2 (z = 0.17, P > 0.05). Between 37 and 39% of the steinernematid isolates were found towards L2 (Fig. 3a). Similar results were observed when comparing the percentage of nematodes that had reached the last section (6–9 cm) (S. lamjungense LMT5: z = 2.82, P < 0.01; S. abbasi CS1: z = 2.03, P < 0.05; S. everestense DKP4: z = 2.74, P < 0.01; S. sp. KL1: z = 2.66, P < 0.01; H. indica CK2: z = 0.10, P > 0.05). 0.5% (S. abbasi CS1) to 2% (S. lamjungense LMT5) of the nematodes were extracted from the last section towards L2; 0.82% (H. indica CK2) to 5.9% (S. lamjungense LMT5) of the nematodes were extracted from the last section towards the L3 (Fig. 3b).
Migration (mean % ± SE) in 24 h at 20°C of five isolates of entomopathogenic nematodes in sand columns (PVC pipes: 2.5 cm diam) in response to concurrent exposure to second (L2) and third (L3) instar larvae of Holotrichia longipennis. Grubs were caged in two opposite ends of PVC pipes, and 1,500 IJs were applied at the centre of the sand column. a Percentage of nematodes found at either side of point of application, b Percentage of nematodes in the section (6–9 cm). For each isolate, bars or figures without asterisk are not significantly different (P > 0.05). LMT5 = Steinernema lamjungense, CS1 = S. abbasi, CK2 = Heterorhabditis indica, KL1 = S. sp. E and DKP4 = S. everestense
Virulence
Mortality rates of larval stages in bioassays
The overall mortality induced by the EPNs to L2 and L3 ranged from 35.6 to 83%. In general, L2 were more susceptible than L3. The greatest mortality of L2 was observed for S. everestense DKP4 (83%), S. lamjungense LMT5 (78.3%) and S. abbasi CS1 (75%) and the lowest for H. indica CK6 (44.8%). In case of L3, the greatest mortality was observed for S. lamjungense LMT5 (72.8% to L3) and the lowest for H. indica CK6 (35.6%) and H. indica CK2 (41%) (Fig. 4a). In overall statistics, significant differences in virulence were observed amongst the isolates (F = 66.09, df = 6, 112, P < 0.001), time of observation (F = 323.47, df = 3, 112, P < 0.001) and stage of host insect (F = 107.16, df = 1, 112, P < 0.001). Significant interactions were observed between isolates * time of observation (F = 3.04, df = 18, 112, P < 0.001), isolates * stage of host insect (F = 6.85, df = 6, 112, P < 0.001) and time of observation * stage (F = 8.59, df = 6, 112, P < 0.001). Regression of corrected mortality on time of observation indicated a significant linear relationship between mortality and time with variation in slopes between isolates (Table 2). Significant differences in LT50 values for L2 (F = 16.80, df = 6, 14, P < 0.001) and L3 (F = 3.46, df = 6, 14 P < 0.05) were observed (Table 2). The analysis of cumulative mortality as observed at the end of the bioassay yielded significant differences in virulence between the isolates (L2: F = 17.65, df = 6, 14, P < 0.001; L3: F = 9.84, df = 6, 14, P < 0.001). Differences in mortality of L2 and L3 were observed for S. lamjungense SS4 (t = 4.85, df = 4, P < 0.01), S. abbasi CS1(t = 3.25, df = 4, P < 0.05), H. indica CK2 (t = 3.20, df = 4, P < 0.05), S. sp. KL1 (t = 3.71, df = 4, P < 0.05) and S. everestense DKP4 (t = 4.45, df = 4, P < 0.05), but not for S. lamjungense LMT5 (t = 1.12, df = 4, P > 0.05) and H. indica CK6 (t = 1.72, df = 4, P > 0.05).
Cumulative corrected mortality (mean % ± SE) caused by seven isolates of entomopathogenic nematodes at 22 ± 2°C to different stages of Holotrichia longipennis after four a or three b weeks exposure to L2 and L3 (a) and, pupae and L3 (b). In the sand filled plastic container (60 ml), 1,000 IJs per insect were administered and observation taken at weekly interval after inoculation. Bars headed (different case used for different stage) by the same letter are not significantly different (P > 0.05). For each isolate, bars without asterisk are not significantly different (P > 0.05). LMT5 and SS4 = Steinernema lamjungense, CS1 = S. abbasi, CK2 and CK6 = Heterorhabditis indica, KL1 = S. sp. E and DKP4 = S. everestense
Mortality rates of L3 and pupae in bioassays
The overall mortality induced by the EPNs to L3 and pupae ranged from 32.3 to 93.3%. In general, pupae were more susceptible than L3. With respect to the mortality of both L3 and pupae, S. lamjungense LMT5 caused the greatest mortality (69% to L3 and 93.3% to pupae); the smallest mortality was observed for H. indica CK2 (32.3% to L3 & 70% to pupae) (Fig. 4b). In overall statistics, significant differences in virulence were observed amongst the isolates (F = 56.15, df = 6, 84, P < 0.001), time of observation (F = 385.50, df = 2, 84, P < 0.001) and stage of host insect (F = 532.27, df = 1, 84, P < 0.001). Insignificant interactions were observed between isolates * time (F = 0.525, df = 12, 84, P > 0.05) and isolates * stage of host insect (F = 0.685, df = 6, 84, P > 0.05), but there was a significant interaction between observation time * stage of host insect (F = 21.06, df = 2, 84, P < 0.001). Regression of corrected mortality of pupae on duration of time indicated a significant linear relationship with variation in slopes (Table 2). Significant differences in LT50 values for pupae (F = 10.96, df = 6, 14, P < 0.001) were observed (Table 2). The trends of isolates causing mortality of L3 were similar as in the previous bioassay; hence the regression analysis and Probit analysis were not done. The analysis of cumulative mortality at the end the bioassay yielded significant differences between the isolates for both L3 and pupae (L3: F = 8.96, df = 6, 14, P < 0.001; pupae: F = 8.16, df = 6, 14, P < 0.001). In general, greater mortality was observed in pupae than in L3. Significant differences in mortality of L3 and pupae were observed for all isolates: S. lamjungense LMT5 (t = 5.42, df = 4, P < 0.01), S. lamjungense SS4 (t = 7.33, df = 4, P < 0.005), S. abbasi CS1 (t = 7.16, df = 4, P < 0.005), H. indica CK2 (t = 6.68, df = 4, P < 0.005), H. indica CK6 (t = 10.58, df = 4, P > 0.001), S. sp. KL1 (t = 4.67, df = 4, P < 0.05), and S. everestense DKP4 (t = 4.79, df = 4, P < 0.05).
Host penetration and multiplication
Significant differences in nematode penetration into L3 of H. longipennis were observed between isolates (F = 6.19, df = 6, 63, P < 0.001). The greatest penetration was found for S. lamjungense LMT5 (7.3%), S. sp. KL1 (6.8%) and S. everestense DKP4 (5.8%); the lowest penetration was observed for H. indica CK2 (3.8%), H. indica CK6 (4.3%) and S. abbasi CS1 (4.9%) (Fig. 5a). Similarly, significant differences between nematode isolates in multiplication were observed on L3 of H. longipennis (F = 107.114, df = 6, 63, P < 0.001), the greatest multiplication was observed for S. lamjungense LMT5 (39.9 IJ/mg) and the smallest for H. indica CK2 (7.9 IJ/mg), H. indica CK6 (8.2 IJ/mg) (Fig. 5b).
Penetration (mean % ± SE) (a) and multiplication per milligram body weight of host (mean number of IJ ± SE) (b) of seven isolates of entomopathogenic nematodes at 22 ± 2°C into last instar larvae of Holotrichia longipennis. 1,000 IJs per insect were administered in the sand filled plastic container containing 1 insect larva (60 ml). Bars headed by the same letter are not significantly different (P > 0.05). LMT5 and SS4 = Steinernema lamjungense, CS1 = S. abbasi, CK2 and CK6 = Heterorhabditis indica, KL1 = S. sp. E and DKP4 = S. everestense
Mortality in pot experiments
Maize
At the last observation (28 days), the greatest mortality (65.63%) was observed for both S. lamjungense LMT5 and chlorpyrifos followed by S. everestense DKP4 (54.91%) (Fig. 6a); the mortality obtained after S. abbasi CS1 was significantly lower. Significant differences in grub mortality were observed between the treatments (F = 12.49; df = 3, 48; P < 0.001) and times of observation (F = 22.69; df = 3, 48; P < 0.001); the interaction between isolates and moment of observation, however, was not significant (F = 0.467; df = 9, 48; P > 0.05). The mortality gradually increased over time; the regression analysis of corrected mortality over time indicated a significant linear relationship (Table 3). Between the nematode isolates, the coefficient indicating the intercept of the regression equation was the highest for S. lamjungense LMT5; the intercept for chlorpyrifos was the double as the one for the nematode.
Corrected mortality of Holotrichia longipennis (mean % ± SE) caused by different treatments in pot experiments using maize (a) and cabbage (b) as a host plant of the insect. Bars headed by the same letter(s) are not significantly different (P > 0.05). Nematodes and chlorpyrifos were used at the rate of 2.5 × 109 IJ/ha and 25 kg/ha, respectively. CS1 = Steinernema abbasi, LMT5 = S. lamjungense and DKP4 = S. everestense
Cabbage
At the last observation (21 days), the greatest mortality (55.71–61.90%) was observed for S. lamjungense LMT5 (55.71%), S. everestense DKP4 (59.05%) and chlorpyrifos (61.90%) (Fig. 6b); the mortality obtained after S. abbasi CS1 was significantly lower. Significant differences in grub mortality were observed amongst the treatments (F = 9.86; df = 3, 48; P < 0.001) and moments of observation (F = 33.55; df = 3, 48; P < 0.001); the interaction between isolates and time of observation was not significant (F = 1.035; df = 6, 48; P > 0.05). As observed in the maize pot experiment, the mortality gradually increased over time; the regression analysis of the corrected mortality over time indicated a significant linear relationship (Table 3). The coefficient indicating the intercept of the regression equation was comparable with the one obtained for S. abbasi CS1 and S. lamjungense LMT5 on maize. The value of the intercept of the equation for S. everestense DKP4, however, was negative and combined with a high value for the slope of the equation.
Population reduction in the field experiments
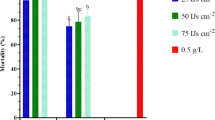
Three weeks after EPN application, treatments differed significantly (F = 3.64; df = 3, 16; P < 0.05) in reduction of the white grub population. Reduction of the grubs was lowest (34.29%) after S. abbasi CS1 and highest (58.61%) after S. lamjungense LMT5, chlorpyrifos (52.85%) and S. everestense DKP4 (43.58%) (Fig. 7). No significant differences (χ² = 0.089; df = 3; P > 0.05) were observed in reduction of white grub population between the four treatments at 19 weeks after EPN application (Fig. 7). Population decline varied between 73 and 80% (S. lamjungense LMT5: 80.19%, chlorpyrifos: 79.58%, S. everestense DKP4: 73.64% and S. abbasi CS1: 78.47%).
Corrected population reduction of Holotrichia longipennis (mean % ± SE) 3 and 19 weeks after different treatments in field experiments using cabbage as a host for the insect. Bars headed by the same letter are not significantly different (P > 0.05). Nematodes and chlorpyrifos were used at the rate of 2.5 × 109 IJ/ha and 25 kg/ha, respectively. CS1 = Steinernema abbasi, LMT5 = S. lamjungense and DKP4 = S. everestense
Discussion
This is the first report on screening of EPN against insects in Nepal. The screening was restricted to indigenous isolates that were previously detected during a survey. A preliminary screening considering all isolates showed clear differences amongst species. This difference was also obvious between isolates of the same species.
In the present study, in both vertical and horizontal migration set-ups and with or without insect, all isolates migrated over 3 cm, but not over 6 cm in the absence of an insect. In the presence of H. longipennis, however, all isolates covered a 6 cm distance over 24 h. This demonstrates a degree of cruising behaviour of the Nepalese isolates. Obviously, this behaviour differs between species/isolates. In vertical as well as in horizontal assays with H. longipennis, both isolates of S. lamjungense (LMT5 and SS4) and S. everestense DKP4 migrated in greatest numbers. Heterorhabditis indica (CK2 & CK6) showed faster migration horizontally than vertically. The positive influence of host insect cues on the migration of EPN is well documented (e.g. Lacey et al. 2001; Koppenhöfer and Fuzy 2008). Nematode attraction in response to insects is reported to be due to host cues like CO2 (Gaugler et al. 1980; Lewis et al. 1993) and/or gut fluids (Grewal et al. 1993a, b). In general, more nematodes migrated towards L3 than to L2. Differences in CO2 production by different insects have been reported by many authors (e.g. Gaugler et al. 1991; Ramos-Rodríguez et al. 2007). Smaller hosts are reported to be less attractive to EPN because of their reduced CO2 output (Kaya 1985). The larger L3 might have higher metabolic activities than the smaller L2; one can predict higher concentrations of CO2 and gut fluids produced by L3 than by L2.
Attraction of nematodes does not always result in higher penetration. As there is no chance of reverse reaction, successful penetration defines the fate of nematodes. Our isolates showed differences in ability to penetrate the host insects. The penetration rates into H. longipennis are greater for most of the steinernematid isolates than for the H. indica isolates. White grubs have developed a number of defence mechanisms (Forschler and Gardner 1991; Cui et al. 1993; Gaugler et al. 1994; Wang et al. 1995). Nematodes that have penetrated into the grubs’ haemocoel may still have to face a strong immune response, viz. melanotic encapsulation (Wang et al. 1994, 1995). The establishment of nematode species and isolates in H. longipennis varied between 3.77% (H. indica CK2) and 7.29% (S. lamjungense LMT5). Similar differences in penetration into other white grub species were observed amongst different other species (Koppenhöfer et al. 2007) and varied from very low 1% (S. scarabaei to Cyclocephala borealis) to as high as 22% (S. scarabaei to Anomala orientalis).
After the successful establishment in the host, nematodes undergo several cycles of multiplication. The success of this process determines their fate to establish in the field. Our observations clearly demonstrated that most of the steinernematid isolates multiplied better than H. indica isolates. Many authors reported factors like initial inoculum density (Selvan et al. 1993; Shapiro-Ilan et al. 1999; Susurluk 2008), host species (Elawad et al. 2001; Phan et al. 2005), size of the host (Flander et al. 1996) and IJs body size of nematodes (Bhatnagar et al. 2004) determining the total number of nematode production of any species or strain.
Virulence to the target host is after all the most important feature of any isolate used as a biological control agent. In the field, the different larval stages (L2 and L3, July–September) and pupae (April–May) of H. longipennis prevail simultaneously for quite some time. Hence, information on preference and virulence of putative biocontrol agents to these different stages is important, especially in view of the application of the correct species/strain at the right time. Nepalese isolates showed differences in virulence to different stages of H. longipennis. Three steinernematids (S. lamjungense LMT5, S. everestense DKP4 and S. abbasi CS1) proved to be more pathogenic than the H. indica and other steinernematid isolates to all stages. Compared with the other species/isolates, S. lamjungense LMT5 killed relatively fast. Immediately after application, S. everestense DKP4 and S. abbasi CS1 were intermediate in virulence but eventually yielded a virulence similar to that of S. lamjungense LMT5. Variations in virulence of different EPN species to different white grub species ranging from 0 to 100% have been observed in many laboratory experiments (Grewal et al. 2005). Susceptibility of different developmental stages differs with the nematode species and white grub species (Fujiie et al. 1993; Smits et al. 1994; Lee et al. 2002; Koppenhöfer and Fuzy 2004; Ansari et al. 2006; Power et al. 2009). We observed a greater virulence to L2 than to L3 for all isolates, except for S. lamjungense LMT5 and H. indica CK6 for which similar mortalities were observed. The immune system of matured larvae may be stronger than earlier stages, increasing their ability to eliminate invading pathogens (Watanabe 1987). In general, pupae of white grubs are more susceptible to EPN than active feeding larvae (Lacey et al. 2001; Lee et al. 2002; Koppenhöfer and Fuzy 2004). Our bioassays yielded similar results for all isolates. These differences might be explained by a weaker defence mechanism of pupae compared to active stages.
In the pot experiments, grub mortality was already at quite high level 1 week after the treatments. At that moment, the effect obtained by applications of S. lamjungense LMT5 did not differ statistically from that obtained with chlorpyrifos. Similar results were obtained in the field experiment 19 weeks after all applications. Unlike applications of chlorpyrifos, the effect of treatments with EPN drastically increased over time in the pot experiment with maize. In the cabbage pots, this dynamic was also observed for chlorpyrifos. The fastest increase was observed after applications of S. everestense DKP4 on cabbage. Obviously, the host of the insect influences the efficacy of the EPN. In field studies with cabbage as host for the insect, insect control comparable to chlorpyrifos was only obtained with S. lamjungense LMT5 and S. everestense DKP4. This explains a part of the results obtained with both nematode species. The reduction of the grub population was almost the same for all tested nematodes, 19 weeks after the applications. Steinernema abbasi CS1 had caught up with the other nematode species and chlorpyrifos. The change can be explained by the fact that S. abbasi CS1 multiplies very well in H. longipennis, whilst it was intermediate in host searching behaviour and pathogenicity. The fact that the grub reduction caused by EPN is comparable to that of chorpyriphos is a good indication of the potentiality to replace chlorpyrifos by EPN application.
In summary, steinernematid isolates generally performed better than did isolates of H. indica and within the steinernematids, S. lamjungense LMT5 and S. everestense DKP4 were generally better than the other isolates in all bioassays, pot and field studies. Both species were isolated from regions comparable in climate and geography with that of H. longipennis; hence, they probably are better adapted to the insect. Moreover, in screening experiments, both isolates have demonstrated the fastest movement when exposed to H. longipennis. It is well known that nematodes having a good cruising behaviour are more suitable for sedentary hosts like white grub control (Grewal et al. 2005). Steinernema lamjungense LMT5 is a member of the ‘glaseri’ group (Khatri-Chhetri et al. 2011b), which has a close relation to scarabaeids. Similarly S. everestense DKP4 is close to S. kushidai (Khatri-Chhetri et al. 2011a) that is also a scarab adapted EPN species (Mamiya 1988; Fujiie et al. 1993). Steinernema everestense DKP4 was recovered from an area where H. longipennis is prevalent. Steinernema abbasi CS1, on the other hand, was recovered from relatively low altitude experiencing higher temperature. As a matter of fact, infectivity might have been reduced at Sidhuwa, a region with lower temperatures are prevailing.
The results of our experiments clearly demonstrate the potential of indigenous EPN isolates for the control the economically important white grub species, H. longipennis, in Nepal. Both, pot and field experiments showed the efficacy of EPN to be comparable with that of the widely practised treatment with chlorpyrifos. Our observations on laboratory bioassays also suggest that an application of the selected species/isolates at the time L2 are present (August-September) may generate a better control of both stages right from the time of application with a further multiplication in the host cadavers.
The positive results we obtained with EPN applications urge further research under different conditions like geographical localities and other grub species. The combination of these isolates with chemicals and or other biologicals like Metarhizium anisopliae may also be a potential area for developing a successful and sustainable management strategy against H. longipennis. Their application may reduce the use of insecticides and guarantee the export of vegetables.
References
Abbott WS (1925) A method for computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Ansari MA, Ali F, Moens M (2006) Compared virulence of the Belgian isolate of Steinernema glaseri (Rhabditida: Steinernematidae) and the type population of S. scarabaei to white grub species (Coleoptera: Scarabaeidae). Nematol 8:787–791
Bhatnagar A, Shinde V, Bareth SS (2004) Evaluation of entomopathogenic nematodes against Maladera insanabilis Brenske. Int J Pest Manag 50:285–289
Cui LR, Gaugler R, Wang Y (1993) Penetration of steinernematid nematodes (Nematoda: Steinernematidae) into Japanese beetle larvae, Popillia japonica (Coleoptera: Scarabaeidae). J Invertebr Pathol 62:73–78
Ehlers R-U (1996) Current and future use of nematodes in biocontrol: practice and commercial aspects in regard to regulatory policies. Biocontrol Sci Technol 6:303–316
Ehlers R-U (2001) Mass production of entomopathogenic nematodes for plant protection. Appl Microbiol Biotechnol 56:623–633
Elawad SA, Goven SR, Hague NGM (2001) Progeny production of Steinernema abbasi in lepidoptera larvae. Int J Pest Manag 47:17–21
Flander KL, Miller JM, Shields EJ (1996) In vivo production of Heterorhabditis bacteriophora ‘Oswego’ (Rhabditida: Heterorhabditidae), a potential biological control agent for soil inhabiting insects in temperate regions. J Econ Entomol 89:373–380
Forschler B, Gardner W (1991) Parasitism of Phyllophaga hirticula (Coleoptera: Scarabaeidae) by Heterorhabditis heliothidis and Steinernema carpocapsae. J Invertebr Pathol 58:396–407
Fujiie A, Yokoyama T, Fujikata M, Sawada M, Hasegawa M (1993) Pathogenicity of an entomopathogenic nematode Steinernema kushidai Mamiya (Nematoda: Steinernematidae) on Anomala cuprea (Coleoptera: Scarabaeidae). Jpn J Appl Entomol Zool 37:53–60
Gaugler R, LeBeck L, Nakagaki B, Boush GM (1980) Orientation of the entomogenous nematode Neoaplectana carpocapsae to carbon dioxide. Environ Entomol 9:649–652
Gaugler R, Campbell JF, Gupta P (1991) Characterization and basis of enhanced host finding in a genetically improved strain of Steinernema carpocapsae. J Invertebr Pathol 57:234–241
Gaugler R, Wang Y, Campbell JF (1994) Aggressive and evasive behaviors in Popillia japonica (Coleoptera: Scarabaeidae) larvae: defenses against entomopathogenic nematode attack. J Invertebr Pathol 64:193–199
GhartyChhetry YD, Keller S, Nagel P, Kafle L (2009) Abundance and diversity of scarabaeid beetles (Coleoptera: Scarabaeidae) in different farming areas in Nepal. Formos Entomol 29:103–112
Grewal PS, Gaugler R, Selvan S (1993a) Host recognition by entomopathogenic nematodes: behavioral response to contract with host faeces. J Chem Ecol 119:1219–1231
Grewal PS, Gaugler R, Lewis E (1993b) Host recognition by entomopathogenic nematodes during contact with insect gut contents. J Parasitol 79:495–503
Grewal PS, Koppenhöfer AM, Choo HY (2005) Lawn, turfgrass, and pasture pests. In: Grewal PS, Shapiro-Ilan DI, Ehlers R-U (eds) Nematodes as biocontrol agents. CAB International, Wallingford, UK, pp 115–146
Herrera RC, Gutiérrez C (2009) A laboratory study on the activity of Steinernema feltiae (Rhabditida: Steinernematidae) Rioja strain against horticultural insect pests. J Pest Sci 82:305–309
Jackson TA (1992) Scarabs: pests of past or future? In: Jackson TA, Glare TR (eds) Use of pathogens in scarab pest management. AgRes Lincoln, New Zealand, pp 1–6
Kaya HK (1985) Susceptibility of early larval stage of Pseudaletia unipuncta and Spodoptera exigua (Lepidoptera: Noctuidae) to the entomopathogenic nematodes Steinernema feltiae (Rhabditida: Steinernematidae). J Invertebr Pathol 46:58–62
Khatri-Chhetri HB, Waeyenberge L, Manandhar HK, Moens M (2010) Natural occurrence and distribution of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in Nepal. J Invertebr Pathol 103:74–78
Khatri-Chhetri HB, Waeyenberge L, Spiridonov S, Manandhar HK, Moens M (2011a) Steinernema everestense n. sp. (Rhabditida: Steinernematidae), a new species of entomopathogenic nematode from Pakhribas, Dhankuta, Nepal. Nematol 13:443–462
Khatri-Chhetri HB, Waeyenberge L, Spiridonov S, Manandhar HK, Moens M (2011b) Steinernema lamjungense n. sp. (Rhabditida: Steinernematidae), a new species of entomopathogenic nematode from Lamjung district, Nepal. Nematology 13:589–605
Koppenhöfer AM, Fuzy EM (2004) Effect of white grub developmental stage on susceptibility to entomopathogenic nematodes. J Econ Entomol 97:1842–1849
Koppenhöfer AM, Fuzy EM (2008) Attraction of four entomopathogenic nematodes to four white grub species. J Invertebr Pathol 99:227–234
Koppenhöfer AM, Grewal PS, Fuzy EM (2007) Differences in penetration routes and establishment rates of four entomopathogenic nematode species into four white grub species. J Invertebr Pathol 94:184–195
Lacey LA, Rosa JS, Simões NO, Amaral JJ, Kaya HK (2001) Comparative dispersal and larvicidal activity of exotic and Azorean isolates entomopathogenic nematodes against Popillia japonica (Coleoptera: Scarabaeidae). Eur J Entomol 98:439–444
Lee DW, Choo HY, Kaya HK, Lee SM, Smittley DR, Shin HK, Park CG (2002) Laboratory and field evaluation of Korean entomopathogenic nematode isolates against the oriental beetle Exomala orientalis (Coleoptera: Scarabaeidae). J Econ Entomol 95:918–926
Lewis EE, Gaugler R, Harrison R (1993) Response of cruiser and ambusher entomopathogenic nematodes (Steinernematidae) to host volatile cues. Can J Zool 71:765–769
Mamiya Y (1988) Steinernema kushidai nsp. (Nematoda: Steinernematidae) associated with scarabaeid beetle larvae from Shizuoka, Japan. Appl Entomol Zool 23:313–320
Mishra PN, Singh MP (1993) Field biology of white grub, Holotrichia longipennis on potatoes in U.P. hills. Potato J 20 Abstr
Phan KL, Tirry L, Moens M (2005) Pathogenic potential of six isolates of entomopathogenic nematodes (Rhabditidae: Steinernematidae) from Vietnam. Biocontrol 50:477–491
Power KT, Ruisheng A, Grewal PS (2009) Effectiveness of Heterorhabditis bacteriophora strain GPS11 applications targeted against different instars of the Japanese beetle Popillia japonica. BiolControl 48:232–236
Ramos-Rodríguez O, Campbell JF, Lewis EE, Shapiro-Ilan DI, Ramaswami SB (2007) Dynamics of carbon dioxide release from insects infected with entomopathogenic nematodes. J Invertebr Pathol 94:64–69
Selvan S, Campbell JF, Gaugler R (1993) Density-dependent effects on entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) within an insect host. J Invertebr Pathol 62:278–284
Shapiro DI, Cate JR, Pena J, Hunsberger A, McCoy CW (1999) Effects of temperature and host range on suppression of Diaprepes abbreviatus (Coleoptera: Curculionidae) by entomopathogenic nematodes. J Econ Entomol 92:1086–1092
Singh MP, Mishra PN, Bisht RS (2004) Nature and extent of damage of white grub Lachnosterna longipennis (Holotrichia longipennis Blanch.) under various farming situations of Uttaranchal hills. Indian J Entomol 66:277–280
Smits PH, Wiegers GL, Vlug HJ (1994) Selection of insect parasitic nematodes for biological control of the garden chafer, Phyllopertha horticola. Entomol Experim Appli 70:77–82
Susurluk A (2008) Potential of entomopathogenic nematodes Steinernema feltiae, S weiseri and Heterorhabditis bacteriophora for the biological control of the sugarbeet weevil Bothynoderes punctiventris (Coleoptera: Curculionidae). J Pest Sci 81:221–225
Susurluk A, Ehlers R-U (2008) Field persistence of the entomopathogenic nematode Heterorhabditis bacteriophora in different crops. Biocontrol 53:627–641
Toepfer S, Kurtz B, Kuhlmann U (2010) Influence of soil on the efficacy of entomopathogenic nematodes in reducing Diabrotica virgifera virgifera in maize. J Pest Sci 83:253–264
Wang Y, Gaugler R, Cui L (1994) Variations in immune response of Popillia japonica and Acheta domesticus to Heterorhabditis bacteriophora and Steinernema species. J Nematol 26:11–18
Wang Y, Campbell JF, Gaugler R (1995) Infection of entomopathogenic nematodes Steinernema glaseri and Heterorhabditis bacteriophora against Popillia japonica (Coleoptera: Scarabaeidae) larvae. J Invertebr Pathol 66:178–184
Watanabe H (1987) The host population. In: Fuxa JR, Tanada Y (eds) Epizootiology of insect diseases. Wiley & Sons, New York, USA, pp 71–112
Woodring JL, Kaya HK (1988) Steinernematid and heterorhabditid nematodes: a handbook of techniques. Southern Cooperative Series Bulletin 331, Arkansas Agricultural Experiment Station, Fayetteville, Arkansas
Acknowledgments
We appreciate the assistance of Prem Adhikari and Sumitra Ghimire in the laboratory at NARC, Khumaltar, Nepal. We appreciate the assistance of Mr GP Timsina and Dhanik Lal Mandal for collecting and transporting white grubs to laboratory. We thank to Dr. VV Ramamurthy and Shaloo Ayri of National Pusa Collection, Division of Entomology, Indian Agriculture Research Institute (New Delhi) for the insect identification. We also thank Dr. W Wesemael for his support on statistical analysis. The Vlaamse Interuniversitaire Raad-University Development Co-operation (VLIR-UOS), Belgium is highly acknowledged for providing a Ph.D. scholarship to the first author.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.-U. Ehlers.
Rights and permissions
About this article
Cite this article
Khatri-Chhetri, H.B., Timsina, G.P., Manandhar, H.K. et al. Potential of Nepalese entomopathogenic nematodes as biocontrol agents against Holotrichia longipennis Blanch. (Coleoptera: Scarabaeidae). J Pest Sci 84, 457–469 (2011). https://doi.org/10.1007/s10340-011-0370-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-011-0370-5