Abstract
Synthetic polymers used in various technical applications were analyzed by asymmetric flow field flow fractionation (AF4) and size exclusion chromatography size exclusion without dash is more frequent (SEC) followed by a multi-angle light scattering (MALS) photometer and the results were compared from the view point of the resolution of the two methods and their ability to determine the molar mass distribution. The analyzed polymers covered broad molar mass range and included linear, randomly branched and star-shaped molecular topologies. Narrow polystyrene standards were used to determine the molar mass dependence of the efficiency. The number-average molar masses obtained by SEC-MALS were systematically lower compared to those from AF4-MALS with the exception of highly branched polymers, whereas the weight-averages and z-averages were well consistent except for polymers containing ultra-high molar mass fractions undergoing shear degradation in SEC columns. The number of theoretical plates in AF4 was molar mass-dependent as predicted by the theory. Using linear cross flow decay, the molar mass dependence of the AF4 selectivity showed an arc-shaped pattern. The AF4 mass recovery was close to 100% for all samples not containing molecules below the cut-off of the channel membrane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the inception of asymmetric flow field flow fractionation (AF4) by the paper by Wahlund and Giddings [1], the AF4 method has been co-existing with size exclusion chromatography (SEC). Despite several limitations described for example in reference [2], SEC has been always the dominant analytical separation method in the area of synthetic polymers soluble in organic solvents. From AF4 literature references, only a minor fraction is dedicated to the applications in the research and development of organic soluble synthetic polymers, whereas most of them are related to the characterization of proteins [3], drug-delivery particles [4], liposomes [5], viruses [6], exosomes [7], polysaccharides [8] and nanoparticles [9] in aqueous carriers. The AF4 coupled with a multi-angle light scattering (MALS) detector has been successfully used for the characterization of paint binders prepared by emulsion polymerization [10,11,12,13] that usually contain branched ultra-high molar mass species formed by the chain transfer to polymer or even crosslinked nano-gels created either by excessive chain transfer or purposefully by addition of a crosslinking agent. Other AF4 applications on technical polymers reported in the literature include narrow polystyrene standards [14], star-shaped polystyrene [15], branched poly(phenyl-acetylene) [16], hybrid polyurethane/acrylic latex [17], hyper-branched polyesters [18], polymers for pressure sensitive adhesives [19], synthetic and natural polyisoprene [20], polybutadiene [21], branched ultra-high molar mass poly-olefins and polybutadiene [22], poly-olefins [14, 23, 24], amphiphilic copolymers based on methacrylates [25], functionalized styrene–butadiene rubbers [26], single-chain nanoparticles [27], and poly(methyl methacrylate) [28]. The published results clearly indicate that AF4 can sufficiently separate synthetic polymer samples that are difficult for traditionally used SEC. The long-term experience gained in the author´s laboratory shows that SEC data can be for some polymers completely misleading since a substantial part of a polymer under investigation can be not only strongly affected by shear degradation, but even also completely retained in SEC columns. Due to recent improvement of AF4 instrumentation, one can expect organic AF4 to become more frequent technique in analytical laboratories dealing with synthetic polymers. The purpose of this study is comparison of the two separation techniques from the view point of their resolution and ability to determine the molar mass distribution of various synthetic polymers.
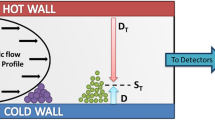
AF4 separates polymer molecules on the basis of their hydrodynamic volume as does SEC. The only difference is using the thin ribbon like channel with one semipermeable wall instead of packed SEC columns. Strictly spoken, due to the absence of stationary phase, AF4 does not belong to a family of chromatographic techniques despite similar applications and chromatographic set-ups.
Although AF4 theory in principle allows the determination of hydrodynamic radius from the retention time, this paper deals exclusively with AF4 combined with a MALS detector. The MALS detector measures molar mass that is preferred by polymer scientists over the hydrodynamic radius. In addition to the molar mass, the size information is obtained as the root mean square (RMS) radius (radius of gyration). The hydrodynamic radius can be measured directly using a MALS detector combined with dynamic light scattering. The fundamental theory of MALS and principles of determining the molar mass and RMS radius have been published many times and can be found in the literature [29, 30] as well as the theoretical background of AF4 [30,31,32] and SEC [30, 33].
Experimental
The conditions used for all AF4 experiments are described in Table 1. Note that the steps 1, 2 and 9 do not directly contribute to the separation process and are used only for the stabilization of flow conditions in the channel and facilitation of the baseline setting. The focusing time of 13 min is a compromise between proper sample focusing to a narrow line and the total run time. Note that complete focusing of high molar mass components can take tens of minutes [1]. To allow easy comparison of the results, the identical conditions were used for all polymers. Based on the author’s experience, they can be used as generic conditions for a great majority of technically important polymers of various molar mass distribution and molecular structure and thus complicated method development for the AF4 characterization of synthetic polymers is mostly unnecessary. As a rule of thumb, five to ten minutes longer steps 7 and 8 can be used for polymer samples containing substantial amount of ultra-high molar mass fractions to achieve their complete elution from the channel, and about five minutes shorter step 6 can speed sample elution. Regenerated cellulose Nadir 5 kDa cut-off membrane was used with the channel of following dimensions: 350 μm spacer, spacer tip-to-tip length = 265 mm, trapezoidal profile of 240 mm length and 22 mm and 6 mm bases.
The AF4 system consisted of an Eclipse AF4 separation module from Wyatt Technology with a 1200 Series isocratic pump and a sampler (both Agilent Technologies) followed by a MALS detector HELEOS II and a refractive index (RI) detector Optilab T-rEX (both from Wyatt Technology). The Eclipse, pump and sampler were controlled by the software VISION and the MALS data were acquired and processed by ASTRA software (both Wyatt Technology). The same chromatographic set-up was used for the SEC analyses with two Agilent PLgel Mixed-C 5 μm 300 × 7.5 mm columns with the flow rate of 1 mL min−1. The columns cover broad molar mass range from oligomers to polymers with molar masses reaching ≈ 107 g mol−1. An alternative set of two Agilent PLgel Mixed-B columns with particle size 10 μm was used for some of the polymers containing ultra-high molar mass fractions (namely samples 13–18) to reduce the possibility of shear degradation. However, minor effect on the results acquired by the two different column types was found. Stabilized tetrahydrofuran (THF) was used as the SEC eluent and AF4 carrier. The analyzed polymers were dissolved in THF in the concentration of about 2.5 mg mL−1 (≈ 5 mg mL−1 for the lowest molar masses) and filtered with 0.45 μm filters. The injected volume was 100 μL in both types of experiments. Substantially lower injected masses were used for narrow polystyrene standards to avoid overloading of the channel; specifically, from 1.1 mg mL−1 for 13,500 g mol−1 standard to 0.025 mg mL−1 for 2,875,000 g mol−1 standard with 10 μL injected volume.
Polymers presented in this study are epoxy resin (EP), polycarbonate (PC), poly(methyl methacrylate) (PMMA), polystyrene (PS), poly(butyl methacrylate) (PBMA), polybutadiene (PBD), and core–shell poly(methyl methacrylate-co-butyl acrylate) (PMMABA). They were selected to cover broad molar mass range and linear, randomly branched and star-branched topology. The samples represent typical examples for each kind of polymer and similar results have been obtained in the author’s laboratory for hundreds of other polymer samples, namely various acrylic and styrene–acrylic emulsion copolymers, polyisoprene, polybutadiene, styrene–butadiene rubber, nitrocellulose, cellulose tri-carbanilate, polyurethanes and poly(phenyl-acetylene). The polymers were obtained either as testing samples from various manufacturers or synthetized at SYNPO. One of the broad polystyrene samples was well-known NIST SRM 706a standard, narrow PS standards were purchased from Agilent Technologies.
Results and Discussion
Resolution
Figure 1 shows the separation of a mixture of six narrow PS standards. The two separation techniques allow nearly baseline separation of the standards, but AF4 needs significantly longer run time to do so. The reason for that is that the actual separation starts after focusing at 17 min and that AF4 peaks are generally broader compared to SEC counterparts due to markedly lower efficiency. The broadening of peaks and thus the efficiency of the chromatographic separation is characterized by the height equivalent to a theoretical plate (plate height). The influence of AF4 operational parameters on the plate height is defined by the following equation [34]:
where H is the non-equilibrium contribution to the plate height, which is the major contribution in AF4; D is the diffusion coefficient; w, V0 and L are the channel thickness, volume and length, respectively; and \(\dot{V}\) and \({\dot{V}}_{c}\) are volumetric detector and cross flow rates, respectively. Another parameter indicative of separation device performance is the number of theoretical plates (N), equal to the length of a separation device divided by the plate height. The diffusion coefficient is related with molar mass through a simple equation:
where KD and b are constants for a given polymer, solvent and temperature. Combination of Eqs. (1) and (2) shows that the plate height is in log–log scale indirectly proportional to the molar mass, or the number of theoretical plates is directly proportional.
The molar mass dependence of number of theoretical plates calculated from the data in Fig. 1 and three additional data points acquired with standards measured separately is for the two separation techniques contrasted in Fig. 2. The number of theoretical plates was calculated using the well-known equation:
where tR is the retention time and W1/2 is the peak width at the half height. The results, despite being affected by the dispersity (polydispersity) of standards, can be used for the comparison of the efficiency of the two techniques. In accordance with Eq. (1), the N in AF4 increases linearly with molar mass, whereas that in SEC is molar mass independent. Another conclusion clearly evident from Fig. 2 is that the efficiency of AF4 is markedly below that of SEC, especially in the region of lower molar mass.
The number of theoretical plates is one of the two major parameters affecting the resolution (RS) of two components in SEC and AF4:
where W is the peak width at baseline and S is the selectivity that can be expressed as:
Here, the absolute value accounts for the fact that retention time in SEC decreases with increasing molar mass. Note that in contrast to liquid chromatography of low molar mass compounds, in the separation of polydisperse polymers the resolution does not describe the ability to resolve two monodisperse compounds, but it is related to the local polydispersity of macromolecules eluting at a given time from the columns or channel (slice polydispersity).
The slice polydispersity in SEC is a result of band broadening primarily by eddy diffusion and mass transfer. In the case of branched polymers and copolymers, the slice polydispersity is further increased by co-elution of molecules of identical hydrodynamic volume, but different chemical composition and/or degree of branching and thus of different molar mass. The anchoring of branched macromolecules in the pores of SEC column packing represents another significant contribution to the slice polydispersity in the case of large branched macromolecules [35]. In addition to the anchoring, branching also decreases the thermodynamic quality of the solvent. Note that the second virial coefficient decreases with increasing molar mass and increasing extent of branching. Consequently, in ultimate case of highly branched macromolecules with ultra-high molar mass, the SEC eluent being for a given polymer thermodynamically good solvent may become poor solvent which increases the possibility of interactions with stationary phase. Another contribution to the SEC slice polydispersity can occur in separation of polymer samples containing fractions exceeding the exclusion limit of SEC columns. In that case, the totally excluded ultra-high molar mass species can exhibit excessive peak tailing reaching far from the exclusion limit [36]. Slalom chromatography is one more possible contribution to the slice polydispersity in SEC that happens when ultra-high molar mass polymers undergo flow-induced extension and start turning frequently around the column packing particles [37, 38]. Moreover, various enthalpic interactions between the solute and stationary phase are frequent in the case of functional polymers, polymers bearing ionic groups or polymers consisting both hydrophobic and hydrophilic parts.
In AF4, the major contribution to the slice polydispersity is the band broadening and, as in the case of SEC, the co-elution of molecules of different branching degree and/or chemical composition. In contrast to SEC, the anchoring and slalom effects are completely eliminated by the absence of stationary phase. The AF4 channel also does not have any exclusion limit as do the SEC columns. Various enthalpic interactions with the membrane are imaginable, yet much less probable than in SEC due to markedly lower membrane surface area compared to the porous SEC packing. Consequently, the resolution is governed mainly by the selectivity and efficiency without significant contribution of other non-AF4 mechanisms.
Examples of selectivity plots are depicted in Fig. 3 for linear and randomly branched PS. The values of selectivity were obtained as the slopes of short sections of the plots log(tR) versus log(M) with log(M) increments equal to 0.2. Figure 3 shows nearly molar mass independent selectivity for SEC, whereas the AF4 selectivity plots exhibit an arc-shaped pattern. The data in Figs. 2 and 3 indicate that one has to expect the lowest AF4 resolution in the region of low molar masses, whereas the decreasing selectivity at high molar masses is partly compensated by increasing number of theoretical plates. The drop of selectivity in the region of high molar masses can be counteracted by markedly longer cross-flow decay time or separation under isocratic cross-flow. Examples of such separation are shown in Fig. 4 where decay time was increased to 50 min or isocratic cross flow of 2 mL min−1 was used. However, none of these is suitable for routine measurements. Especially separation of polydisperse polymers under isocratic cross-flow is difficult or even impossible as with too low cross flows one encounters an insufficient retention of lower molar mass fractions, whereas higher cross-flow values can result in an excessive increase of retention times and strongly tailing peaks where the integration limit of especially RI detector becomes difficult to set.
Molar mass dependence of selectivity for linear PS (lin) in SEC (open circles) and AF4 (open squares), and for branched PS (br) in AF4 (open triangles) using conditions from Table 1
It may be worth noting that in contrast to SEC, where the parameters N and S are constant under giving column selection and flow rate; in AF4, similarly as in the case of gradient HPLC, there are practically indefinite variations of cross flow profiles including the steepness of the cross-flow gradient, the start and end cross-flow values, isocratic or linear or exponential cross-flow patterns or their combinations. However, as already mentioned, almost identical separation conditions often serve well even for markedly different polymers.
Molar Mass Distribution and Mass Recovery
The molecular characteristics, namely the number-average molar mass (Mn), the weight-average molar mass (Mw), and the z-average molar mass (Mz) are listed in Tables 2 and 3. The values of polydispersity and z-average RMS radii are available in Supplementary Information that also shows molar mass versus retention time plots and MALS and RI chromatograms and fractograms of all samples. Table 2 also includes mass recovery in AF4 as an important parameter that shows the fraction of sample lost through the membrane. To avoid errors arisen from possible inaccuracies of the specific refractive index increment and/or calibration constant of RI detector, the mass recovery in AF4 was calculated relative to that in SEC. The lowest sample recovery is for sample 1 (epoxy resin) that contains about 50% fractions with molar mass less than 5000 and ≈ 75% with M < 104 g mol−1. Low molar mass samples of this type are evidently not suitable for AF4 and the method might be useful only to confirm or exclude presence of high molar mass byproducts, aggregates or intentionally added nanoparticles, should they be expected to be present. Another significant sample loss can be observed for sample 2 that consists of narrow arms of M ≈ 6000 g mol−1 (44% of the entire sample), and polydisperse stars synthetized from the arms. However, a part of the arms was retained in the channel despite their molar mass on the verge of the cut-off of the channel membrane. It may be worth noting that the nominal cut-off of the membrane is fixed, but the true cut-off depends on the molecular conformation. An important finding is that for polymers not containing significant amounts of fractions with low molar mass the mass recovery is close to 100%.
By definition, the average Mn counts mainly the fractions with lower molar mass, whereas the averages Mw and especially Mz are sensitive to those with high molar mass. The Mn calculation in SEC-MALS and AF4-MALS is based on the equation:
where ci is the concentration (in g mL−1) of molecules eluting from the separation device at the ith retention time and Mi is their molar mass. Assuming the eluting fractions are strictly monodisperse, the value of Mn calculated from the above equation is correct. However, the quantity Mi is not the molar mass of absolutely monodisperse fractions, but the slice weight average Mw,i as the eluting molecules are to a certain extent polydisperse. Note that MALS measures for polydisperse polymers the weight-average molar mass. Consequently, the Mn values determined by means of SEC-MALS or AF4-MALS are always more or less overestimated because the number-average is calculated from the slice weight-averages. The overestimation increases with the increasing slice polydispersity and thus the higher AF4 Mn values can be explained by the lower resolution of AF4 in the region of low molar masses. The overestimation of Mn can be further enhanced by the loss of oligomeric fractions through the channel membrane. The exceptions are the Mn of highly branched polymers 7, 13, 14, 16, and 17 due to complete elimination of the anchoring effect. For these polymers, AF4 yields lower and obviously more correct values of Mn. For highly branched sample 7, AF4 yields markedly lower Mn despite 10% loss of low molar mass arms.
The average Mw is calculated according to equation:
In contrast to the average Mn, the weight-average calculated according to the above equation is not affected by the slice polydispersity as the weight-average calculated from the slice weight-averages is the weight-average. The AF4 averages Mw for all the analyzed polymers are higher compared to the results from SEC-MALS. Markedly higher results for samples 1 and 2 can be accounted to significant loss of lower molar mass fractions through the membrane. The AF4 results higher by a few percent can be explained by neglecting the term with the second virial coefficient A2 in the basic light scattering equation:
from which the molar mass is expressed as:
In the above equations R0,i is the Rayleigh ratio (intensity of scattered light related to the intensity of incident light and geometry of the instrument) extrapolated to zero scattering angle, ci is the concentration of eluting molecules, Mi is their molar mass, and K* is an optical constant; the subscript i indicates the ith retention time slice. Neglecting the term with A2 mostly does not create substantial errors in molar mass as the concentrations of eluting molecules in SEC are very low (in this study mostly less than ≈ 0.15 mg mL−1 at the peak apex) and A2 is often of the order of magnitude 10–4 mol mL g−2. However, since the injected mass was identical both in SEC and AF4 and the AF4 fractograms were much broader, the maximum concentration in AF4 was usually about one tenth of that in SEC which further decreased the small errors caused by neglecting the second term of Eq. (9). Additional contribution to higher Mw from AF4 may be reduction of shear degradation that is well-known to happen in SEC of samples containing fractions with molar mass is the range of several millions g mol−1 [39]. That is undoubtedly the case of samples 13, 14, 16 and 17. The shear degradation for these samples is even more obvious from the z-average molar mass that is more sensitive to the high molar mass fractions than Mw.
Molar mass distribution curves of linear PS (sample 8) and of highly branched PS (sample 16) are depicted in Figs. 5 and 6, respectively. A slight shift of the AF4 distribution of linear PS toward higher molar masses can be explained by lower resolution of AF4. The shift diminishes toward the high end of the distribution curve due to the increasing efficiency of the AF4 separation. Sample 16 is an example of polymer the molar mass distribution of which cannot be properly determined by SEC. The distribution by SEC is biased both by the anchoring in the region of lower molar masses and by the shear degradation at the high end of the distribution. Similar curves were acquired for other ultra-high molar mass samples 13, 14 and 17.
The delayed elution of branched macromolecules at the end of SEC chromatogram significantly increases the slice polydispersity as shown in Fig. 10 of reference [40]. Consequently, the plot of molar mass versus retention time shows typical upswing. One can say that the MALS detector does not see the majority of small polymer molecules because of a minor fraction of delayed macromolecules with very high molar mass. An example of the comparison of molar mass versus retention time plots acquired for a branched polymer by SEC-MALS and AF4-MALS can be found in reference [41], Fig. 4, and the relations of the RMS radius versus molar mass (conformation plots) are compared in Fig. 5 of ibidem.
Conclusion
Although AF4 allows optimization of the separation conditions by cross-flow profile, many technical polymers can be sufficiently separated by simple operational conditions outlined in this paper. With these conditions, the selectivity of AF4 shows a maximum roughly three times the selectivity of two Mixed-C 5 μm 300 × 7.5 mm columns, while in the region of low and high molar masses the selectivity of the two techniques is approximately equal. The number of theoretical plates in AF4 increases with increasing molar mass, yet even at the molar mass of several millions g mol−1 remains several times below that of SEC. With the exception of samples containing significant amounts of oligomers, the mass recovery in AF4 is close to 100%. More efficient SEC separation of smaller macromolecules results in more accurate averages Mn. The exceptions are samples containing highly branched macromolecules that exhibit delayed elution of branched species. The most powerful application of AF4 is in the characterization of polymers containing ultra-high molar mass highly branched fractions. For such polymers, SEC completely fails in the sense of improper description of low molar mass part of the distribution due to the anchoring and the high molar mass region due to the excessive shear degradation, and also in the sense of inability to yield conformation plots usable for further branching calculations. Although the main power of AF4 is in the area of polymers containing ultra-high molar mass species, branched polymers, polymers containing nanogels or nanoparticles, and polymers with strong interactions with stationary SEC phase, the method gives results well comparable to those from SEC even in the case of many “SEC easy” polymers.
References
Wahlund KG, Giddings JC (1987) Properties of an asymmetrical flow field-flow fractionation channel having one permeable wall. Anal Chem 59:1332–1339
Berek D (2010) Size exclusion chromatography—a blessing and a curse of science and technology of synthetic polymers. J Sep Sci 33:315–335
Wahlund P-O, Lorenzen N, Rischel C (2021) Screening for protein–protein interactions with asymmetrical flow field-flow fractionation. J Pharm Sci 110:2336–2339
Wang Y, Yan L, He S, Zhou D, Cheng Y, Chen X, Jing X, Huang Y (2018) A versatile method to prepare protein nanoclusters for drug delivery. Macromol Biosci 18:1700282
Evjen TJ, Hupfeld S, Barnert S, Fossheim S, Schubert R, Brandl M (2013) Physicochemical characterization of liposomes after ultrasound exposure—mechanisms of drug release. J Pharm Biomed Anal 78–79:118–122
Bousse T, Shore DA, Goldsmith CS, Hossain MJ, Jang Y, Davis CT, Donis RO, Stevens J (2013) Quantitation of influenza virus using feld flow fractionation and multi-angle light scattering for quantifying influenza A particles. J Vir Met 193:589–596
Zhang H, Lyden D (2019) Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat Protoc 14:1027–1053
Nomura K, Sakai M, Ohboshi H, Nakamura A (2021) Extraction of a water-soluble polysaccharide fraction from lentils and its potential application in acidified protein dispersions. Food Hydrocoll 117:106740
Caputo F, Clogston J, Calzolai L, Roesslein M, Prina-Mello A (2019) Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J Control Release 299:31–43
Makan AC, Williams RP, Pasch H (2016) Field flow fractionation for the size, molar mass, and gel content analysis of emulsion polymers for water-based coatings. Macromol Chem Phys 217:2027–2040
Podzimek S, Machotova J, Snuparek J, Vecera M, Prokupek L (2014) Characterization of molecular structure of acrylic copolymers prepared via emulsion polymerization using A4F-MALS technique. J Appl Polym Sci 131:40995
Machotova J, Zgoni H, Podzimek S, Stranska E, Pukova K (2016) Characterization of molecular structure of emulsion acrylic microgels: exploring the impact of molar mass on coating properties of self-cross-linkable latexes. Polym-Plast Technol Eng 55:1807–1818
Podzimek S, Machotova J, Zgoni H, Bohacik P, Snuparek J (2016) Application of A4F-MALS for the characterization of polymers prepared by emulsion polymerization: comparison of the molecular structure of styrene-acrylate and methyl methacrylate-acrylate copolymers. Polym-Plast Technol Eng 55:1365–1372
Otte T, Bruell R, Macko T, Pasch H, Klein T (2010) Optimisation of ambient and high temperature asymmetric flow field-flow fractionation with dual/multi-angle light scattering and infrared/refractive index detection. J Chromatogr A 1217:722–730
Pitkaenen L, Striegel AM (2014) AF4/MALS/QELS/DRI characterization of regular star polymers and their “span analogs.” Analyst 139:5843–5851
Angoy M, Bartolome MI, Vispe E, Lebeda P, Jimenez MV, Perez-Torrente JJ, Collins S, Podzimek S (2010) Branched poly(phenylacetylene). Macromolecules 43:6278–6283
Mehravar S, Ballard N, Agirre A, Tomovska R, Asua JM (2017) Relating polymer microstructure to adhesive performance in blends of hybrid polyurethane/acrylic latexes. Eur Polym J 87:300–307
Erber M, Boye S, Hartmann T, Voit BI, Lederer A (2009) A convenient room temperature polycondensation toward hyperbranched AB2-type all-aromatic polyesters with phenol terminal groups. J Polym Sci Part A Polym Chem 47:5158–5168
Daniloska V, Carretero P, Tomovska R, Asua JM (2014) High performance pressure sensitive adhesives by miniemulsion photopolymerization in a continuous tubular reactor. Polymer 55:5050–5056
Dubascouxa S, Thepchalerma C, Dubreucqa E, Wisunthornc S, Vayssed L, Kiatkamjornwonge S, Nakasonb C, Bonfils F (2012) Comparative study of the mesostructure of natural and synthetic polyisoprene by size exclusion chromatography-multi-angle light scattering and asymmetrical flow field flow fractionation-multi-angle light scattering. J Chromatogr 1224:27–34
Makan AC, Otte T, Pasch H (2012) Analysis of high molar mass branched polybutadienes by SEC-MALLS and AF4-MALLS. Macromolecules 45:5247–5259
Otte T, Pasch H, Macko T, Bruell R, Stadler FJ, Kaschta J, Becker F, Buback M (2011) Characterization of branched ultrahigh molar mass polymers by asymmetrical flow field-flow fractionation and size exclusion chromatography. J Chromatogr A 1218:4257–4267
Mes EPC, de Jonge H, Klein T, Welz RR, Gillespie DT (2007) Characterization of high molecular weight polyethylenes using high temperature asymmetrical flow field-flow fractionation with on-line infrared, light scattering, and viscometry detection. J Chromatogr A 1154:319–330
Otte T, Pasch H, Bruell R, Macko T (2011) Visualization of thermo-oxidative degradation of polyolefins in solution using HT-SEC and HT-AF4-MALS. Macromol Chem Phys 212:401–410
Strehmel V, Wetzel H, Laschewsky A, Moldenhauer E, Klein T (2008) Influence of imidazolium-based ionic liquids on the synthesis of amphiphilic copolymers based on n-butylmethacrylate and a zwitterionic methacrylate. Polym Adv Technol 19:1383–1390
Bang DY, Shin DY, Lee S, Moon MH (2007) Characterization of functionalized styrene–butadiene rubber by flow field-flow fractionation/light scattering in organic solvent. J Chromatogr A 1147:200–205
Engelke J, Boye S, Tuten BT, Barner L, Barner-Kowollik C, Lederer A (2020) Critical assessment of the application of multidetection SEC and AF4 for the separation of single-chain nanoparticles. ACS Macro Lett 9:1569–1575
Zanelle V, Pasch H (2021) Variable temperature asymmetric flow field-flow fractionation for the topology separation of poly(methyl methacrylate). Anal Chim Acta 1144:150–157
Wyatt PJ (1993) Light scattering and the absolute characterization of macromolecules. Anal Chim Acta 272:1–40
Podzimek S (2011) Light scattering, size exclusion chromatography and asymmetric flow field flow fractionation. Wiley
Schimpf M, Caldwell K, Giddings JC (eds) (2000) Field-flow fractionation handbook. Wiley, London
Podzimek S (2012) Asymmetric flow field flow fractionation. Encycl Anal Chem. https://doi.org/10.1002/9780470027318.a9289
Striegel AM, Yau WW, Kirkland JJ, Bly DB (eds) (2009) Modern size-exclusion chromatography. Wiley, London
Wahlund K-G, Winegarner HS, Caldwell KD, Giddings JC (1986) Improved flow field-flow fractionation system applied to water soluble polymers: programming, outlet stream splitting, and flow optimization. Anal Chem 58:573–578
Podzimek S, Vlcek T, Johann C (2001) Characterization of branched polymers by size exclusion chromatography coupled with multiangle light scattering detector. I. Size exclusion chromatography elution behavior of branched polymers. J Appl Polym Sci 81:1588–1594
Busnela JP, Foucault F, Denis L, Lee W, Chang T (2001) Investigation and interpretation of band broadening in size exclusion chromatography. J Chromatogr A 930:61–71
Liu Y, Radke W, Pasch H (2005) Coil-stretch transition of high molar mass polymers in packed-column hydrodynamic chromatography. Macromolecules 38:7476–7484
Liu Y, Radke W, Pasch H (2006) Onset of the chromatographic mode transition from hydrodynamic chromatography to slalom chromatography: an effect of polymer stretching. Macromolecules 39:2004–2006
Barth HG, Carlin FJ Jr (1984) A review of polymer shear degradation in size-exclusion chromatography. J Liq Chromatogr 7:1717–1738
Podzimek S (2019) Molar mass distribution by size exclusion chromatography: comparison of multi-angle light scattering and universal calibration. J Appl Polym Sci 136:47561
Podzimek S (2019) Multi-angle light scattering: an efficient tool revealing molecular structure of synthetic polymers. Macromol Symp 384:1800174
Funding
The author declares that no funds, or other support were received during the study and preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The manuscript has been completely written by the author and all the data used in the manuscript were collected by the author.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Podzimek, S. Comparative Study of SEC and AF4 in the Characterization of Organic Soluble Synthetic Polymers. Chromatographia 85, 1097–1105 (2022). https://doi.org/10.1007/s10337-022-04217-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-022-04217-0