Abstract
Synthetic polymers have complex molecular structures with distributions in molar mass, chemical composition, functionality and molecular topology. For the comprehensive analysis of polymer structures, a number of advanced spectroscopic and fractionation techniques are used. For the fractionation of polymers, most frequently column-based methods are applied. These are of limited value for the separation of very high molar mass and fragile analytes. Thermal field-flow fractionation (ThFFF) as a channel-based method has developed into a powerful alternative and complementary technique to column-based fractionations. This perspective discusses novel applications of ThFFF and highlights its potential for the fractionation of polymer assemblies such as micelles, vesicles and nanogels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Intrinsically, synthetic polymers are complex multicomponent mixtures that exhibit various distributions in molecular properties, including molecular size (molar mass), chemical composition, molecular topology (tacticity, branching) and functionality. To address the different property distributions, sophisticated fractionation methods have been developed over the last 6 decades. Typically, fractionations using column-based experimental protocols are conducted with size-exclusion chromatography (SEC) being the preferred tool for molar mass analysis. Chemical composition and topology are addressed by various methods of interaction chromatography using solvent and temperature gradients. These methods have a number of limitations, in particular for very high molar mass and fragile analytes.
As an alternative to column-based fractionations, channel-based fractionations have been introduced mainly by Giddings and his students where separations are achieved in empty channels by applying external fields [1]. Different fractionation results are obtained depending on the applied external field and the underlying physical phenomena. For example, asymmetric flow field-flow fractionation (AF4) uses a solvent cross flow and the separation is based on the differences in translational diffusion coefficients of different species, which are a function of molecular size/molar mass. In contrast, ThFFF uses thermal gradients and the separation is based on the interplay of translational and thermal diffusion [1, 2].
Thermal diffusion is strongly influenced by the chemical composition of the analyte and, hence, can be used to fractionate chemically different species. In combination with advanced detectors, quantitative molecular information on size/molar mass, chemical composition and molecular topology can be obtained for a variety of different polymer structures and polymer assemblies such as block copolymer micelles, vesicles and nanogels. Finally, ThFFF can be used as one dimension in multidimensional fractionation setups.
A schematic presentation of the ThFFF process is presented in Fig. 1. Similar to all other FFF techniques, ThFFF has an open-channel design that does not contain any stationary phase. The separation takes place in a single liquid phase (mobile phase). The absence of a stationary phase allows ThFFF to characterize a wide variety of macromolecules and particles ranging from a few nanometers up to micrometers in size with high resolution. For ThFFF fractionation, a temperature gradient is applied as the external field by heating one of the FFF channel walls while cooling the other. When analytes are subjected to such temperature gradients they migrate from the hot wall towards the cold wall (accumulation wall) under the process of thermal diffusion. This thermal diffusion is characterized by the thermal diffusion coefficient, DT. As a result of the concentration build-up at the cold wall, the analytes migrate back towards the center of the channel under the process of ‘normal’ or Brownian diffusion, which is characterized by the (translational) diffusion coefficient, D. The interplay between DT and D (known as the Soret coefficient, ST) is responsible for the differential placement of analytes in the parabolic flow velocity profile and thus differential elution from the channel. The parabolic flow profile shown in Fig. 1 is an idealized presentation. It has been shown by Geisler and Lederer that in reality the profile is highly distorted from an ideal parabola [17]. The remarkable characteristic of ThFFF is that retention depends not only on molar mass (through its influence on size and thus D) but, more importantly, also on the chemical composition of the polymer. The sensitivity towards composition is due to the fundamental dependence of thermal diffusion on the composition of both the polymer and the carrier liquid.
Scheme of the ThFFF channel and the fractionation mechanism. Thermal diffusion, DT drives polymers towards the accumulation wall while normal diffusion, D causes migration away from the wall. The Soret coefficient, ST determines the position in the velocity profile and the elution from channel [2].
ThFFF was introduced not much later than SEC but it was not able to develop into a practical laboratory instrument as quickly as SEC and, as a result, was slowly forgotten by the polymer community. The slow development of ThFFF was mainly due to the incomplete understanding of thermal diffusion that still limits quantitative predictions of the retention characteristics of complex polymers. In recent years, ThFFF has started to gain considerable attention for its potential to separate analytes (mostly copolymers) based on composition [3,4,5]. This can be attributed to a combination of three main factors, namely (1) the continuous improvement in ThFFF instrumentation and methodology, (2) the increasing need for new analytical techniques for increasingly complex polymeric materials, and (3) the increasing demand for characterization platforms that can address very high molar mass polymers, polymer aggregates and polymer assemblies, analytes susceptible to shear degradation, and polymer samples with complex branching distributions [6].
Regarding molar mass analysis, the fractionation power of SEC and ThFFF is comparable in the range of 104–105 g/mol while above a molar mass of 105 g/mol, the fractionating power of ThFFF is generally several times higher than that of SEC. Above a molar mass of 106 g/mol, SEC becomes increasingly limited by possible shear degradation whereas ThFFF is capable of separating ultrahigh molar mass polymers without shear degradation. On a larger size scale, gels and particles that tend to block SEC columns can readily be separated by ThFFF with high resolution [2]. One has to keep in mind that SEC separates according to hydrodynamic size in solution. Accordingly, in selected cases such as branched and dendritic polymers that exhibit very compact molecular structures and, thus, small hydrodynamic sizes, SEC separation can be achieved even at very high molar masses.
When it comes to chemical composition fractionation, one might compare ThFFF and solvent/temperature gradient interaction chromatography (IC). There is no doubt that IC is far superior for the chemical composition fractionation of single macromolecules having medium molar masses. If the analyte, however, is a polymer assembly, polymer aggregate or nano-/microgel then in most cases ThFFF is the better choice. First of all, IC is limited with regard to molar mass which is not the case for ThFFF. More importantly, IC like all other liquid chromatography methods requires filtration to prevent undesired precipitation of sample components on the stationary phase. Typically, in the filtration step larger molecules and aggregates are removed and fragile analytes such as micelles, vesicles and other polymer assemblies are destroyed. For such analytes, IC is suitable to a very limited extent.
ThFFF does not have such limitations. A filtration step prior to fractionation is not required and larger molecular species can be injected directly into the channel. ThFFF is a very gentle fractionation technique and (provided the analyte has a certain thermal stability) polymer assemblies, aggregates and other large entities can be fractionated without degradation.
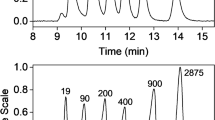
The following few examples shall serve to illustrate the progress and the future perspectives of ThFFF. It has been demonstrated in the past that ThFFF is a useful method for chemical composition fractionation of copolymers [3,4,5,6]. More recently, ThFFF was shown by Greyling et al. to be suitable for microstructure characterization by fractionating 1,4- and 1,2-polybutadiene isomers and 1,4- and 3,4-polyisoprene isomers according to microstructure while syndiotactic and isotactic PMMA were separated according to tacticity, see Fig. 2 [7, 8]. Poly(n-butyl methacrylate) and poly(t-butyl methacrylate) were separated based on topology [9]. This was a significant advancement as traditional column-based techniques, such as SEC, are not suitable for this type of microstructure-based separation.
ThFFF fractionation of polyisoprene and polybutadiene with regard to microstructure [7].
Another recent advancement has been seen with ThFFF being applied to polymeric self-assemblies, such as micelles. Current techniques are not suitable to provide comprehensive information regarding size, molar mass, chemical composition and micelle stability in different environments and it was shown that ThFFF is an excellent technique for this purpose [10, 11]. Among other studies, it was proven by the analysis of micelles with various corona compositions that ThFFF is capable of separating micelles according to corona composition irrespective of size while providing comprehensive information on important micelle characteristics such as size, molar mass, chemical composition and their respective distributions from a single analysis. Moreover, it was shown that ThFFF is currently the only suitable technique to monitor the dynamics of mixed micelle formation in terms of size, molar mass and chemical composition [10]. In addition to corona composition, ThFFF was also demonstrated to be a suitable technique to separate self-assemblies based on morphology. In this case, vesicle, worm and jelly fish morphologies were successfully separated by ThFFF [12], see Fig. 3.

Copyright 2017, adapted from [12] with permission of American Chemical Society
ThFFF fractionation of polystyrene-poly(ethylene oxide) block copolymer assemblies.
An important feature of advanced ThFFF is the fact that the ThFFF channel, very similar to liquid chromatographic columns, can be coupled to multiple information-rich detectors and even a second fractionation device. This was convincingly demonstrated by Muza et al. who coupled ThFFF with five different detectors to obtain quantitative information on the most important molecular parameters [13], see Fig. 4. The online coupling of ThFFF to NMR and FTIR spectroscopy has been presented by Hiller et al. [14] and Radebe et al. [15], respectively.
Schematic presentation of a ThFFF system equipped with five information-rich detectors for the fractionation and analysis of complex polymers [13].
The latest development in multidimensional ThFFF is the comprehensive coupling of ThFFF and SEC developed by Viktor et al. [16]. As complex block copolymers are distributed regarding chemical composition and molar mass, at least two (orthogonal) fractionation techniques must be used to obtain the full picture of molecular heterogeneity. It has been shown by the authors that THFFF as the first dimension in the two-dimensional setup (being sensitive to chemical composition and molecular size) provides information on the chemically different species while SEC as the second dimension provides quantitative molar mass information, see Fig. 5. Chemical composition information can be obtained when a dual-detector system (e.g., ELSD–UV) is used.
Schematic presentation of the comprehensive two-dimensional ThFFF–SEC coupling for the analysis of complex polymers [16].
To summarize, over the last 10 years, ThFFF has developed into an important advanced fractionation technique for complex polymers and polymer assemblies. In its own right, it provides unique separation capabilities for very high molar mass samples, samples with complex molecular topologies and fragile analytes such as micelles, vesicles and other polymer assemblies. At the same time, it can be used as a complementary technique for column-based (SEC, IC) fractionations due to the unique cooperative effects of thermal and translational diffusion that may provide dual information on chemical composition and size. The coupling of ThFFF with column-based fractionations or sets of information-rich detectors promises to be one of the important directions in the further development of advanced analytical methods for complex polymers.
Change history
31 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10337-021-04055-6
References
Schimpf ME, Caldwell K, Giddings JC (2000) Field-flow fractionation handbook. Wiley, New York
Greyling G, Pasch H (2019) Thermal field-flow fractionation of polymers. Springer, Cham (ISBN: 978-3-030-10649-2)
Ponyik C, Wu D, Williams SK (2013) Separation and composition distribution determination of triblock copolymers by thermal field-flow fractionation. Anal Bioanal Chem 405:9033–9040
Runyon JR, Williams SKR (2011) Composition and molecular weight analysis of styrene-acrylic copolymers using thermal field-flow fractionation. J Chromatogr A 1218:6774–6779
Runyon JR, Williams SKR (2011) Characterization of complex polymers using thermal field-flow fractionation coupled with online multiangle and dynamic light scattering and differential refractive index detection. Polym Prepr (Am Chem Soc, Div Polym Chem) 52:230–231
Malik MI, Pasch H (2016) Field-flow fractionation: new and exciting perspectives in polymer analysis. Progr Polym Sci 63:42–85. https://doi.org/10.1016/j.progpolymsci.2016.03.004
Greyling G, Pasch H (2014) Multidetector thermal field-flow fractionation as a novel tool for the microstructure separation of polyisoprene and polybutadiene. Macromol Rapid Commun 35:1846–1851. https://doi.org/10.1002/marc.201400405
Greyling G, Pasch H (2015) Tacticity separation of poly(methyl methacrylate) by multidetector thermal field-flow fractionation. Anal Chem 87:3011–3018. https://doi.org/10.1021/ac504651p
Greyling G, Pasch H (2015) Fractionation of poly(butyl methacrylate) by molecular topology using multidetector thermal field-flow fractionation. Macromol Rapid Commun 36:2143–2148. https://doi.org/10.1002/marc.201500429
Greyling G, Pasch H (2016) Multidetector thermal field-flow fractionation: a unique tool for monitoring the structure and dynamics of block copolymer micelles. Macromolecules 49:1882–1889. https://doi.org/10.1021/acs.macromol.5b02634
Greyling G, Pasch H (2015) Multidetector thermal field-flow fractionation as a unique tool for the tacticity-based separation of poly(methyl methacrylate)-polystyrene block copolymer micelles. J Chromatogr A 1414:163–172. https://doi.org/10.1016/j.chroma.2015.08.023
Muza UL, Greyling G, Pasch H (2017) Characterization of complex polymer self-assemblies and large aggregates by multidetector thermal field-flow fractionation. Anal Chem 89:7216–7224. https://doi.org/10.1021/acs.analchem.7b01445
Muza UL, Pasch H (2019) Thermal field-flow fractionation with quintuple detection for the comprehensive analysis of complex polymers. Anal Chem 91:6926–6933. https://doi.org/10.1021/acs.analchem.9b01384
Hiller W, van Aswegen W, Hehn M, Pasch H (2013) Online ThFFF-NMR: a novel tool for molar mass and chemical composition analysis of complex macromolecules. Macromolecules 46:2544–2552. https://doi.org/10.1021/ma400350y
Radebe NW, Beskers T, Greyling G, Pasch H (2019) Online coupling of thermal field-flow fractionation and FTIR as a new tool for polymer characterization. J Chromatogr A 1587:180–188. https://doi.org/10.1016/j.chroma.2018.12.012
Viktor Z, Pasch H (2020) Two-dimensional fractionation of complex polymers by comprehensive online-coupled thermal field-flow fractionation and size exclusion chromatography. Anal Chim Acta 1107:225–232. https://doi.org/10.1016/j.aca.2020.02.033
Geisler M, Lederer A (2020) Non-parabolicity correction for fifty-nine solvents and a retention study for strongly distorted flow-profiles in thermal field-flow fractionation. J Chromatogr A 1621:461082. https://doi.org/10.1016/j.chroma.2020.461082
Funding
There is no funding to report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to report.
Ethical approval
This article does not contain any studies with animals or human participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pasch, H. Thermal Field-Flow Fractionation as a Powerful Tool for the Fractionation of Complex Synthetic Polymers: A Perspective. Chromatographia 84, 525–530 (2021). https://doi.org/10.1007/s10337-021-04036-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-021-04036-9