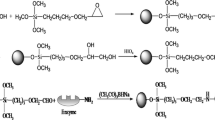
Abstract
In this study, an online capillary electrophoresis-based immobilized enzyme microreactor (CE-IMER) was developed for evaluating inhibitory activity of small-molecule compounds and tea polyphenol extracts on kallikrein (KLK). KLK was immobilized on the inner wall at the inlet of capillary to prepare the KLK-IMER with the aid of chitosan and glutaraldehyde. The immobilized KLK activity and other kinetic parameters were evaluated by measuring the peak area of hydrolysate of chromogenic substrate S-2302 (H-D-Pro-Phe-Arg-pNA). The Michaelis–Menten constant (Km) was determined to be 1.82 mM, and the half-maximal inhibitory concentration (IC50) and inhibition constant (Ki) of nicotinamide (positive control drug) were measured to be 12.07 and 4.31 mM, respectively. The negative control drug tinidazole (5.00 mM) had no inhibitory effect on KLK. Moreover, the activity of the immobilized KLK remained approximately 80.0% of the initial immobilized enzyme activity after 30 runs. The CE-IMER was applied to investigate the inhibitory activity of 10 small-molecule compounds and six tea polyphenol extracts on KLK. The results show that four small-molecule compounds (epicatechin gallate, epigallocatechin, epicatechin and isochlorogenic acid C) (0.25 mM) and six polyphenol extracts (Raw Pu-erh, Mingqian Mao Feng, Fuding white tea, Dark green tea, Biluochun and Black tea) (0.25 mg mL−1) have high inhibitory activity on KLK. In addition, these four small-molecule compounds have binding energies below − 5.0 kcal mol−1 on KLK according to the molecular docking. In short, this study reports a time saving method (0.75 h) for KLK immobilization under mild conditions (25 °C).
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plasma kallikrein (KLK), which is the key enzyme of kallikrein-kinin system [1], plays an important role in inducing endogenous and exogenous coagulation pathway [2, 3]. Inhibition of KLK activity can effectively prevent the clotting reaction caused by the activation of “plasma contact system”, thereby reducing the formation of thrombus in a certain extent. In reality, KLK inhibitors have been developed for the treatment of several diseases, such as hereditary angioedema, microvascular complications of diabetes mellitus and cerebrovascular diseases [4]. Therefore, evaluating the inhibitory effect of small-molecule compounds on KLK is of significant importance for the related drug discovery.
The spectrophotometric, electrochemical and fluorescent methods are simple and easy to perform continuous monitoring of the enzymatic reaction, but they can only be used in offline analysis and would consume large amount of enzyme and substrate. Furthermore, these methods require the substrate and the product of enzymatic reaction have significant difference in spectral properties or possess electrochemical activity [5]. Moreover, the fluorescent signal is susceptible to be interfered by autofluorescence and background absorption of endogenous substances [6]. Alternatively, capillary electrophoresis (CE) has been widely employed in enzyme kinetics assay and inhibitor screening [7], due to its high separation efficiency, short analysis time, low sample consumption, as well as facilitating online enzyme analysis [8]. In general, online enzyme analysis in CE can be divided into homogeneous and heterogeneous assays. The homogeneous assay includes electrophoretically mediated microanalysis (EMMA) and transverse diffusion of laminar flow profiles (TDLFP) strategies. In heterogeneous assay, immobilized enzyme microreactor (IMER) is prepared by immobilizing the enzyme in the capillary wall [9]. CE-IMER, which has many advantages such as improving the stability of enzyme, reducing the cost of enzyme and reusing enzyme without any complicated purification process, has attracted extensive attentions in various fields [10]. The immobilization methods of enzyme in IMER mainly include physical, chemical and affinity binding methods [11]. There were several studies about CE-IMER reported for various research purposes. For example, thrombin IMER and trypsin IMER were constructed by physical adsorption to screening their inhibitors [12, 13]. A cathepsin B IMER was developed via glutaraldehyde method to discover inhibitors in traditional Chinese medicines [14]. An acetylcholinesterase IMER, which was applied in on-line protein digestion, was fabricated using a single-step in situ biphasic sol gel reaction to produce the porous layer followed by aldehyde activation to covalently immobilize the enzymes [15]. Different immobilization methods have their advantages and disadvantages, i.e. the physical methods are simpler but less stable than chemical methods. In addition, there are no reports about evaluation of inhibitory activity of small-molecule compounds on KLK using CE-IMER method.
The aim of this study was to develop a KLK-IMER in capillary for evaluating the inhibitory activity of small-molecule compounds and tea extracts on KLK. The IMER was constructed by covalent crosslinking between chitosan, glutaraldehyde, and enzyme. This is a simple and fast process (0.75 h) for enzyme immobilization under mild conditions (25 °C). The half-maximal inhibitory concentration (IC50) of nicotinamide on KLK and Michaelis–Menten constant (Km) of immobilized KLK using S-2302 (H-D-Pro-Phe-Arg-pNA) as substrate were determined. Meanwhile, the inhibitory activities of ten small-molecule compounds (epicatechin gallate, epigallocatechin, epicatechin, isochlorogenic acid C, epigallocatechin gallate, ferulic acid, gallic acid, caffeic acid, chlorogenic acid and vanillic acid) and six tea polyphenol extracts (Raw Pu-erh, Mingqian Mao Feng, Fuding white tea, Dark green tea, Biluochun and Black tea) on KLK were studied. This is the first report for screening of KLK inhibitors based on CE-IMER assay. Finally, molecular docking was performed to predict the binding sites and binding energy of epicatechin gallate, epigallocatechin, epicatechin and isochlorogenic acid C with KLK.
Materials and Methods
Chemicals and Materials
Kallikrein (EC 3.4.21.34), nicotinamide and tinidazole were purchased from Shanghai Yuanye Biological Technology Co., Ltd. (Shanghai, China). Chromogenic substrate of S-2302 was purchased from Boatman Biotech Co., Ltd. (Shanghai, China). Epicatechin gallate, epigallocatechin, epicatechin, epigallocatechin gallate, ferulic acid and vanillic acid were purchased from Chengdu Biopurify Phytochemicals Co., Ltd. (Chengdu, China). Isochlorogenic acid C and chlorogenic acid were purchased from Chengdu PUSH Bio-Technology Co., Ltd. (Chengdu, China). Gallic acid and caffeic acid were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Sodium hydroxide (NaOH), sodium chloride, sodium dihydrogen phosphate, chitosan, glutaraldehyde, glacial acetic acid and dichloromethane were of analytical reagent grade and purchased from Chengdu Chron Chemicals Co., Ltd. (Chengdu, China). Mingqian Maofeng and Biluochun were purchased from Chongqing Changcheng Tea Co., Ltd. (Chongqing, China). Raw Pu-erh tea was purchased from Yunnan Xiaguan Tuocha Co., Ltd. (Yunnan, China). Fuding white tea was purchased from Fujian White Tea Co., Ltd. (Fujian, China). Black tea was purchased from Fujian Wuyishan Hongfang Industry Co., Ltd. (Fujian, China), and dark green tea was purchased from Hunan Baishaxi Tea Industry Co., Ltd. (Hunan, China). All the samples were deposited at the Pharmaceutical Engineering Laboratory in School of Chemistry and Chemical Engineering, Chongqing University, Chongqing, China.
Apparatus and Instruments
The CE analysis was performed on an Agilent 7100 CE system (Agilent Technologies, Palo Alto, CA, USA), equipped with a diode array detector and Agilent ChemStation software. The bare fused-silica capillary of 75.0 μm id and 42.2 cm total length with 34.0 cm effective length was purchased from Ruifeng Chromatographic Device Co., Ltd., (Hebei, China). The rotary evaporator (RE-52AA) was purchased from Shanghai Yarong Biochemical Instrument Factory (Shanghai, China). The pH of running buffer was measured using a FE28 pH meter (Mettler-Toledo Instruments, Shanghai, China). The solution was degassed by an ultrasonic cleaner (Kunshan Jielimei Ultrasonic Instrument Co., Ltd., Jiangsu, China).
Preparation of Buffer and Sample Solutions
The phosphate buffer (5.00 mM, pH 7.5) was prepared by dissolving sodium dihydrogen phosphate in deionized water and the pH was adjusted by 1.00 M NaOH. Acetic acid was diluted by deionized water to prepare its 0.10 M solution. Glutaraldehyde (50%) was diluted by deionized water to prepare its 12.5% (v/v) solution. Chitosan was dissolved in 0.10 M acetic acid solution to prepare its 0.5% (w/v) solution. The KLK solution with the concentration of 30 U mL−1 was prepared in the phosphate buffer (5.00 mM, pH 7.5) and was stored at − 20 °C before use. The 2.00 mg mL−1 S-2302 (3.27 mM) was prepared by dissolving it in the phosphate buffer (5.00 mM, pH 7.5). The solutions (0.25 mM) of epicatechin gallate, epigallocatechin, epicatechin, isochlorogenic acid C, epigallocatechin gallate, ferulic acid, gallic acid, caffeic acid, chlorogenic acid and vanillic acid were prepared by dissolving them in the phosphate buffer (5.00 mM, pH 7.5). Different concentrations of nicotinamide (2.50, 5.00, 10.00, 15.00, 17.50 and 20.00 mM) were prepared in the phosphate buffer (5.00 mM, pH 7.5).
The extraction of tea polyphenols was according to the reported method [16]. In brief, 50 mL of 70% methanol and 2.00 g dried tea powder were added into a 100-mL flask, and was reflux extracted for 2.0 h in a 70 °C water bath. Then, the supernatant solution was collected and equal volume of dichloromethane (to remove the caffeine) was added. After filtration, the collected supernatant was mixed with equal volume of ethyl acetate. Then, the mixture was evaporated by a vacuum rotary evaporator (RE-52AA) at 50 °C to remove ethyl acetate. Finally, the remaining solution was dried by a vacuum dryer at 45 °C. The crude extracts from Raw Pu-erh, Mingqian Mao Feng, Fuding white tea, Dark green tea, Biluochun and Black tea were dissolved in the phosphate buffer (5.00 mM, pH 7.5) with a concentration of about 0.25 mg mL−1 before tests, respectively.
Preparation of KLK-IMER
A new bare fused-silica capillary with a total length of 42.2 cm was flushed with methanol and deionized water for 30 and 15 min, respectively. The preparation process of KLK-IMER was schematically shown in Fig. 1. Firstly, chitosan (0.5%, w/v) was injected into the capillary with a pressure of 50 mbar for 15 s and was remained for 10 min. Then, the capillary was flushed by phosphate buffer with a negative pressure of 100 mbar for 180 s to flush out chitosan that was not adhered to the inner wall of the capillary. Secondly, glutaraldehyde (12.5%, v/v) was injected into the capillary with a pressure of 50 mbar for 15 s and was remained for 15 min. Then, the capillary was flushed by phosphate buffer with a negative pressure of 100 mbar for 90 s to flush out the unreacted glutaraldehyde. Finally, the solution of KLK (30 U mL−1) was introduced into the capillary with a pressure of 50 mbar for 15 s and was remained for 20 min. Then, the capillary was flushed by phosphate buffer with a negative pressure of 100 mbar for 90 s to flush out the free KLK. The prepared IMER was kept in 5.00 mM phosphate buffer of pH 7.5 at 4 °C before analysis.
Procedure for KLK-IMER Assay
The KLK-IMER capillary was rinsed with phosphate buffer (5.00 mM, pH 7.5) for 150 s, and 2.00 mg mL−1 substrate (3.27 mM) was introduced into the capillary with a pressure of 50 mbar for 15 s and incubated for 120 s. Then, the unreacted substrate (S-2302) and product (p-nitroaniline, pNA) were separated at a voltage of 20 kV. The activity of KLK was determined by measuring the peak area of product (pNA) at 405 nm. The capillary was flushed with phosphate buffer (5.00 mM, pH 7.5) for 120 s between successive assays.
Kinetics Study of KLK
The Km is an important parameter of the enzyme kinetic reaction, which can be calculated by the Lineweaver–Burk equation:
where V and Vmax are the initial and maximum rate of the enzyme reaction, respectively. [S] is the concentration of substrate.
Different concentrations of substrate (S-2302) varied from 0.50 to 3.00 mg mL−1 (0.82–4.91 mM) were injected into the KLK-IMER for the enzyme assays (n = 3) and the peak area of product (pNA) was used to express the initial reaction velocity (V). From the Lineweaver–Burk plots, the Km can be calculated by the slope and the intercept of the equation.
Inhibition Study
Nicotinamide is an inhibitor of KLK [17], which was used as positive control drug in this study. Different concentrations of nicotinamide (2.50, 5.00, 10.00, 15.00, 17.50 and 20.00 mM) were tested in triplicates. The inhibitory activity of negative control drug tinidazole on KLK was tested at the concentration of 5.00 mM. The % of inhibition can be calculated by:
where I (%) represents the % of inhibition, Ai and A0 represent the peak area of the enzymatic reaction product (pNA) with the presence or absence of inhibitors, respectively. IC50 was obtained from dose–response nonlinear regression equation, which can be fitted in Origin pro 8.0 software and inhibition constant (Ki) can be obtained by Cheng–Prusoff equation [18]:
Inhibitor Screening Assay by KLK-IMER
The performance of the assay was evaluated by Z′ factor, which was calculated by Eq. (4) [19]:
where qs and qc represent the peak area when there was no inhibitor and the % of inhibition was 100%, respectively. δs and δc are the standard deviations of the data. When the % of inhibition was 100%, qc and δc were both 0. Therefore, Eq. (4) can be simplified to Eq. (5):
Molecular Docking Study
The molecular docking was performed to investigate the binding modes of inhibitors when acting with KLK. The crystal structure of KLK was acquired from the Protein Data Bank (PDB: 2PKA). Before docking, all the water molecules were removed and the hydrogen atoms were added. The 3D structures of four small-molecule compounds were plotted and the energy minimization was carried out in ChemDraw (Cambridge Soft, USA). The optimal conformation after docking was presented in Discovery Studio to observe the interaction of enzyme and small-molecule compounds.
Results and Discussion
Characterization of KLK-IMER
The surface morphology of KLK-IMER was characterized by scanning electron microscopy (SEM) and the results were shown in Fig. S1. The inner wall of the bare capillary is very smooth (Fig. S1A), and there are some small particles on the inner surface of the capillary after enzyme immobilization (Fig. S1B). These results indicate that KLK was successfully immobilized on the inner wall of the capillary.
Optimization of the Immobilized KLK Microreactor
Both the pH and incubation time play important roles in the enzymatic reaction. The effects of phosphate buffer pH (6.5, 7.0, 7.5, 8.0 and 8.5) and incubation time (60, 90, 120, 150 and 180 s) on the enzyme activity were investigated (the cassette temperature was set at 25 °C). The peak area of the product at pH 7.5 was the highest (Fig. 2A), indicating that the activity of immobilized KLK reached the maximum at this pH. Herein, pH 7.5 was selected for the further experiments. On the other hand, the peak area of product was increased with the increase in incubation time, and the velocity of enzymatic reaction kept constant within 120 s (Fig. 2B). It is very important to control the reaction in its initial stage to determine the enzymatic reaction kinetic parameters. In this study, the velocity of enzymatic reaction kept constant within 120 s, which can be considered to be the initial velocity. Therefore, 120 s was chosen to be the incubation time for further experiments.
Effects of buffer pH (A) and incubation time (B) on the peak area of product (pNA). CE Conditions: injection, pressure of 50 mbar for 15 s; separation voltage, 20 kV; UV detection at 405 nm; cassette temperature, 25 °C; substrate, 2.00 mg mL−1 (3.27 mM) of S-2302 dissolved in phosphate buffer (pH 7.5); enzyme concentration, 30 U mL−1; 5.00 mM phosphate buffer, pH 7.5 of buffer for B; 120 s of incubation time for A
Repeatability and Stability of the Immobilized KLK
The repeatability and stability of the KLK-IMER were investigated by measuring the migration time and peak area of product. The intra-day RSD% (n = 6) of the migration time and peak area are 1.5% and 1.9%, respectively. The RSD% of batch-to-batch (n = 3) in different capillaries of the migration time and peak area are 3.5% and 2.7%, respectively. Moreover, the storage stability of the prepared IMER was tested after being stored at 4 °C for three days, and the relative activity of the immobilized KLK remains 81.3% of its initial activity. In addition, the stability of the prepared IMER was evaluated by consecutive assays. As shown in Fig. 3, the relative activity is remained approximately 80.0% of the initial immobilized KLK activity after 30 runs. These results indicate that the prepared KLK-IMER is stable and reliable.
Stability evaluation of immobilized KLK for 30 consecutive runs. Incubation time, 120 s; 5.00 mM phosphate buffer, pH 7.5. Other CE conditions were the same as Fig. 2
Kinetics Study of the Immobilized KLK
Measurement of Km
The Km value reflects the affinity between the enzyme and substrate, and the lower Km represents higher affinity. Based on Eq. (1), the obtained linear regression equation is y = 0.03569 x + 0.01961 and R2 = 0.9950. The Km value obtained from the slope and intercept is 1.82 mM (Fig. 4A), which is slightly higher than the values determined on affinity purified KLK from human plasma using Cbz-Phe-Arg-AMC (0.83 mM), Cbz-Pro-Phe-Arg-AMC (1.40 mM) and Boc-Leu-Lys-Arg-AMC (0.91 mM) as substrates [20]. In addition, the substrate S-2302 at a concentration of 2.00 mg mL−1 (3.27 mM) was selected to perform the enzyme analysis in the following experiments.
Double reciprocal plot (A) and inhibition curve for nicotinamide on immobilized KLK (B). For A, substrate S-2302 concentrations were from 0.50 to 3.00 mg mL−1 (0.82–4.91 mM); For B, nicotinamide concentrations were from 2.50 to 20.00 mM. Incubation time, 120 s; 5.00 mM phosphate buffer, pH 7.5. Other CE conditions were the same as Fig. 2
Reliability of the KLK-IMER
The IC50 and Ki can be used to verify the suitability of the established IMER for screening and evaluation of inhibitors. IC50 value can be obtained by constructing dose–response nonlinear regression equation using Origin 2018. The IC50 and the Ki of nicotinamide on KLK are calculated to be 12.07 and 4.31 mM, respectively (Fig. 4B). Furthermore, the negative control drug of tinidazole, which does not bind to the KLK, has no inhibitory effect on KLK at the concentration of 5.00 mM. In addition, Z′ factor is a characteristic parameter that indicates the quality of an inhibitor screening method. In this study, according to Eq. (5), Z′ factor is determined as 0.94 (n = 6), indicating that this KLK-IMER assay is accurate and reliable.
Inhibition Assays by CE-based KLK-IMER
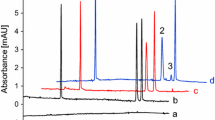
The developed CE-IMER assay was applied to evaluate the inhibitory activity of ten small-molecule compounds and six tea polyphenol extracts on KLK. The related electropherograms of epicatechin gallate, epigallocatechin, epicatechin and isochlorogenic acid C analysis were shown in Fig. 5. As the results shown in Table 1, epicatechin gallate, epigallocatechin, epicatechin, isochlorogenic acid C, epigallocatechin gallate and ferulic acid exhibit good inhibitory effect on KLK (all higher than 40%). The mechanisms of inhibition (competitive, noncompetitive or uncompetitive) of epicatechin gallate, epigallocatechin, epicatechin, isochlorogenic acid C against KLK were also investigated. The inhibition induced by epicatechin gallate was found to be an uncompetitive inhibition, because the KLK activity increased as a function of the concentration of the S-2302 substrate, nevertheless the Vmax and Km values decreased as the concentration of epicatechin gallate increased from 0.00 to 0.20 mM (Fig. 6A). The inhibition induced by epigallocatechin and epicatechin were found to be a competitive inhibition, as the Vmax values remained constant, but the Km values increased (Fig. 6B, C). The inhibition induced by isochlorogenic acid C was found to be a noncompetitive inhibition, for which the Km value remained constant, but the Vmax value decreased (Fig. 6D). Moreover, those compounds such as gallic acid with % of inhibition below 40% at this concentration are considered having no significant inhibitory effect on the KLK. In addition, good inhibitory activity (all higher than 40%) of six tea polyphenol extracts (Raw Pu-erh, Mingqian Mao Feng, Fuding white tea, Dark green tea, Biluochun and Black tea) on KLK were observed at the concentration of 0.25 mg mL−1 (Table 1).
The electropherograms for evaluating the inhibitory activity of small-molecule compounds (0.25 mM) on KLK. a epicatechinn gallate; b epigallocatechin; c epicatechin; d isochlorogenic acid C; e control group. Incubation time, 120 s; 5.00 mM phosphate buffer, pH 7.5. Other CE conditions were the same as Fig. 2
Lineweaver–Burk plots of inhibition assays of inhibitors (0.00, 0.05 and 0.20 mM) on immobilized KLK. A Epicatechinn gallate; B epigallocatechin; C epicatechin; D isochlorogenic acid C. Substrate S-2302 concentrations were from 2.00 to 5.00 mg mL−1 (0.12–0.31 mM). Incubation time, 120 s; 5.00 mM phosphate buffer, pH 7.5. Other CE conditions were the same as Fig. 2
Vascular KLK can activate prorenin to renin, promoting the conversion of angiotensinogen to angiotensin I, which is converted into angiotensin II under the action of angiotensin converting enzyme, and further rising the blood pressure [21]. Furthermore, vascular KLK also plays important roles in microvascular complications of diabetes mellitus including diabetic retinopathy, diabetic macular edema and diabetic nephropathy. Bradykinin released by vascular KLK can increase retinal vascular permeability, leading to retinal edema and vision impairment [22]. Theoretically, KLK inhibitors can block the changes of retinal vascular permeability and improve vision impairment and regulate blood pressure. The good inhibitory of tea polyphenol extracts and small-molecule compounds on KLK may provide a reference for drug discovery in the treatment of these diseases. In addition, the established method in this study can play a guiding and predictive role in the discovery of KLK inhibitors.
Molecular Docking Study
Molecular docking was performed to determine the binding site position and the binding mode of potential ligand with enzyme. Those four small-molecule compounds (epicatechin gallate, epigallocatechin, epicatechin and isochlorogenic acid C) with significant inhibitory activity were docked with KLK. The docking diagrams were shown in Fig. 7. The binding energies and binding sites of these compounds with KLK residues were shown in Table 2. Hydrogen bonds, which are very significant bonding for the interaction of these compounds with KLK, present between epicatechin gallate and THR166, TYR99, VAL176, GLU128, LYS230, GLN41; isochlorogenic acid C and HIS57, TYR99, VAL176, GLU128, LEU95A, ASN95, LYS230; epigallocatechin/epicatechin and TYR166, ASP170, SER195, GLN164, SER226. The van der Waals interactions present between epicatechin gallate, epigallocatechin, epicatechin and isochlorogenic acid C with substrate binding residues of TYR99 and HIS57. Carbon hydrogen bonds present between epicatechin gallate, epigallocatechin, epicatechin and isochlorogenic acid C with substrate binding residue of THR166. In addition, the binding energies of epicatechin gallate, epigallocatechin, epicatechin and isochlorogenic acid C are all below − 5.0 kcal mol−1, which can explain their good inhibitory effects on KLK in CE-IMER assay. In a word, epicatechin gallate, epigallocatechin, epicatechin and isochlorogenic acid C are all located in the enzyme active site, and influence the microenvironmental and conformational change of KLK, leading to the inhibition of enzyme activity.
Concluding Remarks
This study developed a simple method for online evaluation of the inhibitory activity of small-molecule compounds and tea polyphenol extracts on KLK. The enzyme was immobilized with the aid of chitosan and glutaraldehyde. The immobilization process is time saving and under mild conditions, just setting the cassette temperature at 25 °C for all the steps. The performances of the fabricated CE-based KLK-IMER, including Km, IC50, and Ki values, were evaluated. Good repeatability was obtained with RSDs less than 5.0%. Evaluations of inhibitory activity of ten small-molecule compounds and six tea polyphenol extracts on KLK were performed using the CE-based IMER, and results indicate that four compounds (epicatechin gallate, epigallocatechin, epicatechin and isochlorogenic acid C) and six tea polyphenol extracts (Raw Pu-erh, Mingqian Mao Feng, Fuding white tea, Dark green tea, Biluochun and Black tea) have good enzyme inhibitory activity. In short, the developed KLK-IMER and molecular docking analysis is a potential tool for screening of KLK inhibitors.
References
Weidmann H, Heikaus L, Long AT, Naudin C, Schlüter H, Renné T (2017) The plasma contact system, a protease cascade at the nexus of inflammation, coagulation and immunity. BBA-Mol Cell Res 1864:2118–2127
Long AT, Kenne E, Jung R, Fuchs TA, Renné T (2016) Contact system revisited: an interface between inflammation, coagulation, and innate immunity. J Thromb Haemost 14:427–437
Schmaier AH (2016) The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost 14:28–39
Kolte D, Shariat-Madar Z (2016) Plasma Kallikrein inhibitors in cardiovascular disease. Cardiol Rev 24:99–109
Cheng MX, Chen ZL (2018) Recent advances in screening of enzymes inhibitors based on capillary electrophoresis. J Pharm Anal 8:226–233
Jiang H, Shi J, Li YY (2011) Screening for compounds with aromatase inhibiting activities from Atractylodes macrocephala Koidz. Molecules 16:3146–3151
Zhang H, Wu ZY, Wang YZ, Zhou DD, Yang FQ, Li DQ (2020) On-line immobilized trypsin microreactor for evaluating inhibitory activity of phenolic acids by capillary electrophoresis and molecular docking. Food Chem 310:125823
AliAl-OthmanAl-WarthanAsninChudinov IZAALA (2014) Advances in chiral separations of small peptides by capillary electrophoresis and chromatography. J Sep Sci 37:2447–2466
Schejbal J, Glatz Z (2018) Immobilized-enzyme reactors integrated with capillary electrophoresis for pharmaceutical research. J Sep Sci 41:323–335
Wu ZY, Zhang H, Yang YY, Yang FQ (2020) An online dual-enzyme co-immobilized microreactor based on capillary electrophoresis for enzyme kinetics assays and screening of dual-target inhibitors against thrombin and factor Xa. J Chromatogr A 1619:460948
Zhang H, Wu ZY, Yang YY, Yang FQ, Li SP (2019) Recent applications of immobilized biomaterials in herbal analysis. J Chromatogr A 1603:216–230
Li QQ, Yang FQ, Wang YZ, Wu ZY, Xia ZN, Chen H (2018) Evaluation of thrombin inhibitory activity of catechins by online capillary electrophoresis-based immobilized enzyme microreactor and molecular docking. Talanta 185:16–22
Zhang H, Lu M, Jiang H, Wang X, Yang FQ (2020) Evaluation inhibitory activity of catechins on trypsin by capillary electrophoresis-based immobilized enzyme microreactor with chromogenic substrate. J Sep Sci 43:3136–3145
Zhang BF, Chen ZL (2019) Screening of cathepsin B inhibitors in traditional Chinese medicine by capillary electrophoresis with immobilized enzyme microreactor. J Pharm Biomed Anal 176:112811
Liu X, Zhu XD, Camara MA, Qu QS, Shan YC, Yang L (2019) Surface modification with highly-homogeneous porous silica layer for enzyme immobilization in capillary enzyme microreactors. Talanta 197:539–547
Zhang H, Lu M, Jiang H, Wu ZY, Zhou DD, Li DQ, Yang FQ (2020) Tyrosinase-mediated dopamine polymerization modified magnetic alginate beads for dual-enzymes encapsulation: Preparation, performance and application. Colloids Surf B Biointerfaces 188:110800
Mcdonald A, Qian S (2017) Therapeutic inhibitory compounds. US9611252B2
Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108
Zhang JH, Chung TD, Oldenburg KR (1999) A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73
Biltoft D, Sidelmann JJ, Olsen LF, Palarasah Y, Gram J (2016) Calibrated kallikrein generation in human plasma. Clin Biochem 49:1188–1194
Tschope C, Schultheiss HP, Walther T (2002) Multiple interactions between the renin-angiotensin and the kallikrein-kinin systems: role of ACE inhibition and AT1 receptor blockade. J Cardiovasc Pharm 39:478–487
Phipps JA, Feener EP (2008) The kallikrein-kinin system in diabetic retinopathy: lessons for the kidney. Kidney Int 73:1114–1119
Funding
This work was sponsored by Natural Science Foundation of Chongqing, China (cstc2019jcyj-msxmX0074), and was supported by the University of Macau [grant number MYRG2019-00011-ICMS].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declared that they have not conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, GY., Zhang, H., Zhang, CY. et al. Immobilized Kallikrein Microreactor Based on Capillary Electrophoresis for Online Enzyme Kinetics Analysis and Inhibitor Screening. Chromatographia 84, 1141–1150 (2021). https://doi.org/10.1007/s10337-021-04098-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-021-04098-9