Abstract
A micellar electrokinetic capillary chromatography (MEKC) method has been developed and validated for simultaneous analysis of febuxostat and its related substances in active pharmaceutical ingredients and pharmaceutical formulations. Analysis was performed in an uncoated fused silica capillary (34 cm effective length, 50 µm diameter). Method sensitivity was enhanced using an extended light path capillary, with an optical path 150 µm and the choice of 320 nm detector operating wavelength due to lower background noise and higher selectivity. BGE consisted of 20 mM borate buffer pH 9.3 and 50 mM SDS and 2% acetonitrile. Analysis was performed at 30 kV separation voltage and 32 °C capillary temperature. The method was linear over the range 1.0–10.0 µg mL−1 for each impurity corresponding to reporting limits regarding the ICH Q3A guidelines. The method has proven to be selective, precise, accurate and robust. Proposed MEKC method has been successfully applied for the quantitative determination of febuxostat and purity assay of active pharmaceutical ingredient and tablet dosage forms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Febuxostat is a novel non-purine type selective xanthine oxidase inhibitor (Fig. 1) [1]. It was approved by the FDA in 2009, indicated for the chronic management of hyperuricemia in patients with gout, a type of inflammatory arthritis, characterized by sudden attacks of redness, swelling and joint pain. Febuxostat lowers uric acid levels in the blood, and clinical studies have shown that febuxostat is far more effective than the well-established allopurinol [2]. Compared to allopurinol therapy, it is far more efficient in patients with kidney disorders and there is no need for dose adjustments [3].
Analytical methods for identification and quantification of febuxostat are not extensive. Furthermore, analytical methods for purity evaluation, as a key point in assessing safety and quality of a pharmaceutical product, are scarce. A spectrophotometric method for the estimation of febuxostat in pure and tablet dosage form has been published [4], as well as a HPTLC method for simultaneous determination of two binary mixtures containing ketorolac with phenylephrine and febuxostat [5]. Literature review shows only a few HPLC methods have been published for the estimation of febuxostat in bulk drugs and formulations [6, 7]. Several stability-indicating LC [8,9,10,11,12] and UV spectrophotometric [4, 13] methods have been reported for febuxostat determination in formulations. Only a limited number of methods have been published for determination of febuxostat impurities in drug substances [14, 15].
Capillary electrophoresis (CE) is a cost-efficient analytical technique that is often considered complementary to the well-established HPLC technique. CE technology offers a green approach to analytical chemistry, with significantly lower sample and reagents consumption [16]. Due to different separation mechanisms it is fit for purpose for the analysis of both small neutral and ionic molecules, as well as for large biomolecules [17,18,19]. CE methods are characterized by high efficiency and high resolution, enabling separation of molecules with different moieties in a single run in a short analysis time [20].
So far no CE method has been reported for simultaneous determination of febuxostat and its related substances in pharmaceutical formulations or active pharmaceutical ingredient. Only one CE method was published for the application in a stability study of febuxostat [21]. In addition, official pharmacopoeia method for febuxostat has not yet been described in European Pharmacopoeia or in United States Pharmacopoeia.
Impurities are an important class of potential drug substance’s critical quality attributes. Because of their potential impact on drug safety and long-term stability of the drug, controlling potential impurities is important to be considered in all steps of drug development, synthesis and manufacturing of the drug product.
During the febuxostat synthesis process, the last step is the hydrolysis of ester intermediates [15].
Ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylate (F-ethyl) can be found as the synthetic precursor impurity in febuxostat [22]. The hydrolysis of the cyano group can form two additional impurities, such as the 2-(3-carbamoyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylic acid (F-amide) and 2-(3-ethoxycarbonyl-4-isobutoxyphenyl)-4-methylthiazole-5-carboxylic acid, (F-DEE) (Fig. 1).
Due to the lack of official pharmacopoeia monograph, developing a sensitive analytical method for determination of febuxostat and its potential impurities in drug products is of great importance. Therefore, the aim of this study was to develop a green, fast, simple and selective CE method for simultaneous determination of febuxostat and its three impurities, F-amide, F-DEE and F-ethyl, in active pharmaceutical ingredient and in tablet dosage forms.
Materials and Methods
Chemicals and Reagents
Febuxostat analytical standard was purchased from Sigma Aldrich (Steinheim, Germany). Its three related impurities were kindly donated by Pliva d.o.o. (Zagreb, Croatia). Pharmaceutical dosage forms Adenuric® containing 80 and 120 mg of febuxostat (Berlin-Chemie Menarini) were purchased from local pharmacy. Commercially available febuxostat active pharmaceutical ingredients were purchased online. Pravastatin was obtained from Pliva d.o.o. (Zagreb, Croatia). Ketoprofen was purchased from Sigma Aldrich (Steinheim, Germany). Salicylic acid, caffeine and codeine phosphate were obtained from Kemika (Zagreb, Croatia).
Sodium dodecyl sulphate (SDS) was from Poch SA (Gliwice, Poland) and tetraborate buffer solution pH 9.3 was from Agilent Technologies (Santa Clara, USA). Ultrapure water was purified by a SG Water euRO system (SG Wasseraufbereitung und Regenerierstation GmbH, Germany). HPLC grade acetonitrile, ethanol and methanol were purchased from J.T. Baker (Germany). 1 M sodium hydroxide (NaOH) was from Agilent Technologies (Santa Clara, USA). α-cyclodextrine was purchased from Sigma-Aldrich (Steinheim, Germany). All other chemicals, including sodium acetate (Kemika, Zagreb, Croatia), disodium hydrogen phosphate (Merck, Darmstadt, Germany) and sodium dihydrogen phospate (Sigma-Aldrich, Steinheim, Germany), dimethyl sulfoxide (Sigma-Aldrich, Steinheim, Germany), Triton X-100 (Sigma-Aldrich, Steinheim, Germany) and dimethyl formamide (Sigma-Aldrich, Steinheim, Germany) were of analytical reagent grade quality.
Standard, Sample and Working Solutions
Stock solution of febuxostat was prepared by accurately weighing the appropriate amount of the standard in acetonitrile to a concentration of 1.0 mg mL−1. Stock solutions of F-DEE and F-ethyl were prepared in acetonitrile at a concentration of 0.5 mg mL−1. Stock solution of F-amide was prepared by dissolving the appropriate amount in dimethyl sulfoxide to the concentration of 0.5 mg mL−1. Ketoprofen and salicylic acid standard solutions were prepared in acetonitrile, while pravastatin was dissolved in water, to a final concentration of 1.0 mg mL−1. Caffeine and codeine phosphate standard solutions were prepared in ethanol to a final concentration of 1.0 mg mL−1.
All stock solutions were sonicated for 10 min, stored in a refrigerator at 4 °C and were stable for at least two weeks. Working solutions were stable at ambient temperature for at least 24 h. Working standard solution used for method optimization contained 50 µg mL−1 febuxostat, F-DEE, F-ethyl and F-amide, and 100 µg mL−1 salicylic acid as internal standard.
Active pharmaceutical ingredient was prepared by accurately weighing the appropriate amount of the powder in acetonitrile to yield a concentration of 1.5 mg mL−1. After vortexing on an orbital shaker for 2 min, the solution was sonicated for 10 min and filtered through Chromafil Xtra cellulose (Macherey–Nagel, Germany) 0.20 µm pore size, disposable syringe filter.
Tablet sample solutions were prepared by crushing twenty 80 and 120 mg Adenuric® tablets in a ceramic mortar to a fine powder. The powder, equivalent to 20.0 mg of febuxostat, was dispersed in 10 mL of acetonitrile, vortexed on an orbital shaker at 2800 rpm for 5 min and sonicated for 15 min. The solution was centrifuged at 6000 rpm for 5 min and the clear solution was filtered.
Instrumentation and CE Conditions
Capillary electrophoretic analysis was performed on an Agilent High-Performance Capillary Electrophoresis (G1600A) system (Waldbronn, Germany). Agilent 3DCE/MSD ChemStation software was used for system control, data collection and data analysis. Separation was performed on uncoated fused-silica bubble-cell capillaries (Agilent Technologies) of 34.0 cm total length/26.0 cm effective length, 50 µm inner diameter with an extended optical path of 150 µm.
New capillaries were conditioned by rinsing with methanol, 1 M NaOH and water for 10 min and equilibrated with the running buffer for 20 min. Daily conditioning was performed with 0.1 M NaOH followed by water and BGE for 10 min. Capillary was flushed with BGE for 3 min before each run, while the post-conditioning was performed with acetonitrile:water (50:50, v/v) and 0.1 M NaOH for 1 min. Running buffers were replenished every five runs.
The optimized running buffer was a 20-mM tetraborate buffer pH 9.3 containing 50 mM SDS and 2% acetonitrile. A voltage of 30 kV was applied for the separation and constant temperature of 32 °C. Samples were injected hydrodynamically at a pressure of 50 mbar for 6 s. Detection was performed at 320 nm wavelength.
Results and Discussion
Method Development
The aim of the method development was to achieve baseline separation of febuxostat and its three impurities in the shortest time possible. Therefore, all experiments were carried out on a short (34.0/26.0 cm) capillary. Since F-ethyl impurity is neutral, micellar electrokinetic chromatography (MEKC) was chosen. As SDS is one of the most accessible and affordable surfactants with a low UV absorbance, it was added as the BGE additive to enable the analysis of the neutral F-ethyl impurity.
The most important step during CE method development is the choice of the type and pH of the buffer. Febuxostat with a pKa value in water around 3.3 [23] and its two impurities, F-DEE and F-amide, would be in their unionized forms in acidic pH, enhancing their interactions with the surfactant micelles added to the buffer and leading to longer migration times. Sodium tetraborate pH 9.3 buffer was chosen for the initial experiments. In the chosen buffer silanol groups on the inner capillary wall are deprotonated, and the electroosmotic flow (EOF) is very strong leading to shorter analysis times. Buffer concentration was examined in a range from 10 to 50 mM. As expected, with higher buffer ionic strength the migration times were longer, resolution between peaks increased, but higher Joule’s heating is produced resulting in sample band dispersion and peak broadening. Therefore, during further method development phases, buffer ionic strength was kept at low, 10 mM and 20 mM, concentrations. Other common buffers, such as acetate buffer and phosphate buffer were tested, but they provided no improvement in peak shape and resolution, in addition to prolonging method analysis.
The addition of surfactants to the BGE changes method’s selectivity and improves in many cases peak symmetry and resolution of all present analytes. The anionic micelles of SDS interact not only with neutral F-ethyl impurity but also with febuxostat, F-DEE and F-amide, which are present in their ionized forms at high buffer pH 9.3. For the neutral species it is only the partitioning into the micelles effects the separation. On the other hand, for ionized species it is a complex combination of both the electrophoretic mobility of the analyte in the current method conditions and the partitioning into the micelles that result in the final apparent mobility of the specie, enhanced selectivity and improved resolution between analytes. Changing the SDS concentration from 10 to 80 mM was tested during method development. At SDS concentration of 10 mM, which is the concentration of the surfactant at which the micelles are already formed, F-ethyl impurity was still not observed, meaning it was migrating together with EOF peak as a neutral molecule. At lower SDS concentrations (15 – 40 mM) F-ethyl showed a very poor peak shape and symmetry. Further increasing of SDS concentrations provided better peak shape and better resolution, but also prolonged the method analysis time significantly. Therefore, 50 mM SDS concentration was chosen as optimal since it provided a satisfactory peak shape for all the analytes of interest and kept the migration time of the F-ethyl impurity, as the last migrating analyte, under two minutes.
Besides assuring good separation within shortest possible analysis time, providing good peak shape and symmetry for febuxostat present in the sample in high concentration and its potential impurities expected in very low concentrations, was also in focus during early stages of method development. Good solubility, peak shape and symmetry were also ensured through solvent choice for all analytes. Different solvents were tested for dissolving and diluting F-amide impurity. Using only water, only acetonitrile, or a mixture of acetonitrile:water (50:50, v/v) was not sufficient, so dimethyl sulfoxide and dimethyl formamide were also tested. Dissolving F-amide in dimethyl sulfoxide provided a sharp symmetrical peak which migrated first after the EOF. To achieve a satisfactory F-ethyl impurity peak shape within reasonable analysis time, several other additives (dimethyl sulfoxide, α-cyclodextrine, triton and dimethyl formamide) were added to the BGE containing 20 mM sodium borate and 50 mM SDS. However, all these additives either significantly prolonged the analysis time, or resulted in very high current within the capillary and have not improved the peak shape. Possible causes of poor peak shape are longitudinal diffusion happening inside the capillary, Joule heating-related dispersion, as well as sorption–desorption kinetics in micellar solubilization, intermicelle mass transfer in the aqueous phase, intracolumn mass transfer and temperature gradient effect on electrophoretic velocity.
In order to improve method precision and accuracy, different potential internal standards (IS) were tested: pravastatin, ketoprofen, salicylic acid, caffeine and codeine phosphate. Febuxostat and its three impurities have similar UV–Vis absorption spectra with two maximums, around 214 nm and 315 nm. Although most of the potential internal standards do not absorb over 300 nm, higher wavelengths (310–320 nm) proved to be a better choice due to lower baseline noise and thus by enhancing method sensitivity. Hence, salicylic acid was finally adopted as the internal standard because it offered suitable migration time and was well resolved from febuxostat and its impurities.
Since the potential impurities are present in very low concentrations, the sensitivity of the method is crucial. By increasing the injection time or pressure, the amount of the sample injected into the column is higher and respective peak areas increased. Therefore, injection times from 2 to 7 s were tested and peak area, peak height, peak width and peak shape were monitored. Although increasing the injection time, as expected, increased peak area, the peak height did not have a linear response and at higher injection time values were coming to a plateau. Furthermore, peak symmetry for F-ethyl, which had the most challenging peak shape, deteriorated significantly by changing the injection time from 6 to 7 s. To further improve the sensitivity of the method, extended path length capillary, known as a bubble cell capillary, was used. With an increased inner diameter of the capillary exactly at the detector window by a factor of three (150 µm), the sensitivity was increased by approximately the same fold, while preserving the resolution between peaks, and current developed within the capillary.
In order to obtain the shortest possible analysis time, and yet meet the satisfactory resolution criteria, effects of applied voltage and temperature were tested in a range of 20–30 kV and 26–34 °C, respectively. Lower voltage values (20–25 kV) prolonged the analysis time significantly and febuxostat and the internal standard co-migrated, so they were not considered in further experiments. The resolution at the highest possible applied voltage (30 kV) was more than satisfactory (Rs = 2.36) and was chosen as optimal.
Once the applied voltage was chosen, the capillary temperature was optimized in terms of migration time, resolution and efficiency. It can be seen in Fig. 2 that the migration times of all analytes decreased by the increase of applied capillary temperature, enabling shorter analysis time. Furthermore, resolution among closely migrating peaks, febuxostat and IS, improved with higher capillary temperatures, especially for the critically closely migrating peaks of febuxostat and IS. Hence, 32 °C was chosen as optimal applied temperature for further analysis.
The effect of organic modifiers on resolution, selectivity and peak shapes was also tested. Acetonitrile, ethanol and methanol were added to the BGE in different ratios. Adding acetonitrile in small portions (2%) proved to be optimal, providing a stable baseline, good peak shapes and enhancing resolution among closely migrating peaks (Fig. 3a).
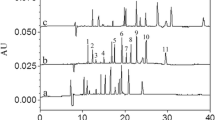
Electropherograms of standard solutions of febuxostat (F), internal standard (IS) and its three impurities F-amide, F-DEE and F-ethyl at optimized MEKC conditions (a) and febuxostat active pharmaceutical ingredient (b). Operating conditions: 20 mM pH 9.3, borate buffer 50 mM SDS, 2% acetonitrile; V = 30 kV; λ = 320 nm, 32 °C, injection time 6 s, pressure 50 mbar
Optimal rinsing procedure, which ensured good migration time reproducibility and no peak broadening due to solute-wall interactions, included a preconditioning step by flushing the capillary with BGE for 3 min, and the postconditioning step by flushing the capillary with acetonitrile:water (50:50, v/v) followed by a flush with 0.1 M NaOH for 1 min.
Method Validation
The newly developed MEKC method for the simultaneous determination of febuxostat and its three impurities was validated according to ICH guidelines [24].
Selectivity of the method was studied by assessment of peak purity to ensure there are no co-migrating peaks. On a synthetic mixture of standard solutions of all analytes in question, purity match factor was assessed for all peaks with the use of 3DCE/MSD ChemStation software (ver. 10.02, Agilent Technologies). Purity match factor was between 997.8 and 1000.0, indicating that the UV spectra of different peaks’ parts were identical, and no co-migrating peaks were observed. The method was further tested on a febuxostat commercial pharmaceutical product solution at the targeted concentration level. By comparing electropherograms obtained by the standard solution and the commercial febuxostat tablet solution it was concluded that no interference from excipients occurred. The same peak purity match factor was determined for the peaks in the febuxostat tablet solution and were found to be between 996.1 and 999.7. Hence, the proposed method is selective and applicable for application on real febuxostat tablet samples.
The results of the method’s linearity, the regression and correlation data of the obtained calibration curves are shown in Table 1.
The detection limit (LOD) and the quantification limit (LOQ) of the proposed analytical procedure were determined based on the standard deviation of the response (σ) and the slope of the calibration curve (a). LOD was calculated as the 3.3 σ/a, while LOQ was calculated as the 10 σ/a. The detection and quantitation limits for all analytes are shown in Table 1.
Method precision was evaluated in terms of repeatability and intermediate precision. The repeatability or intra-day precision was evaluated by six replicates of a mixture of working solutions, febuxostat (1000 µg mL−1), its three impurities at a 0.1% level (1 µg mL−1) and internal standard (Table 2). Low RSD values indicate that the repeatability of the proposed method is acceptable. The intermediate precision (inter-day precision) was determined over three consecutive days with a mixture of working solutions freshly prepared on each day. The obtained % RSD values (Table 2) indicate that the inter-day precision of the proposed MEKC method is suitable.
Accuracy of the proposed method was first determined by comparing measured and known concentration values of the mixture of standard drug solutions. The recovery was found to be in a range from 98.2 to 101.1%. Then the recovery study was performed by spiking the drug substance and the commercially available drug product with known concentrations of the analytes at three different levels (1.0, 5.0 and 10.0 µg mL−1). Recoveries were found to be in a range of 99.1–101.2% for F-amide, 99.2–102.1% for F-DEE and 100.1–103.2 for F-ethyl, showing that the accuracy of the proposed method was good. Slightly higher values were obtained for the F-ethyl impurity, likely due to its peak shape and symmetry.
In robustness testing changes to buffer concentration (20 mM ± 1 mM), SDS concentration (50 mM ± 1 mM), applied separation voltage (30–1 kV) and capillary temperature (32 °C ± 1 °C) were made. The RSD values for migration times for all four evaluated parameters were in a range from 0.68 to 1.87%, while RSD values for peak area were in a range from 1.73 to 2.27%.
Application of the Optimized Method
The newly developed and fully validated MEKC method for the simultaneous determination of febuxostat and its three impurities was successfully applied for the determination of the drug and its related substances in commercially available tablet dosage forms (Adenuric® 80 mg and Adenuric® 120 mg) and in active pharmaceutical ingredients (Fig. 3b). No interferences were observed with excipients present in tablet dosage forms and the peaks of the target analytes were unaffected. The method was used for the quantitative determination of febuxostat, as well as purity evaluation and quantitative determination of febuxostat’s three impurities in real samples.
Quantitative determination was done by calculating the drug concentration from the calibration curve obtained for febuxostat during the validation process. The quantitation of febuxostat in two different batches of active pharmaceutical ingredient had a mean recovery of 98.8% ± 2.1% and 100.89% ± 1.8%, respectively. Results of the febuxostat tablet form analysis showed that the amount of febuxostat found corresponded to the drug label (101.7% ± 1.5%). In order to estimate the recovery percentage, three different concentrations of febuxostat standard were added to febuxostat tablets and analysed in triplicate. The mean recoveries were in a range from 98.61 to 101.12%, with an RSD value of 1.3%. Therefore, the matrix effect of the drug formulation form was not significant, and the accuracy of the proposed MEKC method was satisfactory.
With good recoveries, quantitation of potential impurities is also possible. The sensitivity of the proposed method can detect low concentrations of febuxostat impurities in real tablet samples, with quantification limits enabling determination of impurities lower than 0.05% as requested by the ICH guidelines. In both batches of the active pharmaceutical ingredients, all related impurities have been found in generally very low levels. Significant level has been found only for F-ethyl (0.29%, RSD 2.6%). This is due to the fact that F-ethyl is the final intermediate in the synthesis of febuxostat and is an expected process related impurity. The amount of F-ethyl found in tested tablet dosage forms was under the LOQ. F-amide and F-DEE have been found in low levels (< 0.1%) in both active pharmaceutical ingredients and under the LOD in both tested tablet dosage forms.
Concluding Remarks
A newly developed and validated MEKC method enabled separation and simultaneous determination of febuxostat and its three related substances in active pharmaceutical ingredients and pharmaceutical formulations within only 2 min. The proposed MEKC method is the first capillary electrophoretic method for the determination of febuxostat’s impurities.
Only one stability-indicating CE method has previously been published [21]. However, this method was limited to the quantitative determination of febuxostat in tablet dosage forms and not the process-related impurities, and the analysis time is twice as long.
A limited number of methods have been published for determination of febuxostat impurities in drug substances [14, 15]. LC–MS/MS method for the study of impurity carryover and impurity profile of febuxostat has an extremely long analysis time of 60 min, while the other method uses expensive ultra-performance liquid chromatography technique, and it is still more than twice as long as the proposed MEKC method, with comparable limits of detection and quantification.
Previously reported HPLC methods [8,9,10,11,12] were all stability-indicating analytical methods that could only be applied effectively to separate the drug from its degradation products. These methods all have longer analysis time, comparable accuracy and precision and lower limits of detection and quantification for febuxostat. However, sensitivity of the proposed MEKC method in not a challenge for the quantification of the active ingredient in tablet dosage forms due its high concentrations. Furthermore, these methods require safe disposal of considerable amounts of expensive organic solvents, making them less environmentally friendly. Two reported HPLC methods [6, 7] can only be used for the determination of febuxostat in its bulk and dosage forms. The same goes for the spectrophotometric methods [4, 13] which are not separation techniques and can only for estimation of febuxostat in bulk and tablet dosage forms.
As there are no pharmacopoeia methods for febuxostat available, there is a clear demand for a fast and accurate analytical procedure to be used in routine analysis as a validated assay for purity control. The proposed MEKC method can be used by regulatory authorities to assess the quality dossiers and by the official medicines control laboratories for market surveillance studies.
References
Hu M, Tomlinson B (2008) Febuxostat in the management of hyperuricemia and chronic gout: a review. Ther Clin Risk Manag 4:1209–1220. https://doi.org/10.2147/tcrm.s3310
Becker MA, Schumacher HR, Wortmann RL et al (2005) Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 353:2450–2461. https://doi.org/10.1056/NEJMoa050373
Schumacher HR, Becker MA, Wortmann RL et al (2008) Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Care Res 59:1540–1548. https://doi.org/10.1002/art.24209
Spandana R, Pushpa Latha EJS (2015) Method development and validation of spectrophotometric method for the estimation of febuxostat in pure and tablet dosage form. Int J Pharm Pharmacaeutical Res 3:39–46
El Yazbi FA, Hassan EM, Khamis EF et al (2016) Development and validation of a high-performance thin-layer chromatographic method for the simultaneous determination of two binary mixtures containing ketorolac tromethamine with phenylephrine hydrochloride and with febuxostat. J Chromatogr Sci 54:819–828. https://doi.org/10.1093/chromsci/bmv250
Muvvala SS, Ratnakaram VN, Nadendla RR (2012) A validated RP-HPLC method for the estimation of febuxostat in bulk drugs. Int J Pharm Tech Res 4:1358–1366
Lakade SH, Bhalekar MR, Harde MT (2012) Developed and validated reverse phase HPLC method for the determination of febuxostat in bulk and formulations. Int J Pharm Pharm Sci 4:46–49
Mandle SD, Burade KB (2013) An improved stability-indicating HPLC method for Febuxostat in bulk and pharmaceutical dosage form. Int J Pharm Biol Res 4:1–10
Mukthinuthalapati MA, Bandaru SPK, Bukkapatnam V, Mohapatro C (2013) Development and validation of a stability-indicating RP-HPLC method for the determination of febuxostat (a Xanthine Oxidase Inhibitor). J Chromatogr Sci 51:931–938. https://doi.org/10.1093/chromsci/bms192
Panigrahy UP, Reddy ASKR (2015) Development and validation of stability indicating method for simultaneous estimation of Febuxostat and diclofenac potassium in bulk and tablet dosage form using RP-HPLC. Int J Pharm Sci Res 6:798–807. https://doi.org/10.13040/IJPSR.0975-8232.6(2).798-07
Rajyalakshmi C, Benjamin T, Rambabu C (2013) Stress degradation studies and validation method for quantification of febuxostat in formulations by using RP-HPLC. Int J Res Pharm Biomed Sci 4:138–144
Reddy BS, Bhaskar NV (2013) A stability indicating RP-HPLC method development for determination of Febuxostat in tablet dosage form. Caribb J Sci Technol 1:228–237
Kaur M, Bhardwaj P, Kaur B et al (2017) Development and validation of a novel stability indicating UV spectrophotometric method for estimation of febuxostat in bulk and pharmaceutical formulation (tablets). Pharm Methods 9:24–29. https://doi.org/10.5530/phm.2018.1.5
Reddy Musirike M, Reddy KH (2015) Development and validation of reverse phase-ultra performance liquid chromatographic method for estimation of related substances in febuxostat drug substance. Pharm Anal Acta 6:1–6. https://doi.org/10.4172/2153-2435.1000431
Kadivar MH, Sinha PK, Kushwah D et al (2011) Study of impurity carryover and impurity profile in febuxostat drug substance by LC-MS/MS technique. J Pharm Biomed Anal 56:749–757. https://doi.org/10.1016/j.jpba.2011.07.039
Pedroso TM, Van SA, Salgado HRN (2019) Application of the principles of green chemistry for the development of a new and sensitive method for analysis of ertapenem sodium by capillary electrophoresis. Int J Anal Chem. https://doi.org/10.1155/2019/1456313
Yamamoto S, Fujiwara H, Maruyama K et al (2019) Simultaneous determination of inorganic anions and cations in water and biological samples by capillary electrophoresis with a capacitive coupled contactless conductivity detector using capillary filling method. Anal Sci 35:295–300. https://doi.org/10.2116/analsci.18P422
Scriba GKE, Belal F (2015) Advances in capillary electrophoresis-based enzyme assays. Chromatographia 78:947–970. https://doi.org/10.1007/s10337-015-2912-0
Niedermeier S, Scriba GKE (2020) Quality by design-based development of a chiral capillary electrophoresis method for the determination of dextrodropropizine and 1-phenylpiperazine as impurities of levodropropizine. Chromatographia 83:123–129. https://doi.org/10.1007/s10337-019-03817-7
Gotti R, Furlanetto S, Santagati NA (2012) Analysis of cyclosporin A and main degradation impurities by cyclodextrin-modified micellar electrokinetic chromatography. Anal Lett 45:665–676. https://doi.org/10.1080/00032719.2011.653897
Dalmora SL, Souto RB, Machado FT et al (2014) Validation of a stability-indicating micellar electrokinetic capillary method for the assessment of febuxostat and its correlation with the reversed-phase LC method. Anal Methods 6:3811–3816. https://doi.org/10.1039/c4ay00145a
David P Martin FAS (1998) European Patent Application. EP Pat 0879946A2 1:1–14
Grabowski BA, Khosravan R, Vernillet L, Mulford DJ (2011) Metabolism and excretion of [14C] febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase, in healthy male subjects. J Clin Pharmacol 51:189–201. https://doi.org/10.1177/0091270010365549
ICH (2005) International Conference on Harmonization (ICH) of Technical Requirements for the Registration of Pharmaceuticals for Human Use (2005) Validation of analytical procedures: text and methodology, pp 1–13
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sertić, M., Mornar, A. & Nigović, B. Simple and Rapid Micellar Electrokinetic Chromatography Method for Simultaneous Determination of Febuxostat and its Related Impurities. Chromatographia 83, 993–1000 (2020). https://doi.org/10.1007/s10337-020-03919-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-03919-7