Abstract
A fast and sustainable environment-friendly micellar electrokinetic chromatography coupled with a diode array detector was investigated and validated in accordance with ICH requirements for the simultaneous quantification of ezetimibe and simvastatin in a tablet binary mixture formulation. Drugs’ separation from their internal standard and degradation products was achieved using the optimized electrophoretic conditions: 50 µm i.d. × 30 cm fused-silica capillary, background electrolyte composed of 20 mmol L−1 sodium tetraborate buffer solution (pH 9, adjusted with o-phosphoric acid), 30 mmol L−1 sodium dodecyl sulfate, 12% acetonitrile; with detection at 238 nm and voltage of + 30 kV. Drugs were separated in less than 6 min. The method was linear with determination coefficients 0.9956 and 0.9920 for ezetimibe and simvastatin, respectively; and precise (intra-day 1.05% and 0.38%; inter-day 1.20% and 0.69%) for ezetimibe and simvastatin, respectively. Accuracy was tested through recovery and mean values (101.0 ± 0.77% for ezetimibe and 100.4 ± 0.35% for simvastatin) were obtained. The proposed method is the first that determined ezetimibe and simvastatin in presence of their degradation products. This method can be successfully used in quality control laboratories as an alternative to HPLC technique for the simultaneous quantification of ezetimibe and simvastatin in their combined drug products and could also be used as a stability-indicating method. The proposed method greenness profile was confirmed using Green Analytical Procedure Index and Analytical GREEnness tools with a final score of 11 and 0.81, respectively.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease appears to be the principal cause of death in the world involving hypercholesterolemia as a major risk factor [1]. It should be preferred to prescribe efficacious doses of statins to minimize low-density lipoprotein as per international treatment guidelines on cardiovascular disease prevention [2]. Contrarily, 2nd or 3rd lipid-lowering drugs, i.e., fibrates, bile acid sequestrants, and ezetimibe are preferred over statin monotherapy for the management of high cardiovascular risk patients with hypercholesterolemia [3]. Since 2004, Food and Drug Administration approved ezetimibe/simvastatin combined tablet dosage form with ezetimibe fixed dose (10 mg) and simvastatin variable dose (10/20/40 mg) for both primary and secondary forms of hypercholesterolemia therapeutic management [4]. Last decade, the combination therapy of these drugs has been prescribed to maintain low-density lipoprotein in patients resistant to statin monotherapy or statin dose related side effects that showed successful clinical outcome [5].
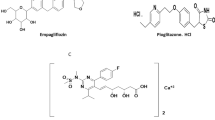
Chemically, ezetimibe (Fig. 1A) named as (3R,4S)-1-(4-fluorophenyl)-3-[(3R)-3-(4-fluorophenyl)-3-hydroxypropyl)]-4-(4-hydroxyphenyl)azetidin-2-one [6] which inhibits cholesterol absorption and decreases excessive accumulation in blood vessels [7]. This distinct mechanism of action results in a synergistic cholesterol lowering effect when used together with statins that inhibits cholesterol synthesis in the liver [5].
Simvastatin (Fig. 1B) named as [(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl] 2,2-dimethylbutanoate [6] which inhibits 3-hydroxy-3-methylglutaryl-coenzyme A reductase for the treatment of hyperlipidemia.
The literature review reveals that ezetimibe and simvastatin were officially listed in the USP [6]. Furthermore, several analytical approaches have been recommended for the simultaneous determination of both drugs in combined pharmaceutical formulations and/or biological fluids by TLC [8], HPLC [8,9,10,11,12], UPLC [13], UPLC–MS/MS [14], UPLC–Q–TRAP/MS [1], LC–MS [15], LC–ESI–MS/MS [16], and HPLC–MS/MS [17]. Some of these methods require extensive instrumentation and time-consuming sample pretreatment methodologies, hence not ideal for routine use. The literature also reveals that the pharmaceutical drugs under investigation were simultaneously analyzed by UV spectrophotometric [18, 19] and capillary electrophoretic [20] methods.
The capillary electrophoresis is rapidly gaining importance as an alternative green analytical separation technique and used in several pharmaceutical and biopharmaceutical analysis [21]. It has various advantages over conventional chromatographic methods such as short analysis time, low reagent consumption, without organic solvents, small sample volume, long-lasting capillary columns, high separation efficiency (millions of theoretical plates), and faster sample development [22], consequently diminishing the impact on environment [23].
At the present, only one capillary electrophoresis method has been described for simultaneous determination of ezetimibe and simvastatin in tablets, and the analytes were determined in a long time, i.e., approximately 10 min [20], this might be due to longer capillary column. Therefore, in this work, we proposed a validated micellar electrokinetic chromatography method, mode of capillary electrophoresis, for the simple and fast simultaneous determination of ezetimibe and simvastatin in binary mixtures under optimized analytical conditions. Moreover, the stability-indicating potential of the proposed method was first time investigated under stress degradation conditions (thermal, oxidative, photolytic, alkaline and acidic hydrolysis) for both ezetimibe and simvastatin, while highlighting the use of green background electrolyte. The proposed method greenness was also determined by using Green Analytical Procedure Index and Analytical GREEnness approaches.
Materials and Methods
Chemicals, Reagents and Samples
The active pharmaceutical ingredients ezetimibe and simvastatin with purities of 100.0% and 99.8%, respectively, were donated by Merck Sharp & Dohme (Sao Paulo, Brazil), internal standard losartan potassium was obtained from Sigma-Aldrich (Sao Paulo, Brazil), and Vytorin® (Merck Sharp & Dohme, Sao Paulo, Brazil) tablet (ezetimibe 10 mg and simvastatin 20 mg) were procured from community pharmacy. Analytical grade methanol, hydrogen peroxide, hydrochloric acid, acetonitrile was brought from Merck (Sao Paulo, Brazil); while o-phosphoric acid, sodium dodecyl sulfate, sodium tetraborate-10-hydrate, dibasic sodium phosphate, and sodium hydroxide were procured from Sigma-Aldrich (Sao Paulo, Brazil). Deionized water was obtained from Milli-Q® (Millipore Corp., Bedford, MA, USA) ultrapure purification system.
Instrument
P/ACE™ MDQ (Beckman Coulter, Inc., Fullerton, CA, USA) capillary electrophoresis system equipped with diode array detector and 32 Karat™ software (ver. 8.0) was employed for data acquisition.
Condition of Electrophoretic Separation
50 µm internal diameter × 42 cm total (30 cm effective) length uncoated fused-silica capillary (Polymicro Technologies, Phoenix, AZ, USA) was used by adjusting temperature (30 °C) and voltage (+ 30 kV) with detection at 238 nm to perform the electrophoretic separation. The samples were injected by applying pressure of 0.3 psi for 3 s (1 psi = 6894.76 Pa) at cathodic side.
Background Electrolyte Preparation
Aqueous stock solutions (100 mmol L−1) of sodium tetraborate buffer (pH 9, adjusted with o-phosphoric acid) and sodium dodecyl sulfate were separately prepared. The pH was measured using Gehaka® Mettler Toledo pH meter (Sao Paulo, Brazil). The optimized background electrolyte (30 mmol L−1 sodium dodecyl sulfate, 20 mmol L−1 sodium tetraborate buffer solution pH 9 and 12% acetonitrile) was prepared by transferring sodium tetraborate (5 mL) and sodium dodecyl sulfate (7.5 mL) stock solutions into a 25 mL volumetric flask, deionized water (8 mL), pH 9 adjusted with o-phosphoric acid solution, then acetonitrile (3 mL) was added, and deionized water was used to complete the volume. This mixture was sonicated for 10 min and filtered through a 0.45 µm polyvinylidene fluoride filter before injected on the capillary electrophoresis instrument.
Capillary Conditioning
Before using, new capillary was conditioned with NaOH 1 mol L−1 for 30 min, followed by deionized water for 20 min and finally with background electrolyte for 30 min. At the beginning of each working day, the capillary was rinsed with NaOH 1 mol L−1 for 15 min, deionized water for 10 min and then 20 min with background electrolyte. Between runs, the capillary was washed with NaOH 1 mol L−1 (1 min) and with the running buffer (1.5 min) to ensure reproducibility of the assay. Finally, at the end of the day, the capillary was flushed with NaOH 1 mol L−1 for 10 min and water for 10 min.
Preparation of Stock Standard and Text-Mixture Solutions
Internal standard, ezetimibe and simvastatin stock standard solutions (1000 μg mL−1) were separately prepared in diluent methanol–water (25:75, v/v) by weighing 10 mg of each one into three 10 mL amber volumetric flasks. The solutions were sonicated for 3 min to aid dissolution then filtered through 0.45 µm polyvinylidene fluoride filters and finally stored at 4 °C. The solutions were found stable for up to 15 days.
For the test-mixture, aliquots from each stock standard solution were transferred into a 2 mL amber volumetric flask and diluted to volume with diluent. The final concentrations were 100 μg mL−1 for ezetimibe and internal standard, while 200 μg mL−1 for simvastatin. The test-mixture was sonicated for 3 min, filtered in a 0.45 µm polyvinylidene fluoride filter and injected on the capillary electrophoresis instrument.
Pharmaceutical and Placebo Stock and Working Preparation
Twenty tablets of Vitoryn® containing 10/20 mg of ezetimibe/simvastatin were weighed and finely powdered. An amount of powder corresponding to 10 mg of ezetimibe and 20 mg of simvastatin was transferred into 10 mL amber volumetric flask and added 5 mL of methanol, the mixture was sonicated for 15 min to aid dissolution with occasional shaking, and diluted to volume with the same solvent. The solution was filtered through a 0.45 µm polyvinylidene fluoride filter. The obtained concentration was 1000 µg mL−1 for ezetimibe and 2000 µg mL−1 for simvastatin.
Placebo matrix was prepared according to the following formula: microcrystalline cellulose (46.8%), lactose monohydrate (45%), magnesium stearate (2%), propyl gallate (0.1%), citric acid (1%), sodium croscarmellose (3%), hydroxypropyl methylcellulose (2%) and butylhydroxyanisole (0.1%).
An aliquot of 200 µL from sample stock solution was volumetrically transferred into a 2 mL amber volumetric flask, and 200 µL of internal standard stock solution was added and the volume was completed with diluent to prepare the working sample solution. The final concentrations were 100 μg mL−1 for ezetimibe and internal standard, while 200 μg mL−1 for simvastatin. The solution was filtered in a 0.45 µm polyvinylidene fluoride filter before being injected.
Method Validation
System Suitability
The system suitability test was carried out to ensure the system resolution and reproducibility. The test was performed by injecting six replicates of working mix standard solutions of simvastatin (200 μg mL−1), ezetimibe and internal standard (100 μg mL−1). The assessed parameters were theoretical plates (N), peak area, migration time, resolution (Rs) and tailing factor.
Specificity/Selectivity
The specificity of proposed method for the determination of ezetimibe and simvastatin was assessed by injecting the Vitoryn® sample, placebo, standard solutions and diluent into the capillary electrophoresis system.
Selectivity and forced degradation stability indicating studies for ezetimibe and simvastatin were applied under hydrolytic (alkaline and acidic), photolytic, thermolytic and oxidative stress conditions according to ICH Q1A(R2) requirements [24]. Ezetimibe and simvastatin standards solutions, Vitoryn® sample solution and placebo were submitted for each forced degradation condition.
The peak purity for both drugs in the obtained electropherograms was evaluated with diode array detector. The interference from tablet excipients was performed by subjecting the placebo to the same stress conditions as Vitoryn® samples.
Alkaline and Acidic Hydrolysis
The concentration of 1 mg mL−1 for ezetimibe and 2 mg mL−1 for simvastatin of Vitoryn® sample was carried out for alkaline (0.1 N NaOH) and acidic (0.1 N HCl) hydrolysis. The solutions were placed at room temperature and under light protection for 7 days.
Thereafter, 200 μL of each solution was transferred volumetrically to 2 mL amber volumetric flask, internal standard stock solution (200 µL) and diluent (1.6 mL) were added, obtaining final concentrations of 100 μg mL−1 of ezetimibe and internal standard, while 200 μg mL−1 of simvastatin. The solutions were sonicated for 5 min then, filtered in a 0.45 µm polyvinylidene fluoride filter before being used. The same procedure was made with placebo tablets.
Thermal and Photolytic Degradation
500 mg of powder from Vitoryn® sample was transferred separately to two Petri plates, one of these was placed in a hot oven at 60 °C (thermal degradation) and the other one, was exposed to direct sunlight (photolytic degradation). After 7 days, an appropriate amount of each powder equivalent to 10 mg of ezetimibe and 20 mg of simvastatin was transferred into two 10 mL amber volumetric flasks. The contents were diluted with 5 mL of methanol and sonicated for 15 min. The volume was completed with the same diluent. The mixtures were submitted at the same condition as in section alkaline and acidic hydrolysis (second paragraph).
Oxidative Degradation
Appropriate amount of Vitoryn® sample powder equivalent to 10 mg of ezetimibe and 20 mg of simvastatin were transferred into a 10 mL amber volumetric flask. The content was diluted with 5 mL of methanol, and 2.5 mL of 3% hydrogen peroxide. The mixture was sonicated for 15 min and submitted at the same condition as in section alkaline and acidic hydrolysis (second paragraph).
Linearity and Range
Linearity was verified by preparing five concentrations by diluting adequate amounts of internal standard, ezetimibe, and simvastatin stock standard solutions in diluent. The concentration ranges from 80 to 120 μg mL−1 for ezetimibe, 160 to 240 μg mL−1 for simvastatin, and fixed concentration of internal standard (100 μg mL−1) were obtained. Three analytical curves for ezetimibe and simvastatin were constructed by plotting the peak area ratio (ezetimibe or simvastatin peak area/internal standard peak area) against ezetimibe or simvastatin concentrations, respectively. Electropherograms were recorded for the solutions injected in triplicate. The method of least squares regression and analysis of variance were used to determine method linearity (p = 0.05). The data homoscedasticity and linearity were analyzed through an F test.
Detection and Quantification Limits
The limit of detection and quantification for ezetimibe and simvastatin were measured using the following equations according to ICH guidelines:
where σ is the standard deviation of the intercept of the mean of three analytical curves, determined by a linear regression model and α is the slope of the corresponding analytical curve [25].
Accuracy
Accuracy was determined in terms of percent recovery. Placebo matrix was spiked with ezetimibe and simvastatin standard solutions at three different concentration levels 80, 100 and 120 μg mL−1 for ezetimibe and 160, 200 and 240 μg mL−1 for simvastatin, equivalent to 80, 100 and 120% of the analytical concentrations, respectively. Each determination was performed in triplicate to obtain the mean and relative standard deviation percentage.
Precision
Method precision was evaluated by repeatability (intra-day) and intermediate (inter-day) analysis. Six determinations from sample solution, on the same day by a single analyst, were performed to study the repeatability. Intermediate precision was evaluated in the same way as repeatability but by different analyst in a different day. Sample Vytorin® (ezetimibe 10 mg and simvastatin 20 mg) solution with constant concentration of internal standard (100 μg mL−1) were used. The results were expressed in terms of relative standard deviation percentage for ezetimibe and simvastatin content in the tablets.
Robustness
The robustness was performed by evaluating slight deliberate changes of the optimal analytical conditions such as hydrodynamic injection time (0.1 s and 0.5 s, optimal 0.3 s) and % of organic modifier (10% and 14% acetonitrile, optimal 12%). The responses were evaluated in terms of system suitability i.e., tailing factor, resolution, and efficiency (theoretical plates).
Results and Discussion
For quality control laboratories, it is very important to develop separation methods for simultaneous quantitation of drugs in combined dosage forms that do not demand pretreatment. Capillary electrophoresis is an alternative technique for this purpose. It offers wide applications [22] and several benefits such as fast analysis, good resolution, low sample consumption, high efficiency and get over several disadvantages of HPLC technique [26]. It is also considered an eco-friendly analytical technique since it uses very small volumes of organic solvents or sometimes does not use them, preventing or reducing the generation of hazardous waste. In addition, it uses long lasting and versatile capillary columns, the same capillary can be used to analyze from small ions to macromolecules. This feature also makes it an economical technique [23].
Method Development and Optimization
Different trials were carried out to optimize the simultaneous separation of ezetimibe and simvastatin within a short analytical time with good resolution. The electrolyte pH adjustment is very important to keep the electroosmotic flow and the migration velocities of weak electrolyte components constant. Thus, a stable and reproducible migration behavior of the analytes can be achieved. The effective mobility of cationic and anionic analytes is strongly dependent on their pKa value related to background electrolyte pH. However, it is worth mentioning that even for substances without effective mobility, it can move through the capillary due to electroosmotic flow, which in turn, is also dependent on background electrolyte pH [27]. In the separation of neutral substances, the pH adjustment has little influence on selectivity; however, a pH value must be selected for the system in which the surfactant micelles are ionized in such a way to allow their migration at a different rate than electroosmotic flow [28].
As ezetimibe and simvastatin drugs are water insoluble, this problem can be overcome by using micelles as additives [29]. Solutes interact with a migrating micellar phase and this mechanism allows separation of neutral and charged species [22]. Additives act as a pseudo-stationary phase for the separation of closely compounds and other unresolved compounds in capillary zone electrophoresis [30].
Then micellar electrokinetic chromatography method was performed by addition of sodium dodecyl sulfate surfactant above its critical micellar concentration. This provides a chromatographic partition between pseudo-stationary phase (micelles), formed when the surfactants are above their critical micellar concentration, and the aqueous background electrolyte. To obtain a good separation, effects of pH, buffer concentration, sodium dodecyl sulfate concentration, organic modifier, applied voltage, injection time and detection wavelength were studied.
Effect of pH Value
The effect of pH was performed from 7 to 9 since pKa values of ezetimibe and simvastatin were between 9.75 and 14.91 [31, 32]. For optimum resolution between analytes, the background electrolyte pH should be in the range of the corresponding pK values of the analytes [22]. The initial sodium tetraborate buffer concentration was set at 20 mmol L−1 as background electrolyte, containing 30 mmol L−1 sodium dodecyl sulfate, the buffer pHs were modified by using o-phosphoric acid and NaOH solutions. As sodium tetraborate buffer pH increased, the electroosmotic flow was also increased which resulted in shorter migration time (pH 9), but the analytes could not be well separated (Fig. 2C). To obtain complete separation, organic modifier was added to the background electrolyte (Fig. 3B). Here, pH was set at 9 under constant conditions, temperature 30 ºC, injection time 0.3 psi/3 s and voltage + 30 kV.
Electropherograms of the effect of pH. A pH 7, B pH 8, and C pH 9 of sodium tetraborate buffer background electrolyte on the separation of standard mixture of ezetimibe, simvastatin and internal standard (100 μg mL−1). Electrophoretic conditions: uncoated fused-silica capillary with 50 µm i.d. × 42 cm total (30 cm effective) length; electrolyte: 20 mmol L−1 sodium tetraborate buffer solution, and 30 mmol L−1 sodium dodecyl sulfate; hydrodynamic injection: 0.3 psi/3 s; applied voltage: + 30 kV; temperature: 30 °C; UV detection at 238 nm. Peaks: (1) losartan potassium (internal standard), (2) ezetimibe, (3) simvastatin, and (EOF) electroosmotic flow
Electropherograms of the effect of sodium dodecyl sulfate concentration. A 20 mmol L−1, B 30 mmol L−1, C 40 mmol L−1, and D 50 mmol L−1 on the separation of standard mixture of simvastatin (200 μg ml−1), ezetimibe and internal standard (100 μg mL−1). Electrophoretic conditions: uncoated fused-silica capillary with 50 µm i.d. × 42 cm total (30 cm effective) length; electrolyte: 20 mmol L−1 sodium tetraborate buffer solution (pH 9), × mmol L−1 sodium dodecyl sulfate and 12% acetonitrile; hydrodynamic injection: 0.3 psi/3 s; applied voltage: + 30 kV; temperature: 30 °C; UV detection at 238 nm. Peaks: (1) losartan potassium (internal standard), (2) ezetimibe, (3) simvastatin, and (EOF) electroosmotic flow
Effect of Organic Modifier
In micellar electrokinetic chromatography, the presence of water-miscible organic solvents such as methanol, 2-propanol and acetonitrile usually improve resolution and/or variation in the selectivity. They reduce capacity factor of highly hydrophobic solutes to within or near the ideal range because of altering the retention mechanism by changing the polarity of the aqueous phase, electrolyte viscosity and zeta potential. The organic solvents contribute to reduce the electroosmotic velocity and expand the migration time window and increase resolution [33]. However, these solvents in high concentrations cannot be used as they can breakdown micellar structure. Generally, the maximum content of the organic solvent that can be used is 20% or so [30]. The influence of acetonitrile and methanol in background electrolyte was performed in concentration range 5 to 15% (v/v). The results showed a decrease of electroosmotic flow mobility giving longer analysis time when used 15% of solvent, especially when methanol was used, since methanol is more viscous than acetonitrile. Therefore, acetonitrile was chosen for further analysis. Ezetimibe and simvastatin were nearly co-eluted in a low amount of acetonitrile ≤ 8%. The separation between the analytes under study was getting increased with the increase in the further levels of acetonitrile. Hence, 12% (v/v) acetonitrile was chosen as the optimum concentration for the complete separation of ezetimibe and simvastatin (Fig. 3B), under constant conditions of pH 9, temperature 30 ºC, injection time 0.3 psi/3 s and voltage + 30 kV.
Effect of Surfactant Concentration
Sodium dodecyl sulfate is the most used surfactant in micellar electrokinetic chromatography. Surfactants are added to background electrolyte in concentrations above their critical micellar concentration to form micelles. Analytes distribute according to their hydrophobicity in and outside of the micelles and they are separated by their different affinity to the micelles [30].
In the present work, the effect of surfactant concentration on the migration times of ezetimibe and simvastatin was studied. Sodium dodecyl sulfate concentration was evaluated in the range of 20 to 50 mmol L−1. The obtained results shown that sodium dodecyl sulfate concentration had high effect in the mobility of the two analytes. The higher sodium dodecyl sulfate concentration the higher migration time. Hence, 30 mmol L−1 sodium dodecyl sulfate was chosen for this study because it gave sharp peaks, short analysis time (approximately 5.8 min) (Fig. 3) compared with Yardimci and Ӧzaltin work [20], they determined the analytes in approximately 10 min.
Effect of Borate Buffer Concentration
The influence of borate buffer concentration on the separation of ezetimibe and simvastatin was analyzed with concentration 20, 30 and 40 mmol L−1 at pH 9. It was observed that migration time increased with increasing borate concentration. The higher buffer concentration the higher background electrolyte viscosity and the lower electroosmotic flow mobility. Furthermore, the current increased (≈ 82 µA) with increasing borate concentration, above 30 mmol L−1. No change of the retention order was observed. Therefore, 20 mmol L−1 borate buffer was chosen for its good symmetric peaks and analysis time (Fig. 4C).
Electropherograms at the finally optimized micellar electrokinetic chromatography method. A background electrolyte, B Vitoryn® placebo sample, C standard mixture of 100 μg mL−1 ezetimibe, 200 μg ml−1 simvastatin and 100 μg mL−1 internal standard, D Vitoryn® sample (100 μg mL−1 ezetimibe and 200 μg mL−1 simvastain) and 100 μg mL−1 internal standard. Electrophoretic conditions are the same as in Fig. 3 with 30 mmol L−1 sodium dodecyl sulfate. Peaks: (1) losartan potassium (internal standard), (2) ezetimibe, (3) simvastatin, and (EOF) electroosmotic flow
Effect of Applied Voltage, Injection Time and Capillary Temperature
Regarding the applied voltage, values from 20 to 30 kV were tested using the optimized background electrolyte. The results showed that by increasing voltage, migration times decreased, it is owing to increase in electroosmotic flow, since the magnitude of electroosmotic flow is directly proportional to the strength of the electric field [33]. Hence, a voltage of 30 kV was chosen which producing best peak shape with short migration time of the analytes.
Another instrumental parameter evaluated was the injection time. The volume injected depends essentially on the injection time and it affects area, width, and height of the peaks. In this way, tests were carried out with times of 1, 3 and 5 s with hydrodynamic injection at 0.3 psi. It was observed that above 3 s there was a widening at baseline peaks, causing their distortion and deformation. Good peak shapes for ezetimibe and simvastatin (Fig. 4C) were observed with 3 s injection time. Therefore, this injection time was used for further experiments.
Capillary temperature was also investigated in the range from 25 to 40 °C. Best results were observed at 30 °C in terms of current generated in the capillary, resolution and run time. Increasing the temperature above 30 °C resulted in shorter run time but poor resolution and high current. Therefore, temperature of 30 °C was selected for further analysis.
Selection of Detection Wavelength
Using diode array detector, absorption spectra of ezetimibe and simvastatin showed maximum absorbance at 238 nm. Figure 1C shows an overlap of ezetimibe and simvastatin spectra. In addition, samples of the tablet matrix do not showed interference at this wavelength (Fig. 4D). Thus, we thereafter used a 238 nm detection wavelength for all experiments.
Internal Standard
Internal standard improves the quantitative performance of capillary electrophoresis methods in terms of linearity, precision, and recovery data. It eliminates error introduced by variability in voltage, injection volume or electroosmotic flow [34]. Some substances such as clozapine, promethazine and losartan potassium were tested as internal standard candidates. Losartan potassium (100 µg mL−1) was the most suitable due to good absorbance in the method wavelength. Complete separation of internal standard, ezetimibe and simvastatin were obtained with migration times of 2.0, 5.4 and 5.8 min, respectively (Fig. 4).
Optimized Electrolyte Composition
The best results were obtained by using the electrolyte composed of 20 mmol L−1 sodium tetraborate buffer solution (pH 9, adjusted with o-phosphoric acid), 30 mmol L−1 sodium dodecyl sulfate, and 12% acetonitrile. The quantitative determination of the analytes was performed at + 30 kV and 30 °C. Under these conditions, ezetimibe and simvastatin were separated below 6 min (Fig. 4).
Method Validation
The validation of an analytical method ensures credibility during routine use, being sometimes referred to as a process which provides documented evidence that the method is suitable for its purpose. The proposed method was fully validated as per ICH guidelines [25].
System Suitability
The obtained results of six replicate injections showed that the parameters tested were within the acceptable range. Ezetimibe and simvastatin were well separated at 5.4 and 5.8 min, respectively, expressing excellent resolution (mean resolution 7). The tailing factor for both ezetimibe and simvastatin peaks never exceeded 1.5 indicating good peak symmetry (acceptance limit is < 2). The theoretical plates were always > 10,000 in all electropherograms which ensured good efficiency throughout the developed separation process [25]. The proposed method offers high sensitivity, and both drugs can be detected accurately. The analytes were well separated from the degradation products. Results are presented in Table 1.
Specificity/Selectivity and Forced Degradation Stability Indicating Capability
The electropherograms of background electrolyte, standards (ezetimibe and simvastatin), placebo and Vitoryn® sample were compared to evaluate the specificity of the proposed method. The specificity was demonstrated by the absence of interference among ezetimibe, simvastatin and excipients from Vitoryn® samples (Fig. 4).
Selectivity and stability indicating capability of the analytical method was performed through forced degradation study. Peak purity was assessed using diode array detector. The analysis was carried out to ensure that the proposed method was able to separate ezetimibe and simvastatin from the degradation products generated during the forced degradation study. The samples were subjected to stress conditions by acidic and alkaline hydrolysis, oxidative, thermal (60 °C) and photolytic degradation for 7 days. It was observed that sample was sensitive to acidic hydrolysis, with the formation of one degradation product in 5.1 min (Fig. 5D). In alkaline hydrolysis, it was observed degradation of ezetimibe and almost total degradation of simvastatin, showing two degradation products at 4.3 and 5.1 min (Fig. 5E). In oxidative condition, it was observed the formation of two degradation products at 5.1 and 5.3 min (Fig. 5F). Under heating test, it was observed the formation of three degradation products at 5.1, 5.7 and 6.2 min (Fig. 5G). Under photolytic stress, there were observed two degradation products at 4.5 and 5.7 min (Fig. 5H). In all cases, ezetimibe, simvastatin and internal standard peaks showed purity factor greater than 99%. In addition, resolution between the analyte peaks and the possible degradation product was greater than 1.5, which shows acceptable degree of method specificity.
Electropherograms of forced degradation study. A placebo, B standard mixture of 100 μg mL−1 ezetimibe, 200 μg mL−1 simvastatin and 100 μg mL−1 internal standard, C Vitoryn® sample (100 μg mL−1 ezetimibe and 200 μg mL−1 simvastatin, D acidic hydrolysis (0.1 mol L−1 HCl); E alkaline hydrolysis (0.1 mol L−1 NaOH), F oxidative conditions (3% hydrogen peroxide), G thermal conditions (60 °C), and H photolytic conditions (visible light) for 7 days for all above conditions. Electrophoretic conditions are the same as in Fig. 2 except pH 9. Peaks: (1) losartan potassium (internal standard), (2) ezetimibe, (3) simvastatin, and (EOF) electroosmotic flow
Linearity and Range
The data obtained from plotting the peak area ratios (ezetimibe or simvastatin/internal standard) versus drug concentrations confirmed the method linearity in the concentration range between 80 and 120 μg mL−1 for ezetimibe; while 160 and 240 μg mL−1 for simvastatin. The correlation equations obtained for the linear model were y = 0.0373x–1.1003 and y = 0.0417x–1.7465 with determination coefficient of 0.9956 and 0.9920 for ezetimibe and simvastatin, respectively. Analysis of variance at 95% was performed to verify the good fitting of the micellar electrokinetic chromatography method, to evaluate its linearity and the validity of linear regression. From the obtained data (Table 2) we can see there was significant regression since calculated F was greater than critical F.
Therefore, analysis of variance showed that the adjustment of the linear model is appropriate for the two curves constructed, since the p values found were lower than Fcritical values, indicating that the variances of the response in Y are independent of the concentration levels in X. The adjustment to the linear model was verified by normal and random distribution of the residuals (Fig. 6A and 6B), thus showing that there is no dependency between the values. Therefore, the homoscedasticity assumption is satisfied indicating a good fit for the linear model.
Limits of Detection and Quantification
Limits of detection and quantification was obtained by using the equations specified in section detection and quantification limits, demonstrate that the method is sensitive and viable to be applied for the determination of ezetimibe and simvastatin in combined dosage forms (Table 2).
Accuracy
The proposed analytical method accuracy was evaluated by determining the mean percent recoveries of ezetimibe and simvastatin standards added to the placebo matrix using three replicate determinations at three different levels (80, 100 and 120% of the working concentration). The obtained mean recoveries were 101.0% for ezetimibe and 100.4% for simvastatin with RSD% < 1, demonstrating the method accuracy (Table 3). Recovery values in the range of 100 ± 2% of the target value are considered acceptable [35].
Precision
The repeatability and intermediate method precision (intra- and inter-day, respectively) were assessed. The repeatability (n = 6) was evaluated as the RSD% of six independent samples, performed in the same day and by the same analyst. The intra-day mean assay was 9.95 ± 0.10 mg/tablet (RSD% 1.05) for ezetimibe and 19.68 ± 0.08 mg/tablet (RSD% 0.38) for simvastatin. The intermediate precision was assessed by the analyses of other six independent samples by a different analyst, which showed mean assay of 10.07 ± 0.12 mg/tablet (RSD% 1.20) for ezetimibe and 19.77 ± 0.14 mg/tablet (RSD% 0.69) for simvastatin. Both repeatability and intermediate precision levels showed low RSD% values (< 2) which confirmed the good method precision. These results also indicated that the mean of the assay 10.01 ± 0.12 mg/tablet (RSD% 1.24) for ezetimibe and 19.73 ± 0.11 mg/tablet (RSD% 0.58) for simvastatin was in good agreement with the label claim for Vitoryn® tablets. The RSD% was < 2, demonstrating that the precision of the analytical method is satisfactory (Table 4).
Robustness
The proposed method robustness test showed deliberate changes in the injection time and in the proportion of the organic solvent that can interfere in the instrumental responses. It was observed broad peaks at baseline for both analytes when injection time decreased (symmetry > 2), on the other hand, when the injection time increased no broad peaks was observed (symmetry < 2). Contrarily, decreasing the proportion of organic solvent makes the asymmetry factor better as compared to increased organic solvent. While increasing the theoretical plates makes the injection time and proportion of organic solvent decreased, on the other hand, when the injection time and proportion of organic solvent increased then the theoretical plates decreased. Resolution was unaffected with deliberate change in injection time and proportion of organic solvent (Table 5).
Stability of Solutions
The stability of sample solutions even as standards working solutions in the diluting solvent (methanol and ultrapure water) was checked, no changes were detected within 8 h at room temperature. On the other hand, the standard stock solutions were stored in refrigerator at 4 ºC, they were stable for at least two weeks. Peaks areas and migration time of the drugs seemingly remained unchanged (RSD% < 2). No significant degradation was observed during that period.
Application of the Proposed Method to the Commercial Pharmaceutical Preparation
Ezetimibe and simvastatin analysis in their combined tablets (Vitoryn®) was performed using the developed and validated micellar electrokinetic chromatography method. The assay of ezetimibe and simvastatin in commercial pharmaceutical formulation indicated a mean value of 10.10 ± 0.77 mg/tablet (101.0%, RSD% 0.76) for ezetimibe and 10.04 ± 0.35 mg/tablet (100.4%, RSD% 0.35) for simvastatin. There was good agreement between the obtained results and the label claim for ezetimibe and simvastatin tablets (Table 3). The results were satisfactory, and they are within tolerance limits (95 to 105% label claim) [6].
Greenness Assessment of the Proposed Method
Green Analytical Procedure Index [36] and Analytical GREEnness [37] were employed in this study to evaluate the method greenness.
According to Green Analytical Procedure Index, three different colored (green, yellow and red) pictograms (five-pentagram) allowed us to determine the method greenness in which green color is considered to be more eco-friendly as compared to other two colors (yellow and red). The result showed that 10 regions are shaded green, 2 yellow, 2 red and 1 white indicating the proposed method greenness (Table 6A). A numerical estimation was also made with HPLC [10] which revealed that 4 regions are green, 4 yellow, 6 red and 1 white making the reported method less green as compared to the proposed micellar electrokinetic chromatography method.
Analytical GREEnness found to be comprehensive, convenient, reliable and straightforward evaluation approach which gathers both quantitative and qualitative aspects of comparison. According to this approach, each of the 12 parameters has a score from 0.0 to 1.0 which is indicated by a specific pictogram color ranging from red (0.0) to dark green (1.0). In this work, the final score was 0.81 indicating the greenness of the proposed micellar electrokinetic chromatography method (Table 6B). A numerical estimation was also made with HPLC [10] which revealed less green (0.67) as compared to the proposed method.
Therefore, both are highly recommended approaches that produces an easily interpretable and informative results for the greenness assessment [36, 37].
Conclusion
A fast, sustainable and green micellar electrokinetic chromatography method has been investigated for the simultaneous quantification of ezetimibe and simvastatin. The method was fully optimized and validated as per ICH guideline and was successfully applied for the analysis of ezetimibe/simvastatin in binary mixture tablet dosage form and in the presence of their degradation products after submitted to different forced degradation conditions (oxidative, thermal, photolytic, alkaline and acidic hydrolysis). The proposed method was the first to be considered stability indicating since it enabled the determination of the active pharmaceutical ingredients without any interference under different forced degradation conditions. To our knowledge, there is no work with this intend, on the other hand there is only one research in which ezetimibe and simvastatin were determined simultaneously using capillary electrophoresis in 10 min. In this work, we determined the analytes in less than 6 min. The proposed micellar electrokinetic chromatography method has several advantages like specific, accurate and cost-effective. Therefore, it can be successfully applied in quality control laboratories for determining ezetimibe and simvastatin in their combined binary mixture. Furthermore, the method consumes less solvent with high separation efficiency. Green Analytical Procedure Index and Analytical GREEnness metrics were used to demonstrate the greenness of the proposed method.
Data Availability
The data that support the findings of this study is available from the corresponding author upon reasonable request.
References
Du Q, Zhang Y, Wang J, Liu B (2021) Simultaneous determination and quantitation of hypolipidemic drugs in fingerprints by UPLC-Q-TRAP/MS. J Chromatogr B Anal Technol Biomed Life Sci 1175:1–9. https://doi.org/10.1016/j.jchromb.2020.122496
Davies JT, Delfino SF, Feinberg CE et al (2016) Current and emerging uses of statins in clinical therapeutics: a review. Lipid Insights 9:13–29. https://doi.org/10.4137/LPI.S37450
Schiele F, Farnier M, Krempf M et al (2018) A consensus statement on lipid management after acute coronary syndrome. Eur Hear J Acute Cardiovasc Care 7:532–543. https://doi.org/10.1177/2048872616679791
Bove M, Fogacci F, Cicero AFG (2017) Pharmacokinetic drug evaluation of ezetimibe + simvastatin for the treatment of hypercholesterolemia. Expert Opin Drug Metab Toxicol 13:1099–1104. https://doi.org/10.1080/17425255.2017.1381085
Kei AA, Filippatos TD, Elisaf MS (2016) The safety of ezetimibe and simvastatin combination for the treatment of hypercholesterolemia. Expert Opin Drug Saf 15:559–569. https://doi.org/10.1517/14740338.2016.1157164
United States Pharmacopeial Convention (2021) United States Pharmacopoeia-National Formulary (USP–NF). United States Pharmacopeial Convention, Rockville
Jeu LA, Cheng JWM (2003) Pharmacology and therapeutics of ezetimibe (SCH 58235), a cholesterol-absorption inhibitor. Clin Ther 25:2352–2387. https://doi.org/10.1016/s0149-2918(03)80281-3
Shrestha B, Stephenrathinaraj B, Sharan Patel S et al (2010) Simultaneous HPTLC estimation of simvastatin and ezetimibe in tablet dosage form. E-J Chem 7:1206–1211
Al-Hashimi NN, Shahin RO, Al-Hashimi AN et al (2019) Cetyl-Alcohol-Reinforced Hollow Fiber Solid/Liquid-Phase Microextraction and HPLC-DAD Analysis of Ezetimibe and Simvastatin in Human Plasma and Urine. Biomed Chromatogr 33:1–8. https://doi.org/10.1002/bmc.4410
Desai PR, Mehta PJ, Ojha SK, Chokshi AB (2018) Simultaneous quantification of related substances of ezetimibe and simvastatin in combined dosage form using a novel stability-indicating liquid chromatographic method. Acta Chromatogr 30:85–94. https://doi.org/10.1556/1326.2017.00273
Fahad AMM, Rasheed AS, Ali HH (2022) Separation and determination of simvastatin on ZIC-HILIC stationary phases by hydrophilic interaction chromatography in pharmaceutical material products. Mater Today Proc 49:2817–2821. https://doi.org/10.1016/j.matpr.2021.09.535
Kurbanoglu S, Esim O, Ozkan CK et al (2019) Development and validation of RP-LC method for the simultaneous determination of simvastatin and ezetimibe in fixed-dose combination tablets and in rabbit serum. Chromatographia 82:279–285. https://doi.org/10.1007/s10337-018-3642-x
Devu S, Gupta A, Srinivas KS et al (2012) Development and validation of stability indicating RP-UPLC method for simultaneous determination in fixed dose combination of ezetimibe and simvastatin. Chromatogr Sep Tech 3:1–7. https://doi.org/10.4172/2157-7064.1000131
Wang D, Qin F, Chen L et al (2008) Determination of simvastatin in human plasma using ultra-performance liquid chromatography-tandem mass spectrometry. Chinese J Chromatogr 26:327–330
Elawady T, Ibrahim F, Belal F (2021) Simultaneous determination of ezetimibe, atorvastatin and simvastatin using quadrupole LC-MS: application to combined tablets and plasma after SPE. Acta Chromatogr 33:245–252. https://doi.org/10.1556/1326.2020.00752
Karanam SR, Katakam P, Chandu BR et al (2014) Simultaneous determination of ezetimibe and simvastatin in rat plasma by stable-isotope dilution LC-ESI-MS/MS and its application to a pharmacokinetic study. J Pharm Anal 4:286–294. https://doi.org/10.1016/j.jpha.2013.08.002
Huang L, Shou L, Hu L, Zhou M (2019) Simultaneous determination of 45 addition chemical drugs in hyperglycemic, hyperlipidemic and hypertensive people’s health foods by HPLC-MS/MS. Chinese J Pharm Anal 39:484–495
Magdy N, Ayad MF (2015) Two smart spectrophotometric methods for the simultaneous estimation of simvastatin and ezetimibe in combined dosage form. Spectrochim Acta Part A Mol Biomol Spectrosc 137:685–691. https://doi.org/10.1016/j.saa.2014.08.042
de Souza FH, Todeschini V, Sangoi MD (2018) Chemometric-assisted spectrophotometric method for the simultaneous quantitative determination of ezetimibe and simvastatin in their combined dosage forms. J AOAC Int 101:1015–1020. https://doi.org/10.5740/jaoacint.17-0124
Yardimci C, Özaltin N (2010) Simultaneous determination of ezetimibe and simvastatin in pharmaceutical preparations by MEKC. J Chromatogr Sci 48:95–99. https://doi.org/10.1093/chromsci/48.2.95
Marina ML, Ríos Castro A, Valcárcel Cases M (2005) Analysis and detection by capillary electrophoresis. Comprehensive analytical chemistry, 1st edn. Elsevier, Amsterdam, pp 1–30
Karger BL (1998) Capillary Electrophoresis: Overview and Perspective. In: Khaledi MG (ed) High performance capillary electrophoresis: theory, techniques, and applications, 1st edn. John Wiley & Sons Ltd, New York, pp 3–23
Ranasinghe M, Quirino JP (2021) Can we replace liquid chromatography with the greener capillary electrophoresis? Curr Opin Green Sustain Chem 31:1–8. https://doi.org/10.1016/j.cogsc.2021.100515
ICH Expert Working Group (2003) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: Stability Testing of New Drug Substances and Products Q1A(R2)
European Medicines Agency (1995) International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: Validation of Analytical Procedures (Text and Methodology) Q2(R1)
Ragab MAA, Abdel-Hay MH, Ahmed HM, Mohyeldin SM (2019) Application of Capillary Zone Electrophoresis Coupled with a Diode Array Detector (CZE-DAD) for Simultaneous Analysis of Ibuprofen and Phenylephrine. J AOAC Int 102:473–479. https://doi.org/10.5740/jaoacint.18-0134
Beckers JL, Boček P (2003) The preparation of background electrolytes in capillary zone electrophoresis: golden rules and pitfalls. Electrophoresis 24:518–535. https://doi.org/10.1002/elps.200390060
Gomes TVD (2012) Desenvolvimento e Validação Intralaboratorial do Método por Cromatografia Eletrocinética Capilar Micelar para Determinação de Vitamina K1 e k3 em chá Verde e em Suplemento Farmacêuticos. Pontifícia Universidade Católica do Rio de Janeiro
Voeten RLC, Ventouri IK, Haselberg R, Somsen GW (2018) Capillary electrophoresis: trends and recent advances. Anal Chem 90:1464–1481. https://doi.org/10.1021/acs.analchem.8b00015
Terabe S, Otsuka K, Ichikawa K et al (1984) Electrokinetic separations with micellar solutions and open-tubular capillaries. Anal Chem 56:111–113. https://doi.org/10.1021/ac00265a031
(2022) Simvastatin: Uses, Interactions, Mechanism of Action. In: DrugBank Online. https://go.drugbank.com/drugs/DB00641. Accessed 11 Aug 2022
(2022) Ezetimibe: Uses, Interactions, Mechanism of Action. In: DrugBank Online. https://go.drugbank.com/drugs/DB00973. Accessed 11 Aug 2022
Otsuka K, Terabe S (1998) Micellar Electrokinetic Chromatography. Mol Biotechnol 9:253–271. https://doi.org/10.1007/BF02915799
Altria KD (2002) Improved Performance in Capillary Electrophoresis Using Internal Standards. LC-GC Eur 1–5
Shabir GA (2003) Validation of high-performance liquid chromatography methods for pharmaceutical analysis: understanding the differences and similarities between validation requirements of the US food and drug administration, the US pharmacopeia and the international Conf. J Chromatogr A 987:57–66. https://doi.org/10.1016/s0021-9673(02)01536-4
Płotka-Wasylka J (2018) A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta 181:204–209. https://doi.org/10.1016/j.talanta.2018.01.013
Pena-Pereira F, Wojnowski W, Tobiszewski M (2020) AGREE-analytical greenness metric approach and software. Anal Chem 92:10076–10082. https://doi.org/10.1021/acs.analchem.0c01887
Acknowledgements
The authors wish to thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), São Paulo, Brazil (Process 2012/50595-8) for financial support, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil (Process 88882.377651/2019-01) for FAGF scholarship.
Author information
Authors and Affiliations
Contributions
AMCS and FAGF: investigation, methodology, formal analysis, data curation, software, writing—original draft. MFMT: conceptualization, visualization, and resources. AR and MSAP: conceptualization, validation, resources, writing—review and editing, supervision, and project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
All contributing authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Souza, A.M.C., Fajardo, F.A.G., Rashid, A. et al. Capillary Electrophoresis Method for Simultaneous Quantification of Hypocholesterolemic Drugs in Binary Mixture Formulation: Fast, Green, and Cost-Effective Alternative to HPLC. Chromatographia 86, 317–331 (2023). https://doi.org/10.1007/s10337-023-04243-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-023-04243-6