Abstract
Owing to the high toxicity of polycyclic aromatic hydrocarbons (PAHs) and their low content in the environment, the development of efficient analysis methods is very important. In this work, an extraction tube was developed through filling barium sulfate nanoparticle-coated basalt fibers (BFs) in a poly(ether ether ketone) (PEEK) tube. According to the characterization result by scanning electron microscopy, BFs were evenly covered with barium sulfate nanoparticles, and their diameter was about 11 µm. To sensitively determine PAHs, the extraction tube was connected to a high-performance liquid chromatography instrument with diode array detector for an online enrichment and detection method. By optimizing sampling volume, sampling rate, the content of methanol in sample and desorption time, the analysis method provided a wide linear range (0.30–20 µg L− 1) and high enrichment ratio (71–2095), and was useful for the efficient detection of PAHs in environmental aqueous samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are important environmental contaminants that are mainly sourced from the incomplete combustion of carbon-containing substance [1] and are highly carcinogenic. Because of very low content of PAHs in samples, a sample preparation method is necessary for sensitive and accurate detection, such as solid-phase extraction (SPE) [2], stir bar sorptive extraction (SBSE) [3], and liquid-phase microextraction (LPME) [4, 5]. Although these techniques can achieve satisfactory extraction efficiency, there are some issues including time consumption, consumption of organic solvent, and only off-line coupling with chromatography. In-tube solid-phase microextraction (SPME) can solve these problems; it can be coupled with a high-performance liquid chromatography instrument (HPLC) for online analysis, which is solvent free, time saving and accurate [6,7,8]. In-tube SPME–HPLC method has been widely used in various fields such as environmental detection [9, 10], food analysis [11], biological and analysis [12]. The analysis performance is mainly dependent on the extraction tube.
Basalt fiber (BF) is a kind of inorganic fiber with micrometer diameter, which is mainly composed of silica, alumina, calcium oxide, iron oxide and titanium dioxide. It has good chemical stability, high tensile strength, high thermal stability and other excellent properties. It is made from molten basalt, so it is a cheap and green material, and it has been defined as a high-performance functional material. In our previous work, it was applied as the support for extraction fiber and satisfactory performance was exhibited [13]. The efficient extraction coating is also a crucial component for SPME fiber. Many nanomaterials with high surface area, good selectivity and stability have been explored as extraction coatings [14,15,16,17,18]. Barium sulfate nanoparticles have a great surface area and high ratio surface energy, and can produce quantum effect and surface effect. Based on these properties, it is a potential material for extraction coating.
Based on these considerations, the aim of this work was to coat BFs with barium sulfate nanoparticles for developing SPME fibers. SPME fibers were packed into a poly(ether ether ketone) (PEEK) tube to get an extraction tube. It was connected to HPLC with diode array detector for the analysis of PAHs in aqueous samples. Four important factors including sampling volume, sampling rate, the content of organic solvent and desorption time were optimized, and an in-tube SPME–HPLC method was established to detect PAHs in the environment.
Experimental
Materials and Reagents
Methanol and acetonitrile were of chromatographic grade from Tedia Company, Inc. (Fairfield, USA). Naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flour) and pyrene (Py) were of analytical reagent grade from Shanghai Pure Reagent Co., Ltd (Shanghai, China). Other chemicals were of analytical reagent grade. Barium sulfate nanoparticles were purchased from Beijing Deco Island Gold Technology Co., Ltd (Beijing, China). BFs were obtained from Zhejiang GBF Basalt Fiber Co., Ltd. (Dongyang, China). PEEK tube (750 µm i.d., 0.16 cm o.d.) was obtained from Haohai Chemical Co. (Wuhan, China). Mobile phase was filtered with a 0.45-µm membrane.
Apparatus
A P102 HPLC pump, bought from Dalian Elite Analytical Instruments Co., Ltd. (Dalian, China), was applied to move sample solution through the extraction tube. All chromatographic tests were performed on an Agilent 1260 HPLC system (Santa Clara, California, USA) equipped with a Zorbax C18 column (250 × 4.6 mm i.d., 5 µm) and a diode array detector (DAD). Field-emission scanning electron microscope (SEM) (SUPRATM55, Carl Zeiss AG, Oberkochen, Germany) was used to characterize fibers.
Preparation of Extraction Tube
After being washed with ethanol, ultrapure water and acetone, BFs were dried. 0.4 g barium sulfate nanoparticles, 1 mL epoxy resin and 150 mL acetone were mixed, and then BFs were immersed into the mixture for 10 min with ultrasound treatment. Barium sulfate nanoparticles were adhered onto the surface of BFs by epoxy resin. BFs were taken out and dried. BFs were washed in ultrapure water under ultrasonic condition. In this way, barium sulfate nanoparticles formed a uniform coating on the surface of BFs. To get the extraction tube, a bundle of BFs coated with nanoparticles was packed into a 30-cm PEEK tube; the mass of BFs was about 191 mg.
Sample Preparation
A mixed PAH solution including Nap, Acy, Ace, Flu, Phe, Ant, Flour and Py was prepared with methanol and stored at 4 °C, and the concentration of each PAH in the stock solution was 100 mg L− 1. Working solutions were prepared daily by diluting the stock solution with ultrapure water to 5 µg L− 1. Real aqueous samples including cigarette ash water and tap water were used to prove the application of in-tube SPME–HPLC method in environmental analysis.
In-Tube SPME–HPLC Online Procedure
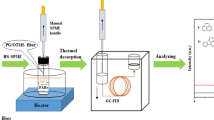
To achieve in-tube SPME–HPLC system, the extraction tube was connected with the HPLC instrument. As shown in Fig. 1, the whole analysis process consists of online extraction, online desorption and detection. As can be seen from Fig. 1a, when the six-port valve is in the “load” position, the sample solution is introduced into the extraction tube. The six-port valve is switched to the “inject” position as shown in Fig. 1b, the mobile phase directly passes through the extraction tube to desorb analytes. After the completion of the desorption process, the six-port valve is returned to the “load” position, and the chromatographic detection and next extraction test are performed at the same time. To get sensitive peak signals, multiple wavelengths were set, including 220 nm for Nap, 225 nm for Acy and Flu, 230 nm for Flour and Py, 250 nm for Phe and Ant, and 260 nm for Ace. A gradient elution (A acetonitrile, B ultrapure water; 0 min A = 70%, B = 30%; 10 min A = 70%, B = 30%; 20 min A = 100%; 20 min stop) and a flow rate of 1 mL min− 1 were used for detecting PAHs.
Results and Discussion
Characterization of Extraction Coating
The fibers were characterized by SEM. As shown in Fig. 2a, b, BFs are uniformly coated with a dense layer of barium sulfate nanoparticles, and the diameter of fibers is about 11 µm. As can be seen from Fig. 2c, d, barium sulfate nanoparticles are under 100 nm in size, and they have a very tight arrangement on the surface of BF.
Optimization of Extraction Conditions
For sensitive analysis of PAHs, four conditions have an important influence including sampling volume, sampling rate, the content of organic solvent in sample and desorption time. To get the best results, these conditions were optimized.
Sampling Volume
The sampling volume is an important factor to influence the extraction efficiency of in-tube SPME. As the extraction volume increases, the target analyte is more fully exposed to the extraction material, thus obtaining high extraction efficiency. After extraction efficiency increases to the maximum, it no longer increases with increasing sampling volume. In general, the optimal extraction volume should be selected for maximum extraction efficiency. In the experiment, sampling rate was fixed as 2.00 mL min− 1, and the extraction volume was investigated at 20, 30, 40, 50 and 60 mL, respectively. As can be seen from Fig. 3a, for Phe, Ant, Flour and Py, the peak area is increased with the increase of sampling volume, indicating that the extraction saturation state was not reached. The sampling volume had no significant effect on the other four analytes. Taking extraction time saving into consideration, 40 mL of sampling volume was adopted.
Sampling Rate
In-tube SPME is a dynamic extraction process, and the sampling rate is one of the main factors. Under the same sampling volume, high sampling rate can help to shorten extraction time, but it is not conducive to the contact between the analyte and extraction coating, which will reduce the extraction efficiency. Furthermore, high sampling rate may cause the damage of extraction tube due to excessive pressure. Sampling volume was fixed as 40 mL, and the sampling rate was tested with 1.00, 1.25, 1.50, 1.75, 2.00 and 2.50 mL min− 1, respectively. As shown in Fig. 3b, peak area of all analytes is slowly reduced along with the increase of the sampling rate. To save extraction time and obtain higher extraction efficiency, a sampling rate of 2.00 mL min− 1 was selected.
Content of Organic Solvent
Adding suitable amount of organic solvents to the sample can improve the uniformity of sample and the analysis accuracy. However, if excessive amount organic solvent is added, the extraction efficiency of hydrophobic analyte will decrease due to their improved solubility in aqueous sample. In this work, different volumes of methanol [0.5, 1.0, 2.0, 3.0, 4.0 and 5.0% (v/v)] were added to the sample solution to investigate the effect. As can be seen from Fig. 3c, for Phe, Ant, Flour and Py, the peak area gradually decreases with increasing methanol content from 0.5 to 5.0% (v/v). For other four analytes, there is little influence of methanol content. So, 0.5% (v/v) of methanol was chosen as the best organic solvent content in sample.
Desorption Time
To achieve accurate detection result, all analytes should be completely released from the extraction tube. In this work, a dynamic desorption process was performed using 1 mL min− 1 of mobile phase (acetonitrile–water, 70:30, v/v). Under the fixed desorption solvent and flow rate, the desorption time directly affects the detection result. Partial amount of analyte is desorbed from the extraction tube with insufficient desorption time, which not only gives inaccurate result but also the residual analyte disturbs greatly the next extraction. If the desorption time is too long, it will delay the next extraction. During the experiment, the desorption time was investigated at 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.5 and 2.0 min, respectively. To prevent the effect of the residue on the next extraction, desorption was carried out once again for 3.0 min before the next test. As shown in Fig. 3d, peak area of Flour and Py is increased from 0.2 to 0.6 min, it becomes stable from 0.6 to 2.0 min. It indicated that desorption of these analytes was inadequate with time less than 0.6 min. For other analytes, their peak area is not changed with desorption time of 0.2–2.0 min. To get adequate desorption of all analytes, 1.0 min was confirmed as the optimum desorption time.
Method Evaluation
After the optimization of extraction conditions, the analysis method was established through extracting a serial of standard solutions. The results of the analytical method including linear range, correlation coefficient, enrichment factor and limit of detection (LOD) are listed in Table 1. The linear ranges of Nap and Acy are 0.30–20 µg L− 1, and the linear ranges of Ace, Flu, Phe, Ant, Flour and Py are 0.30–15 µg L− 1 with correlation coefficient in the range of 0.997–0.999. LOD being 0.10 µg L− 1. These indicated that the analysis method had wide linear ranges and low LOD. The analytical method has high enrichment factors in the range of 71–2095 for eight PAHs. Through the introduction of the extraction tube, the sensitivity of the analysis can be increased to two to three orders than direct detection by HPLC instrument.
Application to Real Samples
To evaluate the analysis method, it was applied to the determination of PAHs in tap water and cigarette ash water. As shown in Fig. 4 and in Table 2, it can be seen that no PAH is found in tap water, and there are 3.67 µg L− 1 of Flour and 4.98 µg L− 1 of Py in the cigarette ash water sample and other six PAHs cannot be quantified. The relative recoveries of analytes spiked at 1.0, 5.0 and 10.0 µg L− 1 in tap water are in the range 81–120, 88–120 and 97–121%, respectively. The corresponding recoveries in cigarette ash water are in the range 79–103, 80–85 and 80–96%. It is obvious that the established in-tube SPME–HPLC method can detect PAHs in real samples very well.
Conclusion
A solid-phase microextraction tube based on barium sulfate nanoparticle-coated basalt fibers was developed. The extraction tube was connected with high-performance liquid chromatography and evaluated using eight PAHs as model analytes. Under the optimized extraction and desorption conditions, a sensitive online analytical method was established. The method provided a wide linear range (0.30–20 µg L− 1), low limit of detection (0.10 µg L− 1) and satisfactory recovery (79–121%). The method was suitable for the efficient detection of polycyclic aromatic hydrocarbons in aqueous sample. In this study, a nanomaterial as the coating was introduced in the field of SPME that not only enriched the kind of extraction sorbents but also expanded the application of barium sulfate nanoparticles. It also gave an inspiration that more nanomaterials should be explored for the development of efficient extraction materials in the future work.
References
Wenzl T, Simon R, Anklam E, Kleiner J (2006) Analytical methods for polycyclic aromatic hydrocarbons (PAHs) in food and the environment needed for new food legislation in the European Union. TrAC-Trend Anal Chem 25:716–725
Cappelinia LT, Cordeiroa D, Brondib SH, Prieto KR, Vieira EM (2012) Development of methodology for determination of pesticides residue in water by SPE/HPLC/DAD. Environ Technol 33:2299–2304
Portugal FCM, Pinto ML, Nogueira JMF (2008) Optimization of polyurethane foams for enhanced stir bar sorptive extraction of triazinic herbicides in water matrices. Talanta 77:765–773
Lasartearagones G, Lucena R, Cardenas S, Valcarcel M (2015) Use of switchable hydrophilicity solvents for the homogeneous liquid-liquid microextraction of triazine herbicides from environmental water samples. J Sep Sci 38:990–995
Hussain I, Ismail RA, Sanagi MM, Wan AWI (2014) Sol-gel coated poly-propylene hollow fiber-based liquid-phase microextraction of triazine herbicides in real water samples. Desalin Water Treat 55:1488–1500
Kataoka H, Narimatsu S, Lord HL, Pawliszyn J (1999) Automated in-tube solid-phase micro extraction coupled with liquid chromatography electrospray ionization mass spectrometry for the determination of beta-blockers and metabolites in urine and serum samples. Anal Chem 71:4237–4244
Kumazawa T, Saeki K, Yanagisawa I, Uchigasaki S, Hasegawa C, Seno H, Suzuki O, Sato K (2009) Automated on-line in-tube solid-phase microextraction coupled with HPLC/MS/MS for the determination of butyrophenone derivatives in human plasma. Anal Bioanal Chem 394:1161–1170
Eisert R, Pawliszyn J (1997) Automated in-tube solid phase microextraction coupled to high performance liquid chromatography. Anal Chem 69:3140–3147
Gonzalez-Toledo E, Prat MD, Alpendurada MF (2001) Solid-phase microextraction coupled to liquid chromatography for the analysis of phenolic compounds in water. J Chromatogr A 923:45–52
Liompart M, Lik FM (1999) Head space solid phase microextraction (HSSPME) for the determination of volatile and semivolatile pollutants in solis. Talanta 48:451–459
Jia M, Zhang QH, Min DB (1999) Pulsed electric field processing effects on flavor compounds and microorganisms of orange juice. Food Chem 65:445–451
Lee M-R, Song Y-S, Hwang B-H, Chou C-C (2000) Determination of amphetamine and methamphetamine in serum via headspace derivatization solid-phase microextraction-gas chromatography-mass spectrometry. J Chromatogr A 896:265–273
Feng J, Tian Y, Wang X, Luo C, Sun M (2018) Basalt fibers functionalized with gold nanoparticles for in-tube solid-phase microextraction. J Sep Sci 41:1149–1155
She X-K, Wang X, Zhou J-B, Zhao R-S (2015) Porous lead(II)-based metal organic nanotubes as an adsorbent for dispersive solid-phase extraction of polybrominated diphenyl ethers from environmental water samples. J Chromatogr A 1423:31–38
Wen C, Li M, Li W, Li Z, Duan W, Li Y, Zhou J, Li X, Zeng J (2017) Graphene deposited onto aligned zinc oxide nanorods as an efficient coating for headspace solid-phase microextraction of gasoline fractions from oil samples. J Chromatogr A 1530:45–50
Xu J, Zheng J, Tian J, Zhu F, Zeng F, Su C, Ouyang G (2013) New materials in solid phase microextraction. Trends Anal Chem 47:68–83
Aziz-Zanjani MO, Mehdinia A (2014) A review on procedures for the preparation of coatings for solid phase microextraction. Microchim Acta 181:1169–1190
Wang L, Hou X, Li J, Liu S, Guo Y (2015) Graphene oxide decorated with silver nanoparticles as a coating on a stainless-steel fiber for solid-phase microextraction. J Sep Sci 38:2439–2446
Acknowledgements
This research work was financially supported by the National Natural Science Foundation of China (NSFC, Nos. 21777054 and 21405061) and the Shandong Provincial Natural Science Foundation of China (No. ZR2017MB043).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the authors.
Rights and permissions
About this article
Cite this article
Feng, J., Sun, M., Wang, X. et al. Barium Sulfate Nanoparticles as a Coating for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons in Aqueous Samples. Chromatographia 81, 1287–1292 (2018). https://doi.org/10.1007/s10337-018-3568-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3568-3