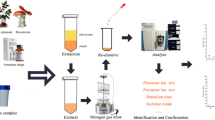
Abstract
In the present study, liquid chromatography coupled to an Orbitrap mass spectrometer (HPLC–Q-Orbitrap MS) was used as an approach for identification and quantification of 113 drugs simultaneously in biological samples (whole blood/plasma/serum). Samples were prepared using liquid–liquid extraction conducted using a trizma/isopropanol/butyl chloride buffer system. Reversed-phase separation employing a column (50 × 2.1 mm) packed with 2.6-μm C18 particles was then performed under gradient elution with mobile phase composition consisting of acetic acid and aqueous-acetonitrile mixtures with the acetonitrile content ranging from 10 to 100% v/v. Compounds were detected with high-resolution MS operated in full scan mode having a mass accuracy < 5 ppm. In this study, isobaric compounds (same nominal mass) were easily distinguished and identified by their different retention times. Extracted ion chromatograms (XICs) with narrow mass tolerance window (5 ppm) provided analysis with acceptable linearity (r2) ranged from 0.9530 to 1, low limits of detection (LOD) (0.02–39 ng mL−1) and low limit of quantification (LOQ) (0.1–130 ng mL−1). The developed method was applied to successfully analyse drugs in 26 blood samples from positive forensic cases and proved that this technique was able to detect analytes at trace level.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Simultaneous screening of a broad range of drugs (here simultaneous is intended to mean in one sample, using a single analytical separation method) is essential in a forensic analysis environment. The analysis should rapidly and reliably identify target or important non-target analytes in biological samples on a routine basis. However, deficiencies may exist in particular methods that are required to provide routine analysis of biological samples, especially when the complexity of the samples presents difficulty in detection selectivity (i.e., the ability to adequately identify a specific drug), and when compounds cannot be detected at a sufficiently low amount.
Numerous analytical techniques have been employed in forensic laboratories to comprehensively analyse a broad range of drugs in complex samples, usually based on the separation techniques gas chromatography–mass spectrometry (GC–MS) [1] and liquid chromatography–mass spectrometry (LC–MS), although direct MS analysis approaches have also been proposed, where the sample is analysed directly by the use of MS without prior chromatographic separation [2, 3]. GC–MS has proven to be a reliable technique for drug analysis because of its high selectivity, sensitivity and the possibility of positive identification provided by drug MS libraries. However, low volatility or thermal stability of some drug substances and/or the need to perform derivatisation steps are method limitations [4]. LC–MS is one of the most important techniques for routine screening of substances in biological samples because of its anticipated sensitivity, potential detection specificity provided by appropriate MS strategies, and its suitability toward molecules of variable polarity, molecular mass and thermal stability [5]. Therefore, it enjoys the position of being a preferred analytical tool for screening and identifying drugs in clinical and forensic toxicology [6].
Contemporary studies are often conducted using LC coupled with tandem mass spectrometry (LC–MS/MS) for drug screening and identification in biological samples [7, 8]. There are some drawbacks to LC–MS/MS due to (i) the many transitions (for quantification and qualification ions) which must be selected for multi-component samples, especially when employing fast chromatographic analysis, reducing the sensitivity or comprehensiveness of the method, and (ii) the technique does not allow identification of unknown molecules, since this is necessarily a targeted method, and ions for known compounds will need to be selected—conceptually a difficult task [9]. More recently, considerable interest from the analytical and forensic toxicology area has focused on high-resolution MS (HRMS) techniques [10]. Selectivity for HRMS combines both mass resolution and mass accuracy considerations. In addition, HRMS instruments such as time of flight (TOF) and Orbitrap MS [11, 12] offer various advantages for drug screening and identification. For routine operations, they provide resolutions between 40,000 and 100,000 full width at half maximum (FWHM), or even higher for some Orbitrap MS instruments. These techniques offer the possibility of increasing the number of compounds which can be analysed in a given time, and importantly allow for retrospective data searching to investigate unknown or unexpected compounds present in the samples, for instance as a result of the emergence of designer drugs. In fact, based on the previous study, LC–Q-TOFMS technique allows the identification via library search based on accurate mass fragment spectra of all essential components in a single run using the “Auto-MS–MS mode” [13]. Several studies report using HRMS techniques to identify drug compounds in various samples [14,15,16,17].
The Orbitrap HRMS mass analyser was first described in 2000 by Makarov [18]. The Exactive-Orbitrap MS (Orbitrap MS) provides high selectivity and sensitivity for the identification and quantification of drug compounds; thus, this analyser allows selective detection of compounds in complex samples such as in biological [19,20,21] and in water samples [22]. The Q-Exactive is a hybrid arrangement of the Orbitrap MS, coupling the high resolving Orbitrap sector with the added selectivity of a quadrupole unit mass resolution sector [23]. The Q-Exactive permits identification, confirmation and quantification with high confidence in a single analysis, with up to 100,000 FWHM mass resolution, to improve the sensitivity and selectivity of the method [24]. It allows non-target screening in a full scan mode with targeted MS/MS analysis in a single analysis, or it may operate separately [25]. High-resolution full scan (FS) mode, in which all ions are detected and analysed by the mass spectrometer, is the standard method used [26]. Full scan HRMS is adequate to achieve chemical specificity and acquire qualitative and quantitative data for many chemical substances [27].
The purpose of the present study was to develop a HPLC–Q-Orbitrap MS method for the simultaneous screening and identification of a broad range of drugs, and then to apply this method to blood samples. This method permits the simultaneous identification and confirmation of a broad range of drugs in a single analysis with full scan mode, which proves to be suitable for a wider range of compounds. An exact mass database including the accurate mass and retention time for each drug was developed using the results of the HRMS measurements, with a heated electrospray ionisation (HESI) source in positive ion mode. The proposed method was applied to the analysis of 26 biological samples (mainly whole blood, but some serum and plasma) from forensic cases.
Experimental
Reagents and Materials
Acetonitrile (HPLC grade) was purchased from Biolab Scientific Pty Ltd. (Mulgrave, Australia). Water was distilled and deionised with a Milli-Q system (Merck Millipore, Bedford, MA). Acetic acid (100% v/v) was obtained from BDH Chemicals Australia Pty Ltd (Merck). A standard mixture of drugs containing 113 drugs was prepared by the Victorian Institute of Forensic Medicine (VIFM) with variable concentrations of each drug (refer to Table 1). The standard mixture was stored at 4 °C before analysis.
Extraction Procedure
A total of 26 forensic cases (blood/plasma/serum) obtained from VIFM were labelled by numbers from 1 to 26. The samples consisted of 22 postmortem cases (case no. 1–8, 10–21, 23, 26) and 4 antemortem cases (case no. 9, 22, 24, 25). For the extraction procedure, the samples were prepared and extracted using the routine method used by the VIFM in their casework. Briefly, liquid–liquid extraction (LLE) with 100-μL Trizma buffer (pH 9) was added to 100 μL of the sample. Then, 1 mL of 10% isopropanol/butyl chloride was added to the buffer and the organic phase was transferred to autosampler vials following a brief centrifugation. The organic phase was evaporated using nitrogen. Finally, the extracts were reconstituted in 50 μL 0.1% of formic acid in acetonitrile and 450 μL of 50 mmol ammonium formate in water at a pH of 3.5.
Instrumentation
Standards and samples were analysed by ultra-high performance liquid chromatography (UHPLC) in combination with a Q-Exactive Plus Orbitrap mass spectrometer (Thermo Fisher Scientific, Scoresby, Australia) which was equipped with a heated electrospray ionisation source (HESI), binary pump, and autosampler. The HESI source was operated in positive ion mode for the ionisation of target compounds. Chromatographic separations were performed on a Thermo Accucore C18 phase column (50 × 2.1 mm) with 2.6-μm particle size. Thus, although the HPLC instrument was capable of ultra-high resolution (UHPLC) due to pump pressure capability, the 2.6-μm particle size does not correlate with conditions that define UHPLC, such as a particle size < 2.0 μm. Subsequently, the system will be referred to as LC. Samples were separated at 25 °C. 0.1% v/v acetic acid in water and acetonitrile were used as mobile phases A and B, respectively. Gradient elution started at 10% v/v of mobile phase B for 2 min, then linearly increased to 27% v/v B at 5 min, then 27–50% v/v B at 10 min and from 50–100% v/v B at 14 min. The mobile phase content was held at 100% v/v B for 1 min and then decreased to 10% v/v B. The column was equilibrated at 10% v/v B for 4 min; the total analysis turnaround time was 20 min. The flow rate was 0.30 mL/min, with injection volume of 15 μL.
The MS conditions were as follows: the sheath gas flow was set at 35, and the auxillary gas unit flow at 10 (both arbitrary units), with a spray voltage of 3.0 kV. Capillary and auxillary gas heater temperatures were set to 320 and 300 °C, respectively. Full scan MS analysis (m/z 50–600) was performed with resolution (70,000 FWHM), AGC target (1 × 106) and maximum IT (200 ms). The Orbitrap was calibrated daily by direct injection of the calibration solution in both positive and negative modes. Detection of analytes in samples was based on calculated exact mass and retention time of standard compounds, presented in Table 1. The mass tolerance window was set to 5 ppm for the analysis of each compound.
Data Analysis
For data evaluation, Xcalibur 3.0.63 (Thermo Fisher Scientific) software was used to control the instrument and for data processing. TraceFinder 3.0 software (Thermo Fisher Scientific) was utilised for confirmation and quantification analysis with Microsoft Excel 2010.
Results and Discussion
LC-Orbitrap MS
Although use of a monolith sorbent has the advantage of higher flow rates with reduced backpressures compared to conventional packed bed columns [28], here a C18 column (50 × 2.1 mm) with 2.6-μm particle size was installed in the LC system as the analytical column. Simultaneous detection of all analytes in one analysis was performed using the Orbitrap MS operated in full scan mode, with positive ionisation mode; in some cases, simultaneous detection arises for unresolved analytes. Each chromatographic run was completed within a total cycle time of 20 min (including column re-equilibration). Acceptable chromatographic resolution or detection based on unique mass measurements was achieved, where all targeted peaks were eluted in less than 12 min (Fig. 1). Peak width at half peak height for a representative analyte was approximately 4.5 s, and peak capacity (nc) of the column under the conditions used here was approximately 70 (implying the column can resolve to baseline a maximum of 70 compounds). Since the reference standard comprised 113 drugs, considerable overlap of chromatographic peaks is anticipated, even on a pure statistical basis. The high mass resolving power provided by this technique is crucial in mass-domain separation of overlapping or closely adjacent peaks, and in ensuring that only one specific ion for each analyte contributes to the measurement. It is particularly useful when handling complex samples such as biological samples [21]. In this study, 70,000 FWHM was selected for the mass resolution, which achieved a satisfactory number of points per chromatographic peak [28].
The increasing demand for analysis of many classes of drugs in a single chromatographic analysis using full scan accurate mass techniques (e.g., TOFMS and Orbitrap MS) supports high throughput analysis [11, 12]. Here, a full scan with a mass tolerance window of 5 ppm was used. The resulting total ion chromatogram (TIC) is shown in Fig. 1. Subsequently, target ions can be presented using extracted ion chromatogram (XIC) plots. Good peak shapes of each analyte were attained with a mass resolution of 70,000 FWHM within 5 ppm mass accuracy of the protonated exact mass [M + H]+. The retention time for each analyte was estimated from triplicate analysis at one concentration level. Although the chromatographic result in Fig. 1 might appear to exhibit broad peaks, this is a consequence of multiple peak overlaps due to relatively low total peak capacity. The use of extracted ion presentations for individual compounds illustrates that they all have good narrow peak responses.
Identification and Detection of Target Compounds
The screening of the 113 targeted component was performed at full scan, with each compound identified using the accurate mass measurements of the [M + H]+ ion, with evaluation of the retention time. A retention time window of ± 0.2 min was applied for all analytes as determined from triplicate injections. Table 1 indicates the list of target drug compounds and their retention times, including exact mass for the given molecular formula (calculated mass from the molecular formula with appropriate number of decimal places), accurate mass (experimental mass that obtained from the actual measurement using the HRMS method), and mass accuracy for each compound.
Confirmation of 113 analytes was based on the mass accuracy operated at full scan (± 2.5 ppm) and provides method selectivity, with little interference, as shown in Fig. 2, for citalopram at tR 6.29 min, with an accurate mass of m/z 325.1716 and good peak shape. Table 1 demonstrates an excellent mass accuracy from − 2.9 to 4.8 ppm, which will reduce the probability of false positives.
High resolution specificity was capable of successfully differentiating 13 sets of nominally isobaric compounds (defined by having the same unit mass value) but of different elemental composition (Table 2). Three examples involved multiple compounds with nominal masses of m/z 316 (4 compounds), m/z 264 (3 compounds) and m/z 340 (3 compounds). All isobaric cases were easily discriminated by their tR, and with the applied high-resolution MS, they were able to be differentiated according to their accurate mass values (Table 2). As depicted in Fig. 3, chlorpromazine and fluvoxamine (with the same nominal mass of m/z 319) result in tRs of 7.59 and 6.99 min, respectively. In addition, it was noted that all assumptions about specificity of identification are limited to only 113 targeted substances. However, there are possibilities of thousands other compounds or metabolites to be present including isomers (same elemental composition but different arrangement of atoms) which cannot be distinguished by full scan mode of HRMS.
Performance of the Method
Method performance was assessed regarding selectivity, specificity, linearity, limit of detection (LOD) and limit of quantification (LOQ) using TraceFinder 3.0 software. Selectivity is a crucial parameter for complex samples such as biological samples. In HRMS detection, accurate mass operation enhances selectivity and reduces interferences [25]. In some conditions, an increased window from 5 ppm to 10 ppm may be used to identify more compounds and obtain better results [29]. In this study, virtually all of the ions generated were monitored with almost zero background in the XICs when setting a narrow mass extraction window (5 ppm), illustrating good specificity of the method (see, Figs. 2 and 3).
Based on XIC ion data, peak areas were generated for all the compounds in standard mixtures of different concentrations according to the software calculated values, to allow construction of calibration curves. Linearity was studied for different analytes based on a five-point concentration calibration. Calibration curves were obtained with r2 values > 0.99 for most of the analytes (Table 1). By using TraceFinder data, analyte concentrations in real samples were established, according to the calibration curves and analyte peak areas.
The LOD was determined as the lowest concentration giving a signal–noise ratio that was at least threefold (S/N > 3), and LOQ as the lowest concentration of the calibration curve, giving a signal–noise ratio at least tenfold (S/N > 10). This method showed excellent sensitivity with low LODs obtained (Table 1). The lowest LODs were 0.02 ng mL−1, for chlorphenamine, hydroxyrisperidone, MDMA, nicotine, nortriptyline, zopiclone and zuclopenthixol.
Application of the Method
To evaluate the applicability of LC–Orbitrap MS with full scan operation, it was employed to screen for a broad range of drugs in 26 blind biological samples, provided by the VIFM, extracted as detailed in the Experimental section. A blank sample was analysed after each extract sample to check for possible carryover; no carryover was noted. The sample data were imported to TraceFinder software to match the sample information with that of the exact mass database of the standards. Although this study was provided with suspected positive drug samples, the results confirmed that all of these forensic cases were indeed positive, with agreement of all positive drugs found according to a parallel LC–MS/MS prior analysis; in addition, this study identified further positive drug hits attributed to its improved lower LOD and LOQ. Table 3 displays the number of positive cases, along with the average concentration of drugs detected, while the detailed information of each sample are shown in electronic supplementary material (Table S1). Cotinine is the most common compound found in 21 cases. In addition, some drugs were detected at concentrations below their LOQ values, but above their LOD values.
Of the 26 forensic cases, sample case no. 7 reported the highest number of basic drugs (17 drug compounds) in the sample, and a further four drugs detected at concentrations below the LOQ values (clozapine, doxepin, perazine and perphenazine). Figure 4 illustrates the XIC of methadone using full scan mode in standard solution and in positive sample case no. 7 with a retention time deviation of 0.02 min. A comparison between the XIC of standard solutions and the positive samples was conducted to reduce any potential for false positives in the screening results. The very clean XIC trace indicates that there were no interferences in the chromatogram at this mass accuracy; the method positively identified this compound in the biological matrix at trace level. Although the accurate mass XIC display gives a ‘clean’ result without interference, the full mass spectrum of the peak at the respective retention time can display other ions detected at that time should there be either other drugs or chromatographic interferences overlapping the target drug. In addition, it should be noted that MDA was detected in three forensic samples (Table 3). Since MDA is the metabolite of MDMA, the absence of MDMA may indicate the possibility of false-positive identification.
Conclusion
The application of LC–Q-Orbitrap MS for analysis of 113 targeted drugs in blood samples with high-performance separation in reversed-phase mode and high-resolution MS analysis has been demonstrated. HRMS screening produces high selectivity and specificity, based on the full scan exact mass measurement of [M + H]+ ions at a narrow mass extraction window. Since HRMS without confirmation by MS/MS has a tendency to obtain false-positive identification, thus it is only suited for screening purposes. The exact mass of an analyte combined with retention time data facilitates the identification of each target component, with excellent mass accuracy. In general, isobaric compounds were easily distinguished and identified by their different retention times, and accurate mass data. The reliable analysis of a broad range of drugs may be performed with acceptable low LODs obtained with little interference in the extracted ion chromatogram. The method allowed identification and quantification of target compounds at low levels. The results of this study represent a promising tool for large-scale screening and quantification, feasible for use in routine analysis.
References
Paterson S, Cordero R, Burlinson S (2004) Screening and semi-quantitative analysis of post mortem blood for basic drugs using gas chromatography/ion trap mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 813:323–330
Guinan TM, Kirkbride P, Della Vedova CB, Kershaw SG, Kobus H, Voelcker NH (2015) Direct detection of illicit drugs from biological fluids by desorption/ionization mass spectrometry with nanoporous silicon microparticles. Analyst 140(23):7926–7933
Liu P, Hu Y, Chen J, Yang Q (2015) Direct detection of the anti-cancer drug 9-phenylacridine in tissues by graphite rod laser desorption vacuum-ultraviolet post-ionization mass spectrometry. Rapid Commun Mass Spectrom 29(14):1328–1334
Leung GNW, Chung EW, Ho ENM, Kwok WH, Leung DKK, Tang FPW, Wan TSM, Yu NH (2005) High-throughput screening of corticosteroids and basic drugs in horse urine by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 825:47–56
Peters FT (2011) Recent advances of liquid chromatography-(tandem) mass spectrometry in clinical and forensic toxicology. Clin Biochem 44:54–65
Lynch KL, Breaud AR, Vandenberghe H, Wu AHB, Clarke W (2010) Performance evaluation of three liquid chromatography mass spectrometry methods for broad spectrum drug screening. Clin Chim Acta 411:1474–1481
Remane D, Meyer MR, Peters FT, Wissenbach DK, Maurer HH (2010) Fast and simple procedure for liquid–liquid extraction of 136 analytes from different drug classes for development of a liquid chromatographic-tandem mass spectrometric quantification method in human blood plasma. Anal Bioanal Chem 397:2303–2314
Di Rago M, Saar E, Rodda LN, Turfus S, Kotsos A, Gerostamoulos D, Drummer OH (2014) Fast targeted analysis of 132 acidic and neutral drugs and poisons in whole blood using LC–MS/MS. Forensic Sci Int 243:35–43
Moulard Y, Bailly-Chouriberry L, Boyer S, Garcia P, Popot MA, Bonnaire Y (2011) Use of benchtop exactive high resolution and high mass accuracy orbitrap mass spectrometer for screening in horse doping control. Anal Chim Acta 700:126–136
Ojanperä I, Kolmonen M, Pelander A (2012) Current use of high-resolution mass spectrometry in drug screening relevant to clinical and forensic toxicology and doping control. Anal Bioanal Chem 403:1203–1220
Boix C, Ibáñez M, Sancho JV, León N, Yusá V, Hernández F (2014) Qualitative screening of 116 veterinary drugs in feed by liquid chromatography-high resolution mass spectrometry: potential application to quantitative analysis. Food Chem 160:313–320
Vogliardi S, Favretto D, Tucci M, Stocchero G, Ferrara SD (2011) Simultaneous LC-HRMS determination of 28 benzodiazepines and metabolites in hair. Anal Bioanal Chem 400:51–67
Broeker S, Herre S, Wust B, Zweigenbaum J, Pragst F (2011) Development and practical application of a library of CID accurate mass spectra of more than 2500 toxic compounds for systematic toxicological analysis by LC-QTOF-MS with data dependent acquistion. Anal Bioanal Chem 400:101–117
Pelander A, Ojanperä I, Laks S, Rasanen I, Vuori E (2003) Toxicological screening with formula-based metabolite identification by liquid chromatography/time-of-flight mass spectrometry. Anal Chem 75:5710–5718
Thomas A, Guddat S, Kohler M, Krug O, Schänzer W, Petrou M, Thevis M (2010) Comprehensive plasma-screening for known and unknown substances in doping controls. Rapid Commun Mass Spectrom 24:1124–1132
Gómez-Pérez ML, Romero-González R, Plaza-Bolaños P, Génin E, Vidal JLM, Frenich AG (2014) Wide-scope analysis of pesticide and veterinary drug residues in meat matrices by high resolution MS: detection and identification using Exactive-Orbitrap. J Mass Spectrom 49:27–36
Strano-Rossi S, Odoardi S, Castrignano E, Serpelloni G, Chiarotti M (2015) Liquid chromatography-high resolution mass spectrometry (LC–HRMS) determination of stimulants, anorectic drugs and phosphodiesterase 5 inhibitors (PDE5I) in food supplements. J Pharm Biomed Anal 106:144–152
Makarov A (2000) Electrostatic axially harmonic orbital trapping: a high-performance technique of mass analysis. Anal Chem 72:1156–1162
Concheiro M, Castaneto M, Kronstrand R, Huestis MA (2015) Simultaneous determination of 40 novel psychoactive stimulants in urine by liquid chromatography-high resolution mass spectrometry and library matching. J Chromatogr A 1397:32–42
Marzinke MA, Breaud A, Parsons TL, Cohen MS, Piwowar-Manning E, Eshleman SH, Clarke W (2014) The development and validation of a method using high-resolution mass spectrometry (HRMS) for the qualitative detection of antiretroviral agents in human blood. Clin Chim Acta 433:157–168
Thomas A, Geyer H, Schänzer W, Crone C, Kellmann M, Moehring T, Thevis M (2012) Sensitive determination of prohibited drugs in dried blood spots (DBS) for doping controls by means of a benchtop quadrupole/Orbitrap mass spectrometer. Anal Bioanal Chem 403:1279–1289
Althakafy JT, Kulsing C, Grace MR, Marriott PJ (2017) Liquid chromatography-quadrupole Orbitrap mass spectrometry method for selected pharmaceuticals in water samples. J Chromatogr A 1515:164–171
Hu Q, Noll RJ, Li H, Makarov A, Hardman M, Cooks RG (2005) The Orbitrap: a new mass spectrometer. J Mass Spectrom 40:430–443
Solliec M, Roy-Lachapelle A, Sauvé S (2015) Quantitative performance of liquid chromatography coupled to Q-Exactive high resolution mass spectrometry (HRMS) for the analysis of tetracyclines in a complex matrix. Anal Chim Acta 853:415–424
Fedorova G, Randak T, Lindberg RH, Grabic R (2013) Comparison of the quantitative performance of a Q-exactive high-resolution mass spectrometer with that of a triple quadrupole tandem mass spectrometer for the analysis of illicit drugs in wastewater. Rapid Commun Mass Spectrom 27:1751–1762
Rajski L, Gomez-Ramos Mdel M, Fernandez-Alba AR (2014) Large pesticide multiresidue screening method by liquid chromatography-Orbitrap mass spectrometry in full scan mode applied to fruit and vegetables. J Chromatogr A 1360:119–127
Zhang NR, Yu S, Tiller P, Yeh S, Mahan E, Emary WB (2009) Quantitation of small molecules using high-resolution accurate mass spectrometers—a different approach for analysis of biological samples. Rapid Commun Mass Spectrom 23:1085–1094
Kaufmann A (2014) Combining UHPLC and high-resolution MS: a viable approach for the analysis of complex samples? TrAC Trends Anal Chem 63:113–128
Del Mar Gomez-Ramos M, Rajski L, Heinzen H, Fernandez-Alba AR (2015) Liquid chromatography Orbitrap mass spectrometry with simultaneous full scan and tandem MS/MS for highly selective pesticide residue analysis. Anal Bioanal Chem 407:6317–6326
Acknowledgements
The authors are very grateful to the Victorian Institute of Forensic Medicine (VIFM) for providing drug standards and forensic cases for analysis. We also thank Thermo Fisher for provision of UHPLC with Q-Exactive MS facilities, together with the Xcalibur 3.0.63 and Trace Finder 3.0 software.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mokhtar, S.U., Kulsing, C., Althakafy, J.T. et al. Simultaneous Analysis of Drugs in Forensic Cases by Liquid Chromatography–High-Resolution Orbitrap Mass Spectrometry. Chromatographia 83, 53–64 (2020). https://doi.org/10.1007/s10337-019-03814-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03814-w