Abstract
A simple, rapid and green ultrasound-assisted ionic liquid solid–liquid extraction coupled with aqueous two-phase extraction method was developed and applied to the extraction of shikonin, acetylshikonin and β,β′-dimethylacrylshikonin in Arnebia euchroma (Royle) Johnst. The separation and determination of the analytes was performed by high-performance liquid chromatography coupled with diode array detection. In the study, 1-butyl-3-methylimidazolium tetrafluoroborate ([C4MIM][BF4]) was used as extraction solvent. Ionic liquid-based solid–liquid extraction and aqueous two-phase extraction were performed simultaneously in one tube under the assistance of ultrasound. The liquid–liquid–solid three-phase system, namely surfactant-rich phase, water-rich phase and sample-rich phase, was formed. The target analytes were enriched in the surfactant-rich phase. Under the optimal experimental conditions, the calibration curves of target analytes showed good linear relationship (r > 0.9997). The inter-day and intra-day precision were in the range of 3.42–8.53 and 1.98–3.14%, respectively. The LOD and LOQ for the target analytes were in the range of 5.0–19.2 and 16.7–64.2 ng mL−1, respectively. The recoveries of naphthoquinone pigments ranged from 90.00 to 97.73% and relative standard deviations were less than 4.50%. Compared with heat reflux extraction and ultrasonic-assisted extraction, the proposed method is simpler, faster and more effective, because the mass transfer process for the analytes was always performed in one centrifugal tube and the steps such as filtration, concentration and redissolution were avoided. The present method was demonstrated to be efficient and satisfactory for the extraction of naphthoquinone pigments in medicinal plants.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arnebia euchroma (Royle) Johnst., also known as Xinjiang Zicao, is a perennial herb widely distributed in Japan, North Korea and China [1]. Its dried roots are dark purple and can be used as medicine. It has been proven to treat hepatitis, vaginitis, cervicitis, dermatitis, empyrosis, pathopyretic ulcer, purpura, eczema, pityriasis rosea, flat wart and psoriasis [1,2,3,4,5]. The prime pharmacological components in Arnebia euchroma (Royle) Johnst. are naphthoquinone pigments such as shikonin, acetylshikonin and β,β′-dimethylacrylshikonin [2], which were used not only in pharmaceutical products, but also in textile, cosmetic and food industries as a natural pigment [2, 4, 6]. Hence, it is significant to develop an efficient method for the determination of naphthoquinone pigments in Zicao.
So far, several analytical techniques have been investigated for the determination of naphthoquinone pigments from medicinal plant materials, such as HSCCC [1], CE [7], infrared spectroscopy [8], TLC–densitometry [6], UV–Vis spectrophotometry [9] and HPLC [2, 3]. Among the above analytical methods, HPLC proved to be a wise choice due to its fast analysis speed, high separation efficiency and high sensitivity. However, an extraction step prior to chromatographic analysis is usually required. Various traditional sample pretreatment methods, such as solid-phase extraction (SPE) [10], ultrasonic-assisted extraction (UAE) [3, 11], microwave-assisted extraction (MAE) [12], matrix solid-phase dispersion extraction (MSPD) [13] and supercritical fluid extraction (SFE) [14], have been developed for the extraction of naphthoquinone compounds. Although these methods are effective, they are laborious and time-consuming. In particular, a large amount of volatile, poisonous and harmful organic solvent is often required. Therefore, green, simple, rapid and miniaturized sample pretreatment methods have aroused great interest. In recent years, some novel sample preparation methods based on ionic liquid sprang up.
Ionic liquids (ILs) are low-temperature molten salts composed entirely of anions and cations [15]. They are liquid substances at or near room temperature. In the field of pharmaceutical analysis, IL is often regarded as a “green solvent” and used for the extraction and enrichment of active ingredients from herbal plant. IL-based MAE [16,17,18,19], UAE [20,21,22,23], pressurized liquid extraction (PLE) [24, 25] and ultrahigh-pressure extraction (UHPE) [26] have been established and applied to extraction of flavonoids, tanshinones, alkaloid, lignans, terpenoids and other active constituents. By means of strong irradiation, high temperature and high pressure, the target analytes can be effectively extracted by IL, and satisfactory results were achieved. Moreover, some novel miniaturized sample preparation methods such as temperature-assisted ionic liquid-dispersive liquid–liquid microextraction (TA-IL-DLLME) [27, 28] and ionic liquid–water–organic solvent three-phase microextraction (ILWDS-3P-ME) [29] have also been developed for the extraction and preconcentration of the target constituents from sample solution. However, the naphthoquinone compounds in Zicao belong to a kind of natural pigments, which are easily degraded by exposure to light, high temperature, metal ions, oxidant, strong acid or strong alkaline [30]. Apparently, a mild pretreatment method for solid samples is more suitable for the extraction of naphthoquinone pigments in Zicao.
Ionic liquid-based aqueous two-phase system (IL-ATPS) first proposed by Rogers et al. [31, 32] is a novel, efficient and green separation system. According to the different objects, it can be divided into three types: IL–inorganic salt [33], IL–carbohydrate [34] and IL–polymer-based ATPS [35]. In IL–polymer-based ATPS, both IL and polymer can be salted out from water depending on their structure, functionality, molar mass and solubility [35]. However, the ATPS consisted of IL and polymer are only studied with two classes of polymers namely PEG and PPG. In recent years, a new kind of ATPS formed by different ILs and anionic or cationic surfactants in aqueous media was reported [36]. In IL–surfactant-based ATPS, the mixtures of IL and surfactants are often used as extraction solvent. Because the mixtures can spontaneously form vesicles, they can be easily separated from the water to form microstructural phases. These vesicles are densely packed in the upper phase and sparsely in the lower phase [37], which is due to not only the electrostatic interaction between surfactants [36] but also the interaction between surfactants and IL [35]. The separation and enrichment of the target analytes can be realized according to the difference of the distribution coefficient between the two phases. Due to the characteristics of short phase separation time, mild separation conditions, low viscosity and simple operation, IL–surfactant-based ATPS has been applied in the extraction and separation of colorant [38], antibiotics [39], biomolecules and metal ions [35, 40, 41] and so on. So far, this technique has not been applied to the extraction of active constituents from medicinal plants.
Therefore, a novel ultrasound-assisted ionic liquid solid–liquid extraction coupled with aqueous two-phase extraction high-performance liquid chromatography method (UAIL-SLE/ATPE–HPLC) was established and applied to the determination of naphthoquinone pigments in Zicao. IL-based SLE and IL–surfactant-based ATPE were performed simultaneously in one tube under the assistance of ultrasound. The proposed method does not require the removal of solid sample matrix prior to ATPE, nor does it require the steps of evaporation, concentration and redissolution, which simplified the experimental procedure and shortened the extraction time.
Experimental
Chemicals and Materials
The standards of shikonin, acetylshikonin and β,β′-dimethylacrylshikonin were obtained from Shanghai Yuanye Bio-Technology Co. Ltd. (Shanghai, China). The structures of the naphthoquinone compounds are shown in Fig. 1. The standard stock solutions for naphthoquinone pigments at the concentration of 500 μg mL−1 were prepared by dissolving the standards in chromatographic-grade acetonitrile and stored in brown volumetric flask at 4 °C. The working solutions were obtained by diluting the stock solutions with chromatographic-grade acetonitrile. 1-Butyl-3-methylimidazolium tetrafluoroborate ([C4MIM][BF4]), 1-hexyl-3-methylimidazolium tetrafluoroborate ([C6MIM][BF4]) and 1-octyl-3-methylimidazolium tetrafluoroborate ([C8MIM][BF4]) were obtained from Cheng Jie Chemical Reagent Co. Ltd. (Shanghai, China). Sodium dodecyl benzene sulfonate (SDBS) and trisodium citrate dihydrate (C6H5Na3O7·2H2O) were obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Chromatographic-grade acetonitrile was obtained from Fisher Scientific (Pittsburgh, PA, USA). Pure water was obtained with a Milli-Q water purification system (Millipore MA, USA). Analytical-grade NaHCO3, (NH4)2SO4, Na2HPO4·12H2O, K2HPO4·3H2O, NaCl, KCl and K2CO3 were purchased from Beijing Chemical Factory (Beijing, China).
Instruments
A 1100 series liquid chromatograph (Agilent Technologies Inc., USA) equipped with DAD was employed. The chromatographic separation of the analytes was carried out on a Zorbax Eclipse Plus C18 column (150 mm × 4.6 mm, 3.5 μm, Agilent, USA). KQ-5200DE ultrasonic generator (Kunshan Ultrasonic, Jiangsu, China) and XW-80A vortex mixer (Qingpu, Shanghai, China) were used in the extraction procedure. RE-52AA rotary evaporator (Yarong, Shanghai, China) was employed. The phase separation was performed on a R404A high-speed freezing centrifuge (Eppendorf, Hamburg, Germany).
Phase Diagram Determination
The three components of ATP (IL, SDBS and water) were separately set as three vertices of the ternary phase diagram. The phase diagram was prepared by the turbidity method at 298.2 K. The amount of [C4MIM][BF4] and SDBS for the determination of the phase diagram were in the range of 3.0–3.8 and 0.2–1.0 g, respectively. They were directly mixed in the test tube according to the required mass ratios, then pure water was continuously added and stirred constantly. At the same time, the changes of the solution were observed carefully and the quality of the water was recorded accurately. The criterion for determining the presence of an ATPS is the appearance of a clear interface between two phases. ATPS is considered as an equilibrium system when the interface between the two phases is clearly visible and the volume of the upper and lower phases is no longer changed.
Sample Preparation
Zicao samples (1–5) from different planting area were purchased from local pharmacy (Changchun, China). All samples were dried thoroughly in the cabinet drier at 60 °C for 24 h. Then the samples were triturated with a pulverizer and passed through a 120-mesh stainless steel sieve. The resulting sample powder was stored in a desiccator before use. The spiked samples containing three naphthoquinone pigments were prepared by spiking the standard solutions into samples. To ensure the standard solutions to be well dispersed, a moderate amount of acetonitrile was added until all the sample powder was immersed in acetonitrile. All experimental results were obtained with Sample 1 except for the real sample analysis results.
UAIL-SLE/ATPE
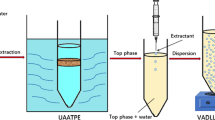
The schematic procedure of UAIL-SLE/ATPE is shown in Fig. 2. 0.01 g of sample powder, 4 mL of pure water and 440 μL of [C4MIM][BF4] were added to a 10-mL centrifuge tube and mixed with a vortex mixer. Then 0.14 g of SDBS and 0.44 g of C6H5Na3O7·2H2O were also added to the tube. After mixing, the sample mixture was extracted by ultrasound for 10 min and centrifuged for 5 min at 2000 rpm. A liquid–liquid–solid three-phase system, namely top surfactant-rich phase, middle water-rich phase and bottom sample-rich phase, was formed. The top phase solution containing the target analytes was precisely withdrawn with a syringe and diluted to 0.5 mL with acetonitrile. The resulting solution was filtered with 0.22-μm membrane filter for HPLC analysis.
Comparison of Methods
UAE
UAE is a standard method recommended by Chinese Pharmacopoeia [42]. 0.50 g of sample powder and 25 mL petroleum ether were accurately measured and placed in a conical flask with cover. The conical flask containing sample and extraction solvent was weighed and ultrasonic extraction was carried out for 30 min at 250 W. After cooling to room temperature, the conical flask was weighed again and the lost weight was made up with petroleum ether. Subsequently, the mixture of sample and solvent was shaken and filtered through a filter paper. 10 mL of subsequent filtrate was accurately collected and evaporated to dryness. The residue was dissolved with the mobile phase and transferred into a 10-mL volumetric flask. Then the mobile phase was added to the volumetric flask scale. The resulting solution was shaken well and filtered with 0.22-μm membrane filter for HPLC analysis.
HRE
HRE is widely applied for a long time. For comparison, HRE is also performed. 0.50 g of sample powder and 80 mL of ethanol were placed into a 500-mL round-bottom flask. After extraction for 2 h, the mixture of sample and solvent was transferred into a 100-mL volumetric flask. The ethanol was added to the volumetric flask. The sample solution was shaken and filtered through a filter paper. 10 mL of subsequent filtrate was accurately collected and evaporated to dryness. Then the residue was dissolved with 10 mL of acetonitrile. The resulting solution was filtered with 0.22-μm membrane filter for HPLC analysis.
Chromatographic Conditions
The mobile phase consisted of acetonitrile (A) and aqueous solution containing 0.5% (v/v) formic acid (B). Gradient program was as follows: 0–5 min, 70% A; 5–8 min, 70–80% A; 8–12 min, 80% A; 12–15 min, 80–85% A; 15–20 min, 85% A; 20–23 min, 85–70% A; 23–30 min, 70% A. The flow rate of the mobile phase was maintained at 0.5 mL min−1. The injection volume of the analytical solution was 10 μL. The temperature of the column was controlled at 35 °C. The detection wavelength was 516 nm.
Determination of Extraction Yield
where C was the concentration of analyte in the sample solution and V was the volume of the sample solution. The concentration of analyte (C) was calculated from the measured chromatographic peak area by the calibration curve in Table 1.
Results and Discussion
Selection of IL
In this study, IL is not only the extraction solvent of SLE, but also the cationic surfactant of ATPE, which has an important influence on the formation of three-phase system and extraction of naphthoquinone pigments. To evaluate the effect of IL type on extraction yields, [C4MIM][BF4], [C6MIM][BF4] and [C8MIM][BF4] were investigated, when the concentration of SDBS was 0.1 mol L−1 and the volume of IL was 440 μL. The experimental results indicated that the liquid–liquid–solid three-phase system cannot be formed when [C6MIM][BF4] and [C8MIM][BF4] were used. The reason may be that the increase in the length of alkyl chain reduces the water solubility and increases the viscosity of IL [43], so that a portion of ILs adhered to the surface of the solid sample particles and cannot form large aggregates with SDBS, which results in the failure to form ATPS. Therefore, [C4MIM][BF4] was selected as the extraction solvent.
ATPS Formation
As shown in Fig. 3, the ternary phase diagram of [C4MIM][BF4], SDBS and water mixture was obtained at 298.2 K. The ternary phase diagram was divided into three different regions: heterogeneous region (L + S), single-phase region (L) and ATP region (2L). The L + S region occupies a larger area, and is located in the region that SDBS accounts for larger proportion. The L + S region containing sediment may be composed of SDBS precipitate and its saturated solution of [C4MIM][BF4] and water. The L region is a continuous extension phase area near the [C4MIM][BF4]–water side. The constituent solution in the L region was transparent, isotropic and less viscous. The 2L region (ATP region) was the smallest area. The concentration ranges of [C4MIM][BF4] and SDBS used to form ATPS were 10.5–56.0 and 2.0–16.2%, respectively.
Optimization of UAIL-SLE/ATPE
To achieve the highest extraction yield, the influence of several experimental parameters was investigated, including volume of IL, amount of SDBS, ionic strength, sample amount, pH value of the extraction medium, ultrasound power and time, centrifugal speed and time. All experiments were performed in triplicate.
Effect of IL Volume
The volume of [C4MIM][BF4] is critical to the formation of ATPS and extraction of the naphthoquinone pigments. The effect of the volume of [C4MIM][BF4] ranging from 360 μL to 500 μL on extraction yields of target analytes was investigated since the ATPS cannot be formed when the volume of [C4MIM][BF4] was less than 360 μL. As shown in Fig. 4a, when the volume of [C4MIM][BF4] increased from 360 μL to 440 μL, the extraction yields of naphthoquinone pigments increased gradually. The highest extraction yields were achieved when 440 μL of [C4MIM][BF4] was used. However, as the volume of [C4MIM][BF4] continued to increase, the extraction yield of shikonin remained almost unchanged, while the extraction yields of acetylshikonin and β,β′-dimethylacrylshikonin reduced slightly. This may be because the increase of IL enhanced the solution viscosity and thereby weakened the mass transfer process. Therefore, 440 μL of [C4MIM][BF4] was used.
Effect of IL volume (a), amount of SDBS (b), type of salt (c), concentration of salt (d), sample amount (e) and pH value of extraction medium (f) on the extraction yields. The experimental parameters of each condition optimization experiment are as follows: a IL type, ([C4MIM][BF4]); SDBS amount, 0.15 g; salt concentration, 7.5% (NH4)2SO4; sample amount, 10 mg; pH value of extraction medium, 7.0. b IL volume, 440 μL of [C4MIM][BF4]; salt concentration, 7.5% (NH4)2SO4; sample amount, 10 mg; pH value of extraction medium, 7.0. c IL volume, 440 μL of [C4MIM][BF4]; SDBS amount, 0.14 g; salt concentration, 7.5%; sample amount, 10 mg; pH value of extraction medium, 7.0. d IL volume, 440 μL of [C4MIM][BF4]; SDBS amount, 0.14 g; salt type, C6H5Na3O7·2H2O; sample amount, 10 mg; pH value of extraction medium, 7.0. e IL volume, 440 μL of [C4MIM][BF4]; SDBS amount, 0.14 g; salt concentration, 11% C6H5Na3O7·2H2O; pH value of extraction medium, 7.0. f IL volume, 440 μL of [C4MIM][BF4]; SDBS amount, 0.14 g; salt concentration, 11% C6H5Na3O7·2H2O; sample amount, 10 mg
Effect of SDBS Amount
SDBS is a necessary condition for IL–surfactant-based ATPE. The amount of SDBS affects the formation of IL–SDBS aggregates and the distribution of target analytes. The effect of amount of SDBS (0.12–0.25 g) on the extraction yields was investigated when the volume of [C4MIM][BF4] was 440 μL. If the amount of SDBS was less than 0.12 g, ATPS cannot be formed. The reason may be that the total concentration of surfactant is too low to form the IL–SDBS aggregates and ATPS [43]. As can be seen from Fig. 4b, the extraction yields of naphthoquinone pigments increased gradually with the increase of SDBS ranging from 0.12 to 0.14 g. This means that with the increase of the SDBS amount, the number of IL–SDBS micelle aggregates for enriching target analytes rose effectively, and eventually the extraction yields increased. When the amount of SDBS was 0.14 g, the extraction yields of target analytes reached the maximum. At the same time, the molar ratio of SDBS and IL is approximately 1:6. When the SDBS amount was increased from 0.14 g to 0.25 g, the volume of the surfactant-rich phase rose gradually, which leads to the decrease of the concentrations and extraction yields of analytes [41]. Hence, 0.14 g of SDBS was chosen.
Effects of Salt Type and Concentration
The addition of salt can effectively improve the extraction performance of the IL–surfactant-based ATPS. First, compared with the salt-free system, the regions of ATPS are much broader and the minimum concentrations for forming an ATPS are much lowered when salt is used [37, 44]. Second, the phase separation time of ATPS is much shortened when salts are added [44]. Finally, the addition of salt can significantly improve the extraction efficiency of the target analytes. For example, Yang et al. [39] reported an IL–surfactant-based ATPS for the extraction of antibiotics in honey. In their research, 0.08 g of NaCl was added to the ATPS so as to obtain higher extraction recoveries. This confirmed the important role of salt in improving extraction efficiency. In addition, an interesting phenomenon was also observed in this study. When no salt was added to the ATPS, the volume of the surfactant-rich phase was larger and the obtained extraction yields were lower. It can be seen that the addition of salt promoted phase separation and improved extraction efficiency, which was consistent with the literature [38, 39]. Therefore, the effects of salt type and amount on the extraction yields of naphthoquinone pigments were investigated. However, when K2HPO4 was used, some dark gray flocculent precipitates appeared at the bottom of the centrifuge tube and the phase separation interface of the ATPS was rather indistinct. As shown in Fig. 4c, when C6H5Na3O7·2H2O, (NH4)2SO4, NaH2PO4 and NaHCO3 were used, a stable ATPS can be formed, and the highest extraction yields were obtained by C6H5Na3O7·2H2O. On the one hand, this phenomenon may be related to the strong salting-out capacity of C6H5Na3O7·2H2O. The binding ability of [C6H5O7]3− to water molecule is far stronger than that of [BF4]− and [C12H25C6H4SO3]− to water molecule, thereby promoting the separation of the micellar aggregates for [C4MIM][BF4] and SDBS from the water-rich phase [39, 45]. On the other hand, a large amount of C6H5Na3O7·2H2O in the solution competes with naphthoquinones for water molecule, which leads to a decrease in the hydration degree of naphthoquinones and then promotes their transfer from the water-rich phase to the surfactant-rich phase [45]. Moreover, the addition of salt can increase the density difference between the two phases and make the phase separation faster [46].Therefore, C6H5Na3O7·2H2O was selected in this study.
To optimize the concentration of C6H5Na3O7·2H2O, when 440 μL of IL and 0.14 g SDBS were used to form the ATPS, the effect of salt concentration (w/v) from 5 to 14% on the extraction yields was investigated. The experimental results shown in Fig. 4d indicated that when the concentration of C6H5Na3O7·2H2O was 11%, the extraction yields of target analytes reached the maximum.
Effect of Sample Amount
As shown in Fig. 4e, the extraction yields of the target analytes decreased gradually with the increase of sample amount from 10 mg to 40 mg. The reason may be that the amounts of [C4MIM][BF4] and SDBS were insufficient to extract the target analytes from sample. In addition, with the increase of sample amount, some sticky IL tends to adhere to the surface of solid particles, which may affect the mass transfer and distribution of the target analytes. However, when the sample amount was less than 10 mg, the extraction yields did not improve significantly. Moreover, the smaller the sample amount, the greater the experimental error. To ensure that the sample is representative, the sample amount was selected as 10 mg.
Effect of pH Value of Extraction Medium
The pH value of extraction medium influences the ionization status and solubility of naphthoquinone pigments. The effect of pH value in the range of 2–13 on the extraction yields was examined. The experimental results shown in Fig. 4f indicated that the extraction yield of shikonin was almost unchanged with the increase of pH value. However, higher extraction yields of acetylshikonin and β,β′-dimethylacrylshikonin were obtained when the pH value of the extraction medium was 5–8, which was consistent with the results reported in the literature [47]. In acidic solution, the solubility of the target analytes reduced and the extraction yields decreased accordingly. In alkaline solution, due to the dissociation of phenolic hydroxyl groups on the naphthoquinones, the red shift of the maximum absorption wavelength for naphthoquinones was generated, which leads to a decrease in the extraction yields. Therefore, pH 7.0 was selected.
Effects of Ultrasound and Centrifugal Conditions
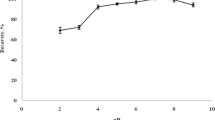
Ultrasound can not only destroy cell wall and accelerate the penetration of solvents into plant tissues, but also affect the distribution efficiency of target analytes and the formation of ATPS [48]. For this reason, the effects of ultrasound power in the range of 40–200 W and ultrasound time in the range of 0–30 min on the extraction yields were studied. As shown in Fig. 5, the extraction yields of naphthoquinone pigments increased gradually with the increase of ultrasound power from 40 to 120 W, and then remaining unchanged up to 200 W except for acetylshikonin. In addition, the extraction yields of analytes were relatively low when ultrasound irradiation was not applied. When the ultrasound time rose to 10 min, the highest extraction yields were obtained, which means that the extraction balance could be achieved within 10 min. However, longer extraction time would affect extraction efficiency of analytes. It was probably because excessive ultrasound irradiation would change the composition of the top and bottom phases of the ATPS, thus affecting the formation of larger micellar aggregates and the distribution of analytes [49]. Based on these results, the ultrasound irradiation at 120 W for 10 min was selected.
However, since the sample residue was not removed prior to ATPE, which greatly influenced the formation of ATPS. The phase equilibration time was prolonged. The phase equilibration could be achieved for at least 1 h. To improve efficiency and completely separate surfactant-rich phase, water-rich phase and sample-rich phase from each other, centrifugation was used. The effect of centrifugal speed from 1000 to 4000 rpm and centrifugal time from 3 to 20 min on the extraction yields was examined (Fig. 5). The experimental results indicated that the phase separation and partition equilibrium could be attained when the centrifugal speed reached 2000 rpm and the centrifugal time exceeded 5 min. Hence, centrifugation was carried out at 2000 rpm for 5 min.
Through optimization of experimental conditions, the liquid–liquid–solid three-phase system can be formed and the highest extraction yield can be obtained. At this time, the concentrations of [C4MIM][BF4] and SDBS used to form the ATPS were 10.41% and 2.74% (w/w), respectively. An interesting phenomenon was observed from Fig. 3. Under optimal experimental conditions, the composition of the ATPS is not in but close to the 2L region of the phase diagram. This may be related to the addition of salt and the presence of solid sample residues in the ATPS, which causes the phase diagram to be modified.
Evaluation of the Method
Analytical Performance
The standard curves were constructed by plotting the peak areas measured versus the concentrations of target analytes. It can be seen from Table 1 that good linearities were achieved in the range of concentrations examined (correlation coefficient higher than 0.9997). The limits of detection (LODs) and quantification (LOQs) refer to the minimum concentration at which the analytes can generate signal-to-noise ratio (SNR) of 3 and 10, respectively. The LOD and LOQ for the target analytes were in the range of 5.0–19.2 and 16.7–64.2 ng mL−1, respectively. The concentrations of target analytes in analytical solution were higher than LOQs and lower than the upper limit of determination of the present method. Therefore, LODs, LOQs and linear range were suitable for the aim of the present method. The calibration curve, correlation coefficient, linear range, LODs and LOQs are presented in Table 1.
Precision was evaluated by measuring intra-day and inter-day relative standard deviations (RSDs). The intra-day precision was obtained by analyzing the samples five times in one day. The inter-day precision was obtained by analyzing the samples once a day for five consecutive days. As indicated in Table 1, the intra-day and inter-day RSDs were in the range of 1.98–3.14 and 3.42–8.52%, respectively. Thus, the present method showed an acceptable repeatability.
To evaluate the accuracy of the present method, the spiked sample 1 was analyzed and the results are listed in Table 2. The recoveries of naphthoquinone compounds range from 90.00 to 97.73% with the RSDs lower than 4.50%. This indicates that the present method can provide acceptable recoveries.
Analysis of Samples
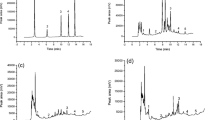
To estimate the applicability of the present method, a total of five real samples from different planting areas were analyzed. The target analytes in samples were identified by comparing their retention times and absorption spectra with those of authentic standards (Fig. 6). The experimental results are listed in Table 3. It can be seen that the contents of shikonin, acetylshikonin and β,β′-dimethylacrylshikonin in Zicao are in the range of 0.52–0.88, 1.03–2.00 and 2.48–5.43 mg g−1, respectively. Because of its high content, β,β′-dimethylacrylshikonin is often considered to be the marker component in Zicao, which is consistent with the Chinese Pharmacopoeia [42]. Moreover, the content of naphthoquinones in Zicao samples from different regions varies greatly, which has important influence on the quality of medicinal herbs. This may be related to different climatic and geographical conditions, such as temperature, precipitation, light and soil [50].
Comparison of UAIL-SLE/ATPE with Other Methods
To further evaluate the performance of the present method, it was compared with the commonly used HRE method and the standard method recommended by the Chinese Pharmacopoeia (UAE) [42]. The results are shown in Table 4. The extraction yields obtained by the present method were significantly higher than those obtained by HRE and slightly lower than those obtained by UAE. However, there was no significant difference between the present method and the standard pharmacopoeia method by t test (p > 0.05). In addition, in this study, IL instead of traditional organic solvent was used as extraction solvent, which reduced the consumption of solvent and protected the environment. Compared with HRE and UAE, the present method does not require the removal of solid sample matrix prior to ATPE, nor does it require the steps of filtration, evaporation and redissolution, which simplified the experimental procedure and shortened the extraction time. Therefore, the UAIL-SLE/ATPE is considered to be a comparatively satisfactory sample pretreatment method.
Conclusion
A simple, rapid and green UAIL-SLE/ATPE coupled with HPLC-DAD method was first established for the extraction and determination of naphthoquinone pigments in Arnebia euchroma (Royle) Johnst. In present method, IL-based SLE and ATPE were performed simultaneously in one tube with the aid of ultrasound, which integrated extraction, separation and preconcentration into one step. Compared with UAE and HRE, the present method has some advantages in terms of experimental operation, extraction time and consumption of extraction solvent. The results indicate that the present method has a great potential for the extraction and determination of naphthoquinone pigments in herbal medicine and pharmaceutical products.
References
Lu HT, Jiang Y, Chen F (2004) Preparative high-speed counter-current chromatography for purification of shikonin from the Chinese medicinal plant Lithospermum erythrorhizon. J Chromatogr A 1023:159–163
Hu YN, Jiang ZH, Leung KSY, Zhao ZZ (2006) Simultaneous determination of naphthoquinone derivatives in Boraginaceous herbs by high-performance liquid chromatography. Anal Chim Acta 577:26–31
Xiao Y, Wang Y, Gao SQ, Zhang R, Ren RB, Li N, Zhang HQ (2011) Determination of the active constituents in Arnebia euchroma (Royle) Johnst. by IL-based ultrasonic-assisted extraction high-performance liquid chromatography. J Chromatogr B 879:1833–1838
Kim JY, Jeong HJ, Park JY, Kim YM, Park SJ, Cho JK, Park KH, Ryu YB, Lee WS (2012) Selective and slow-binding inhibition of shikonin derivatives isolated from Lithospermum erythrorhizon on glycosyl hydrolase 33 and 34 sialidases. Bioorg Med Chem 20:1740–1748
Yang RF, Huang PP, Qiu TQ (2013) Ultrasound-enhanced subcritical water extraction of naphthoquinone pigments from purple Gromwell (Lithospermum erythrorhizon) to higher yield and bioactivity. Food Sci Biotechnol 22:671–676
Bozan B, Baser KHC, Kara S (1999) Quantitative determination of naphthoquinones of Arnebia densiflora by TLC-densitometry. Fitoterapia 70:402–406
Dresler S, Kubrak T, Kocka AB, Szymczak G (2015) Determination of shikonin and rosmarinic acid in Echium vulgare L. and Echium russicum J.F. Gmel. by capillary electrophoresis. J Liq Chromatogr Relat Technol 38:698–701
Glazunov VP, Tchizhova A, Pokhilo ND, Anufriev V, Elyakov GB (2002) First direct observation of tautomerism of monohydroxynaphthazarins by IR-spectroscopy. Tetrahedron 58:1751–1757
Ozgen U, Miloglu FD, Bulut G (2011) Quantitative determination of shikonin derivatives with UV-Vis spectrophotometric methods in the roots of Onosma nigricaule. Rev Anal Chem 30:59–63
Noula E, Samanidou VF, Assimopoulou AN, Papageorgiou VP, Papadoyannis IN (2010) Solid-phase extraction for purification of alkannin/shikonin samples and isolation of monomeric and dimeric fractions. Anal Bioanal Chem 397:2221–2232
Sharma N, Sharma UK, Gupta AP, Devla Sinha AK, Lal B, Ahuja PS (2009) Simultaneous densitometric determination of shikonin, acetylshikonin, and β-acetoxyisovaleryl-shikonin in ultrasonic-assisted extracts of four Arnebia species using reversed-phase thin layer chromatography. J Sep Sci 32:3239–3245
Gao SQ, You JY, Wang Y, Zhang R, Zhang HQ (2012) On-line continuous sampling dynamic microwave-assisted extraction coupled with high performance liquid chromatographic separation for the determination of lignans in Wuweizi and naphthoquinones in Zicao. J Chromatogr B 887–888:35–42
Zhang Q, Cai DF, Wang L, Yang XT, Fan SJ, Zhang KY (2018) Rapid and sensitive determination of shikonin and its derivatives in the roots of Arnebia euchroma (Royle) Johnst using matrix solid-phase dispersion extraction and ultrahigh-performance liquid chromatography with photodiode array detector. J Liq Chromatogr Relat Technol 41:489–497
Feng L, Ji HW, Gu HX, Wang DC, Cui JC, Liu RM, Zhai J (2009) An efficient method for extraction, separation and purification of naphthoquinone pigments from Lithospermum erythrorhizon Sieb. et Zucc. by SFE and HSCCC. Chromatographia 70:1197–1200
Flieger J, Czajkowska AZ (2008) Ionic liquids in separation techniques. J Chromatogr A 1184:6–18
Wei ZF, Zu YG, Fu YJ, Wang W, Luo M, Zhao CJ, Pan YZ (2013) Ionic liquids-based microwave-assisted extraction of active components from pigeon pea leaves for quantitative analysis. Sep Purif Technol 102:75–81
Ma WY, Lu YB, Hu RL, Chen JH, Zhang ZZ, Pan YJ (2010) Application of ionic liquids based microwave-assisted extraction of three alkaloids N-nornuciferine, O-nornuciferine, and nuciferine from lotus leaf. Talanta 80:1292–1297
Ma CH, Liu TT, Yang L, Zua YG, Chen XQ, Zhang L, Zhang Y, Zhao CJ (2011) Ionic liquid-based microwave-assisted extraction of essential oil and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J Chromatogr A 1218:8573–8580
Bhan M, Satija S, Garg C, Dureja H, Garg M (2017) Optimization of ionic liquid-based microwave assisted extraction of a diterpenoid lactone-andrographolide from Andrographis paniculata by response surface methodology. J Mol Liq 229:161–166
Hou KX, Chen FL, Zu YG, Yang L (2016) Ionic liquids-lithium salts pretreatment followed by ultrasound-assisted extraction of vitexin-4″-O-glucoside, vitexin-2″-O-rhamnoside and vitexin from Phyllostachys edulis leaves. J Chromatogr A 1431:17–26
Wang WC, Li QY, Liu YH, Chen BB (2015) Ionic liquid-aqueous solution ultrasonic-assisted extraction of three kinds of alkaloids from Phellodendron amurense Rupr and optimize conditions use response surface. Ultrason Sonochem 24:13–18
Zhang H, Chen XQ, Jiang XY (2011) Determination of phthalate esters in water samples by ionic liquid cold-induced aggregation dispersive liquid-liquid microextraction coupled with high-performance liquid chromatography. Anal Chim Acta 689:110–111
Lin HM, Zhang YG, Han M, Yang LM (2013) Aqueous ionic liquid based ultrasonic assisted extraction of eight ginsenosides from ginseng root. Ultrason Sonochem 20:680–684
Vallecillos L, Borrull F, Pocurull E (2012) Determination of musk fragrances in sewage sludge by pressurized liquid extraction coupled to automated ionic liquid-based headspace single-drop microextraction followed by GC-MS/MS. J Sep Sci 35:2735–2742
Wu HW, Chen ML, Fan YC, Elsebaei F, Zhu Y (2012) Determination of rutin and quercetin in Chinese herbal medicine by ionic liquid-based pressurized liquid extraction–liquid chromatography–chemiluminescence detection. Talanta 88:222–229
Liu F, Wang D, Liu W, Wang X, Bai AY, Huang LQ (2013) Ionic liquid-based ultrahigh pressure extraction of five tanshinones from Salvia miltiorrhiza Bunge. Sep Purif Technol 110:86–92
Wu H, Zhang LB, Du LM (2011) Ionic liquid sensitized fluorescence determination of four isoquinoline alkaloids. Talanta 85:787–793
Zhang HF, Shi YP (2010) Temperature-assisted ionic liquid dispersive liquid-liquid microextraction combined with high performance liquid chromatography for the determination of anthraquinones in Radix et Rhizoma Rhei samples. Talanta 82:1010–1016
Zhang LS, Hu S, Che X, Bai XH, Li QS (2013) A new ionic liquid-water-organic solvent three phase microextraction for simultaneous preconcentration flavonoids and anthraquinones from traditional Chinese prescription. J Pharm Biomed Anal 86:36–39
Sharma N, Sharma UK, Malik S, Bhushan S, Kumar V, Verma SC, Sharma N, Sharma M, Sinha AK (2008) Isolation and purification of acetylshikonin and β-acetoxyisovalerylshikonin from cell suspension cultures of Arnebia euchroma (Royle) Johnston using rapid preparative HPLC. J Sep Sci 31:629–635
Gutowski KE, Broker GA, Willauer HD, Huddleston JG, Swatloski RP, Holbrey JD, Rogers RD (2003) Controlling the aqueous miscibility of ionic liquids: aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations. J Am Chem Soc 125:6632–6633
Kaler EW, Murthy AK, Rodriguez BE, Zasadzinski JAN (1989) Spontaneous vesicle formation in aqueous mixtures of single-tailed surfactants. Science 245:1371–1374
Tan ZJ, Li FF, Xu XL (2012) Isolation and purification of aloe anthraquinones based on an ionic liquid/salt aqueous two-phase system. Sep Purif Technol 98:150–157
Wu B, Zhang YM, Wang HP (2008) Aqueous biphasic systems of hydrophilic ionic liquids + sucrose for separation. J Chem Eng Data 53:983–985
Shukla SK, Pandey S, Pandey S (2018) Applications of ionic liquids in biphasic separation: aqueous biphasic systems and liquid-liquid equilibria. J Chromatogr A 1559:44–61
Liu Q, Chen XX, Guo Y, Han CH, Li J, Jia LH, Liu J, Wei XL (2019) Thermodynamic study of the aqueous two-phase systems of 1-butyl-3-methylimidazolium tetrafluoroborate and sodium dodecylbenzenesulfonate. J Mol Liq 279:18–22
Nan YQ, Liu HL, Hu Y (2006) Interfacial tension in phase-separated aqueous cationic/anionic surfactant mixtures. J Colloid Interface Sci 293:464–474
Yu W, Liu ZL, Li Q, Zhang HQ, Yu Y (2015) Determination of sudan I-IV in candy using ionic liquid/anionic surfactant aqueous two-phase extraction coupled with high-performance liquid chromatography. Food Chem 173:815–820
Yang X, Zhang SH, Yu W, Liu ZL, Lei L, Li N, Zhang HQ, Yu Y (2014) Ionic liquid-anionic surfactant based aqueous two-phase extraction for determination of antibiotics in honey by high-performance liquid chromatography. Talanta 124:1–6
Li FF, Liu Y, Lin WP (2018) Phase equilibrium and protein partitioning in aqueous two-phase systems containing imidazolium ionic liquids and surfactant at low voltage levels. J Mol Liq 256:372–379
Vicente FA, Lario LD, Pessoa A Jr, Ventura SPM (2016) Recovery of bromelain from pineapple stem residues using aqueous micellar two-phase systems with ionic liquids as co-surfactants. Process Biochem 51:528–534
Pharmacopoeia Committee of China (2015) Chinese pharmacopoeia. Chemical Industrial Press, Beijing
Martins MAR, Neves CMSS, Kurnia KA, Carvalho PJ, Rocha MAA, Santo LMNBF, Pinho SP, Freire MG (2016) Densities, viscosities and derived thermophysical properties of water-saturated imidazolium-based ionic liquids. Fluid Phase Equilib 407:188–196
Shang Y, Liu H, Hu Y, Prausnitz JM (2007) Effect of salts on the aqueous two-phase system in mixed solutions of Gemini (12-3-12,2Br−) and sodium dodecyl sulfate. Colloids Surf A 302:58–66
Yang X, Yu R, Zhang SH, Cao BC, Liu ZL, Lei L, Li N, Wang ZB, Zhang LY, Zhang HQ, Chen YH (2014) Aqueous two-phase extraction for determination of triazine herbicides in milk by high-performance liquid chromatography. J Chromatogr B 972:111–116
Yu W, Liu ZL, Gao SQ, Cui SS, Yang X, Qiu W, Zhang HQ, Yu AM, Huan YF (2013) Determination of sulfonamides in blood using acetonitrile-salt aqueous two-phase extraction coupled with high-performance liquid chromatography and liquid chromatography-tandem mass spectrometry. Anal Methods 5:5983–5989
Guan LY, Luo Q, Shi JY, Yu W (2018) Application of ionic-liquid-magnetized stirring bar liquid-phase microextraction coupled with HPLC for the determination of naphthoquinones in Zicao. J Sep Sci 4:868–876
Guo YX, Han J, Zhang DY, Wang LH, Zhou LL (2012) An ammonium sulfate/ethanol aqueous two-phase system combined with ultrasonication for the separation and purification of lithospermic acid B from Salvia miltiorrhiza Bunge. Ultrason Sonochem 19:719–724
Yu W, Li K, Liu ZL, Zhang HQ, Jin XQ (2018) Novelty aqueous two-phase extraction system based on ionic liquid for determination of sulfonamides in blood coupled with high-performance liquid chromatography. Microchem J 136:263–269
Woo YA, Kim HJ, Cho JH, Chung H (1999) Discrimination of herbal medicines according to geographical origin with near infrared reflectance spectroscopy and pattern recognition techniques. J Pharm Biomed Anal 21:407–413
Acknowledgements
This study was financially supported by the Education Department of Jilin Province (Grant no. JJKH20181014KJ) and the Department of Science and Technology of Jilin Province (Grant no. 20190304011YY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Q., Du, B., Wang, C. et al. Ultrasound-Assisted Ionic Liquid Solid–Liquid Extraction Coupled with Aqueous Two-Phase Extraction of Naphthoquinone Pigments in Arnebia euchroma (Royle) Johnst.. Chromatographia 82, 1777–1789 (2019). https://doi.org/10.1007/s10337-019-03804-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-019-03804-y