Abstract
A new in-tube pretreatment method based on ultrasound assisted dispersive solid–liquid microextraction using self-assembly and solidification of an alkanol-based floating organic droplet was developed for the determination of eight pyrethroid insecticides in chrysanthemum by gas chromatography with electron capture detection. This method fully utilized the restricted access property of a 1-decanol/acetonitrile mixture for effective extraction of the analytes from chrysanthemum under ultrasonication, and the self-assembly and coacervation process of 1-decanol by adding water. The 1-decanol phase aggregated and floated on the surface, solidified in an ice bath, and thus was easily collected. For the first time, extraction, separation and preconcentration were combined in a tube, not requiring stepwise preparation for a solid matrix. The recoveries ranged from 75 to 104% with the relative standard deviations of < 8%. The limits of quantification were in the range of 0.15–1.5 µg kg− 1 up to 52-fold compared with the conventional QuEChERS-based, SPE, and solid–liquid dispersive microextraction methods. The results demonstrated that the proposed method was time-saving, sensitive, and environmentally friendly for pyrethroids analysis in chrysanthemum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chrysanthemum, Dendranthema grandiflora, is a popular Chinese herbal medicine containing abundant nutrients of amino acids, flavonoids, alkaloids, and essential oils, and minerals, and thus widely consumed or processed to commercial medicines or beverages [1]. Pesticides application is considered as an effective measure to safeguard the quality of chrysanthemum crops, however, the exposure risk to humans associated with pesticide residues on chrysanthemum plants underlines the need to develop efficient and sensitive analytical methods.
Due to the complexity of the chrysanthemum matrix and the trace level of residues, using effective purification and enrichment procedures is critical to simultaneously determine target compounds. Most of the efforts made for chrysanthemum samples analysis are time consuming and involve laborious pretreatments, including gel permeation chromatography [2], liquid liquid extraction (LLE) with sulfuric acid [3], solid phase extraction (SPE) [4] and dispersive SPE [5]. Large amounts of samples, organic solvents, SPE cartridges, and DSPE adsorbents are required, resulting in costly monitoring and generation of large residues [6]. An additional consideration is the non-compatibility of pretreatment simplification and high concentration factor. In this way, during the past 10 years, dispersive liquid liquid microextraction (DLLME) based on LLE has been introduced and widely used to analyze compounds in aqueous samples, such as water and beverages [7,8,9,10]. The distinct advantages of DLLME with rapid analysis, simple operation, and high enrichment have promoted its combination with other extraction techniques such as QuEChERS [11], SPE [12], supercritical fluid extraction [13], and stir-bar sorptive extraction [14] for the analysis of solid matrices. However, the mentioned approaches still have to be operated in multiple steps, resulting in a time-consuming procedure and potential residue loss [15].
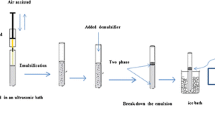
To solve the difficulty of traditional DLLME for its application on analysis of complex solid matrices, an alkanol-based composite solvent was introduced in this work. The composite solvent consisting of a medium-chain alkanol and an organic solvent (e.g. acetonitrile, acetone) was used as the extraction solvent. The ultrasound irradiation was employed to accelerate the analytes extraction from the solid matrix to the composite solvent. When the extraction was complete, water was added to promote self-assembly of the alkanol and caused the spontaneous formation of coacervate droplets, which facilitated creaming and phase separation of the alkanol from the bulk solution [16]. During the process, the restricted access property of the alkanol-based composite solvent that can be switched reversibly from a miscible-in-water status to an immiscible status, achieves the enrichment of pesticides while excluding high molecular mass components in solid matrices [17]. An additional advantage of using the alkanol is its lower density compared with water, low melting point close to room temperature and minimization of toxicity when chosen as an alternative of the hazardous solvents in conventional DLLME, such as chlorobenzene, carbon tetrachloride, toluene and chloroform [18, 19]. The floating droplet of the alkanol can be solidied by immersing the sample tube in an ice bath and easily removed for further analysis, which is defined as a solidification of floating organic droplet process [20, 21]. The entire operation provides a mixed-mode mechanism for extracting the pesticides from the solid sample to the alkanol-based composite solvent, then concentrating the pesticides in the alkanol via its self-assembly and coacervation, and finally collection of the floating phase by solidification.
The new method, namely in-tube ultrasound assisted dispersive solid–liquid microextraction (UA-DSLME) based on self-assembly and solidification of an alkanol-based floating organic droplet, was proposed for the first time to determine eight pyrethroid insecticides in chrysanthemum by gas chromatography with electron capture detection (GC–ECD). The entire process combines the extraction with a 1-decanol/acetonitrile mixture under sonication, cleanup with primary secondary amine (PSA), phase separation and preconcentration by self-assembly and solidification of 1-decanol in a tube which did not require stepwise procedures. The effects of experimental parameters, such as the type and volume of the alkanol-based composite solvent, sonication time and salt addition, were thoroughly assessed. Furthermore, special attention was devoted to the comparison of the optimized approach and conventional QuEChERS-based, SPE, and solid–liquid dispersive microextraction methods. The good performance demonstrated its future application potential for pesticides analysis in solid complex matrices.
Materials and Methods
Reagents and Materials
Eight pyrethroid insecticides standards of bifenthrin (98%), fenpropathrin (98.5%), lambda-cyhalothrin (97%), permethrin (99%), cypermethrin (98%), flucythrinate (97.5%), fenvalerate (98.5%), and deltamethrin (98.7%) were purchased from Agro-Environmental Protection Institute, Ministry of Agriculture (Tianjin, China). The chromatography-grade solvents including 1-decanol, 1-undecanol, 1-dodecanol, toluene, hexadecane, and 1-chlorooctadecane were provided by Aladdin biochemical Polytron Technologies Co. Ltd (Shanghai, China). Acetonitrile, acetone and methanol with analytical grade were from Tedia Co. (Fairfield, USA). Distilled water was obtained using a Milli-Q water purification system. Sodium chloride (NaCl) with analytical-reagent grade was provided by J&K Chemical Co. Ltd (Shanghai, China). PSA was obtained from Agela Technologies, China.
An RJ-TDL-40B low-speed desktop centrifuge was purchased from Ruijiang Co. (Jiangsu, China). A KQ-5200 ultrasonic machine was supplied by Shumei Ultrasonic Instrument Co. Ltd (Zhejiang, China). A pipettor in a range of 0.1 mL was supplied by Meifeng Chemical Co. Ltd (Sichuan, China).
The chrysanthemum samples provided by Institute of plant protection in Anhui (China), were shelled into slices prior to pretreatment.
Preparation of Standards
Individual 1000 mg/L stock solution was prepared by dissolving approximately 0.010 g of each pesticide in 10 mL of acetonitrile. The mixed working solution of the eight pyrethroids was obtained daily by appropriately diluting the stock solution with acetonitrile to obtain a final concentration of 10 mg L− 1. All solutions were stored at − 20 °C in the dark.
In-Tube UA-DSLME Procedure
A homogenized sample (0.2 g, weighed to a precision of 0.01 g) was placed in a 15-mL screw cap polyethylene tube containing 50 mg of PSA. A mixture containing 50 µL of 1-decanol and 2.5 mL of acetonitrile was added. The tube was immersed immediately in an ultrasonic bath (40 kHz, 100 W) for 5 min at 25 ± 2 °C. Then 10 mL of water with 2% NaCl was rapidly added in the mixture. After centrifugation at 4000 rpm for 2 min, the tube was rapidly immersed into an ice bath and the 1-decanol layer solified after 3 min. The solidified droplets floating on the surface could be readily removed to a 1.5-mL plastic tube by a small scoop, and then melted quickly at room temperature. The final 1-decanol was transferred into a 250 µL glass insert-pipe placed in a vial prior to the GC–ECD analysis. The entire extraction procedure is shown as a schematic in Fig. 1.
Instrumental and Analytical Conditions
Chromatographic analysis of the eight pyrethroids was performed on a Shimadzu 2010 Plus GC system (Shimadzu, Japan) equipped with an ECD detector, a Shimadzu AOC20i autosampler and a 30 m × 0.25 mm DB-5 fused-silica capillary column (0.25 µm film thickness). The injector and detector were held at 250 and 300 °C, respectively. The carrier gas was helium at a flow rate of 1.0 mL min− 1. The oven temperature was initially at 120 °C for 1 min, increased to 240 °C at a rate of 30 °C min− 1 and held for 2 min, and finally increased at a rate of 5 °C min− 1 to 280 °C and kept for 15 min. The sample injection volume was 2 µL and the injection mode was splitless.
Method Validation
The linearity of the developed method was evaluated by analyzing the 8 pyrethroids in sample extract at concentrations ranging from 2 to 500 µg kg− 1. The accuracy and precision of the method were determined by calculating the recovery and relative standard deviation (RSD), respectively, at three levels (5, 50, and 200 µg kg− 1) in the blank samples for each analyte. The limit of quantification (LOQ) were estimated at the lowest concentration level for the signal-to-noise (S/N) ratios of 10:1 in the GC–ECD analysis. The extraction recovery (ER) is defined as the percentage of total analyte amount moved to the microextraction solvent from the acetonitrile extract and can be calculated according to the following equation:
where Cmic and C0 are the analyte concentration in the microextraction solvent and sample, respectively, Vmic is the volume of the microextractant, and M0 is the the amount of sample.
Results and Discussion
This study focused first on optimization of the analytical parameters that affect the performance of in-tube UA-DSLME, including type and ratio of the alkanol-based composite solvent (including the microextractant and dispersive solvent), sonication time, volume of added water and concentration of NaCl. Each parameter was assessed on 0.2 g of blank chrysanthemum samples spiked at 200 µg kg− 1 of each of the eight pyrethroids and examed in four replicates. The average of the results was calculated to evaluate the effect of each factor. In the second part, the performance and merits of the proposed method was compared with QuEChERS-based, SPE, and vortex-assisted matrix solid–liquid dispersive microextraction (VA-MSLDME) methods published in our previous works, and other reported methods.
Optimization of In-Tube UA-DSLME
Selection of the Alkanol-Based Composite Solvent
In this study, with introducing the concept of self-assembly and solidified floating, an alkanol-based composite solvent was used as the extraction solvent consisting of a microextractant and dispersive solvent, which is required to fulfill the following characteristics: strong extraction ability, miscibility between the microextractant and dispersive solvent, immiscibility between the microextractant and water, low melting point and density of the microextractant [22, 23]. For these considerations, three medium-chain alkanols (1-undecanol, 1-dodecanol, 1-decanol), and two alkane solvents (n-hexadecane, chlorooctadecane) selected for comparison, were tested as the microextractant, while three commonly used dispersive solvents, acetonitrile, acetone, and methanol, were selected.
A series of mixtures containing 100 µL microextractant (1-undecanol, 1-dodecanol, 1-decanol, n-hexadecane, chlorooctadecane) and 2 mL dispersive solvent (acetonitrile, acetone, methanol), respectively, were examed for extracting the target analytes from chrysanthemum by ultrasonication for 15 min. When acetone or methanol mixture was added, the final collected volume of the five microextractants was much smaller with 50–60 µL. The great reduction of the microextractant might be caused by an excessive emulsification obtained from mixing acetone or methanol with the microextractant. The eight pyrethroids are considered to be highly fat soluble (octanol–water partition coefficient values from 4.5 to 6.6), and thus may be contained in the emulsification phase, contributing to the recoveries less than 40%. This was consistent with our previous works [11]. In contrast, the five microextractants were retrieved at 90–100 µL, and the extract solution was visible with a good phase separation using acetonitrile mixtures as the extraction solvent, which presented higher recoveries for all pyrethroids. Hence, the acetonitrile mixtures were further investigated.
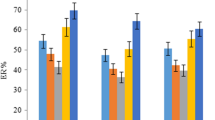
As shown in Fig. 2a, three medium-chain alkanols (1-undecanol, 1-dodecanol, 1-decanol) attained better results than the other two alkane solvents (n-hexadecane, chlorooctadecane). The difference could be due to the higher solubility of the alkanols in acetonitrile compared with the two alkanes, thus showing a better dispersion and extractability. Due to the stronger polarity than the other two alkanols, the use of 1-decanol in the extraction mixture was able to recover the majority of eight pyrethroids with highest recoveries (78–101%). The results demonstrated that the concept of self-assembly and coacervation properties for an alkanol-based composite solvent is feasible for its application in extraction. For reasons of environmental friendliness, compared with 1-decanol to 1-undecanol or 1-dodecanol, a combination of 1-decanol and acetonitrile was chosen as extraction solvent.
Effect of the Volume of the Alkanol-Based Composite Solvent
The volume and ratio of the microextractant and dispersive solvents affect the dispersion degree and cloudy state of the solvents, that determines the method performance [24]. Lower volumes generally constitute substantial enrichment factors, whereas insufficient extractant volumes may lead to lower recoveries. In this regard, different volumes of 1-decanol and acetonitrile in the ranges of 40–100 µL and 2–4 mL were investigated, respectively. First, 2, 2.5, 3, 3.5 or 4 mL of acetonitrile, containing 100 µL of 1-decanol, was subjected and the mixture sample was sonicated for 15 min. The collected volumes of 1-decanol were measured at 98, 95, 90, 80, and 70 µL, respectively, with the acetonitrile amount ranging from 2 to 4 mL. The results in Fig. 2b indicated that the extraction recoveries increased slightly when the acetonitrile volume increased from 2 to 2.5 mL, whereas decreased within acetonitrile at 3–4 mL. In low volumes of acetonitrile, the contact area between the extraction solvent and chrysanthemum sample was not sufficient for pyrethroids extraction. However, the higher volumes of acetonitrile resulted in the decreased volumes of 1-decanol, indicating an unsufficient extraction of the analytes and difficulty to transfer to 1-decanol [25]. Consequently, 2.5 mL of acetonitrile was chosen as the optimum volume.
Usually, the microextractant volume must be sufficient to achieve satisfactory ERs for the target analytes, however, its volume is expected small to obtain high concentrations and enough for subsequent chromatographic analysis. For this consideration, various volumes of 1-decanol (40, 50, 60, 70, 80, 90, and 100 µL) dissolved in 2.5 mL acetonitrile were investigated under ultrasonication for 15 min. Negligible differences of 1-decanol volume at 45–95 µL compared with the added volumes of 50–100 µL were shown, whereas obvious reduction for the collected volume of 1-decanol droplets with 25 µL was presented employing 40 µL of 1-decanol as the microextractant. Therefore, low recoveries of 56–78% were obtained from 40 µL of 1-decanol, and this volume was not studied further. It could be seen in Fig. 2c that slightly increased recoveries of all the pyrethroids were observed when the volume of 1-decanol rose from 50 to 100 µL, However, there was a gradual reduction of the peak areas for the eight pyrethroids when the 1-decanol increased from 50 to 100 µL, which may be caused by the dilution effect with a larger volume [26]. For these reasons, 50 µL of 1-decanol was used since good recoveries and peak areas were both obtained, and subsequently mixed with 2.5 mL of acetonitrile in the following studies.
Effect of Ultrasonication Time
The extraction time is another important parameter, since a relatively long extraction time could led to the equilibrium between the analytes and the extractant phase. However, spending too much time on the extraction process may reduce the overall time efficiency of the procedure [27]. Compared with hand shaking or vortexing, ultrasound assisted shaking shortened extraction time and saved labour force. Ultrasonic energy provided microenvironments with high pressure and high temperature, accelerating the dispersion and mass transfer of target analytes to the organic phases [28]. To find a suitable ultrasonication time for effective extraction, 2.5 mL of acetonitrile containing 50 µL of 1-decanol was mixed with the chrysanthemum sample by ultrasonication at different times of 5, 10 15, 20, 25 min, respectively. Similar volumes of 45 µL could be recovered after different ultrasonication treatments. The ERs in Fig. 2d revealed that ultrasonic extraction at the range of 5–25 min produced similar efficiency, indicating the complete transfer of the pyrethroids to the extraction solvent phase after 5 min. In this case, the ultrasonication time was set at 5 min for extraction.
Effect of Salt Addition
Salt addition could strongly affect the solubility of extraction solvent and analytes in the water phase as a result of the salting-out effect [29]. A series of NaCl concentrations (0–20% w/v at the interval of 4%) were tested to estimate the effect of salt on the extraction. The experiments were carried out by adding 10 mL salt water containing different amounts of NaCl, respectively, for phase separation in the extraction solvent (50 µL of 1-decanol and 2.5 mL of acetonitrile) and sample after ultrasonication for 5 min. When the NaCl concentration was higher than 4%, the acetonitrile solution was isolated from the aqueous solution, leading to acetonitrile mixing with 1-decanol, and thus 1-decanol could not be separated or retrieved from the aqueous phase. A range of 0, 1, 2, 3, and 4% NaCl in water was further examined to achieve effective analytes transfer and 1-decanol phase separation. The volume of 1-decanol was collected at 45 µL under the treatments with 0–4% NaCl aqueous solution. As illustrated in Fig. 2e, the recoveries kept generally constant with the NaCl concentration at 0, 1, or 2%, then decreased obviously when the NaCl concentration increased. This was assumed to be attributed by higher aqueous phase viscosity causing lower diffusion of the analytes [30]. The presence of NaCl in water resulted in a more convenient collection of the solidified droplets on the top. Therefore, water with 2% NaCl was added to ensure the extraction and separation performance.
Performance of In-Tube UA-DSLME
Linearity and LOQ
Linearity was examined for the eight pyrethroids using a single curve with six calibration points. The matrix-matched calibration curve was made by extracting chrysanthemum samples spiked at six concentrations ranging from 2 to 500 µg kg− 1, and the signal intensities used for each data point were averaged from three repeated injections. A good linearity for the eight pyrethroids with correlation coefficients (r2) of 0.9972–0.9993 was obtained. The LOQs were set at values of ten times the background noise obtained for blank chrysanthemum samples were in the ranges of 0.15–1.5 µg kg− 1, as shown in Table 1.
Recovery Studies
Accuracy of the proposed methodology was evaluated in terms of ERs. Recovery assays were performed with the blank chrysanthemum samples spiked at three concentration levels of 5, 50, and 200 µg kg− 1 for all of the pyrethroids. Each level was carried out at six replications as described in the section “Method Validation”. As listed in Table 1, the ER values based on matrix-matched calibration were between 75% and 104% with RSDs of 4–8%, which was calculated using the equations mentioned in section “Method Validation”.
Comparison of the Proposed Method with Other Analytical Techniques
The practical performance of this in-tube UA-DSLME for chrysanthemum samples was further evaluated by comparing with conventional QuEChERS-based, SPE, VA-MSLDME, and other reported techniques for herbal medicines. The comparison study was carried out in terms of LOQs, organic solvent volume, and analysis time, as summarized in Table 2. As a microscale approach, in-tube UA-DSLME has fulfilled environmental considerations by only using a total organic solvent amount of 2.55 mL per sample, whereas 10–90 mL of organic solvent was typically required by most of other methods. Furthermore, 1-decanol was adopted as an alternative of commonly used hazardous solvents, such as toluene, and thus this method met the principle of green analytical chemistry and grenerally reduced environment pollution. Without the stepwise procedures or long-time extraction taking more than 20 min, such as solvent elution and evaporation as with SPE, supercritical fluid extraction, accelerated solvent extraction, or gel permeation chromatography, this proposed method greatly accelerates the analysis by simultaneous extraction/transfer/proconcentration within 10 min. The simplicity allows high sample throughput in routine monitoring.
Generally, good accuracy and precision were obtained from these techniques, however, in term of enrichment, the LOQs of the proposed method were much lower or comparable with most mentioned methods, implying it could achieve higher sensitivity. This was also demonstrated by the results obtained from SPE, QuEChERS, and VA-MSLDME, which were carried out at the same spiked concentration of 50 µg kg− 1, respectively, following the steps developed in our previous works [1, 31]. As observed in Fig. 3, UA-DSLME showed the highest signal responses for all of the pyrethroids, whereas lower responses were obtained from SPE or QuEChERS, especially for permethrin, cypermethrin, flucythrinate, fenvalerate, and deltamethrin (identified as No. 4–8 peaks) with no obvious peaks in the chromatograms. The peak areas obtained from UA-DSLME exhibited up to 1.5 times more than VA-MSLDME, 25 times more than SPE, and 52 times more than QuEChERS, respectively. Based on the comparison, the proposed method is recommended as a sensitive, rapid, easy, and environmentally friendly approach to perform the analysis of pyrethroid pesticides in chrysanthemum.
The GC chromatograms of 50 µg kg− 1 spiked chrysanthemum samples using a in-tube UA-DSLME (the proposed method), b VA-MSLDME, c SPE, and d QuEChERS. Peaks (retention time): (1) bifenthrin (13.6 min), (2) fenpropathrin (14.7 min), (3) lambda-cyhalothrin (16.7 min), (4) permethrin (17.4 min), (5) cypermethrin (21.7 min), (6) flucythrinate(22.2 min), (7) fenvalerate (22.9 + 24.2 min), and (8) deltamethrin (26.7 min)
Conclusions
A new method, in-tube UA-DSLME based on the properties of self-assembly and solidification of a medium-chain alkanol-based composite solvent was developed for analyzing eight pyrethroid insecticides in chrysanthemum by GC–ECD. The composite solvent consisting of 1-decanol and acetonitrile was introduced for pyrethroids extraction from chrysanthemum under ultrasound assistance, followed by adding salt water to convert the acetonitrile-miscible 1-decanol into an immiscible form. Then, the 1-decanol droplets flocculated and floated on the surface due to its lower density than water, and could be collected easily by exposing in an ice bath. This approach represents a new contribution to simultaneous extraction, separation, and preconcentration in one tube, thus avoiding stepwise pretreatment for solid samples. In comparison with other reported methods for solid complex sample, the proposed approach exhibited significant merits, such as lower cost of organic solvent, shorter consumption of analysis time, higher sensitivity, and being more eco-friendly. In addition, the good accuracy and precision demonstrated its strong potential of application in pyrethroid insecticides analysis from solid complex matrix.
References
Xue JY, Chen XC, Jiang WQ, Liu FM, Li HC (2015) Rapid and sensitive analysis of nine fungicide residues in chrysanthemum by matrix extraction-vortex-assisted dispersive liquid–liquid microextraction. J Chromatogr B 975:9–17
Xue JY, Xu YJ, Liu FM, Xue J, Li HC, Peng W (2013) Comparison of different sample pre-treatments for multi-residue analysis of organochlorine and pyrethroid pesticides in chrysanthemum by gas chromatography with electron capture detection. J Sep Sci 36:1311–1316
Xue J, Hao LL, Peng F (2008) Residues of 18 organochlorine pesticides in 30 traditional Chinese medicines. Chemosphere 71:1051–1055
Xue JY, Li HC, Liu FM, Xue J, Chen XC, Zhan J (2014) Transfer of difenoconazole and azoxystrobin residues from chrysanthemum flower tea to its infusion. Food Addit Contam Part A 31:666–675
Chen HP, Gao GW, Liu PX, Pan ML, Chai YF, Liu X, Lu CY (2017) Development and validation of an ultra performance liquid chromatography exactive orbitrap mass spectrometry for the determination of fipronil and its metabolites in tea and chrysanthemum. Food Chem 246:328–334
Petrarca MH, Ccanccapa-Cartagena A, Masiá A, Godoy HT, Picó Y (2017) Comparison of green sample preparation techniques in the analysis of pyrethrins and pyrethroids in baby food by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1497:28–37
Ccanccapa-Cartagena A, Masiá A, Picó Y (2017) Simultaneous determination of pyrethroids and pyrethrins by dispersive liquid-liquid microextraction and liquid chromatography triple quadrupole mass spectrometry in environmental samples. Anal Bioanal Chem 409:4787–4799
Amde M, Tan ZQ, Liu R, Liu JF (2015) Nanofluid of zinc oxide nanoparticles in ionic liquid for single drop liquid microextraction of fungicides in environmental waters prior to high performance liquid chromatographic analysis. J Chromatogr A 1395:7–15
Wang HZ, Hu L, Li WZ, Yang XL, Lu RH, Zhang SB, Zhou WF, Gao HX, Li J (2017) In-syringe dispersive liquid-liquid microextraction based on the solidification of ionic liquids for the determination of benzoylurea insecticides in water and tea beverage samples. Talanta 162:625–633
Zhang Y, Zhang X, Jiao B (2014) Determination of ten pyrethroids in various fruit juices: comparison of dispersive liquid-liquid microextraction sample preparation and QuEChERS method combined with dispersive liquid-liquid microextraction. Food Chem 159:367–373
Xue JY, Li HC, Liu FM, Jiang WQ, Chen XC (2014) Determination of strobilurin fungicides in cotton seed by combination of acetonitrile extraction and dispersive liquid-liquid microextraction coupled with gas chromatography. J Sep Sci 37:845–852
Samadi S, Sereshti H, Assadi Y (2012) Ultra-preconcentration and determination of thirteen organophosphorus pesticides in water samples using solid-phase extraction followed by dispersive liquid-liquid microextraction and gas chromatography with flame photometric detection. J Chromatogr A 1219:61–65
Jowkarderis M, Raofie F (2012) Optimization of supercritical fluid extraction combined with dispersive liquid-liquid microextraction as an efficient sample preparation method for determination of 4-nitrotoluene and 3-nitrotoluene in a complex matrix. Talanta 88:50–53
Farajzadeh MA, Djozan D, Nouri N, Bamorowat M, Shalamzari MS (2010) Coupling stir bar sorptive extraction-dispersive liquid-liquid microextraction for pre-concentration of triazole pesticides from aqueous samples followed by GC-FID and GC-MS determinations. J Sep Sci 33:1816–1828
Bazregar M, Rajabi M, Yamini Y, Saffarzadeh Z, Asghari A (2016) Tandem dispersive liquid-liquid microextraction as an efficient method for determination of basic drugs in complicated matrices. J Chromatogr A 1429:13–21
Ballesteros-Gómez A, Rubio S (2012) Environment-responsive alkanol-based supramolecular solvents: Characterization and potential as restricted access property and mixed-mode extractants. Anal Chem 84:342–349
Ballesteros-Gómez A, Lunar L, Sicilia MD, Rubio S (2018) Hyphenating supramolecular solvents and liquid chromatography: tips for efficient extraction and reliable determination of organics. Chromatographia. https://doi.org/10.1007/s10337-018-3614-1
Saraji M, Boroujeni MK (2014) Recent developments in dispersive liquid-liquid microextraction. Anal Bioanal Chem 406:2027–2066
Ho Y-M, Tsoi Y-K, Leung KS-Y (2013) Highly sensitive and selective organophosphate screening in twelve commodities of fruits, vegetables and herbal medicines by dispersive liquid–liquid microextraction. Anal Chim Acta 775:58–66
Vera-Avila LE, Rojo-Portillo T, Ovarrubias-Herrera R, Peña-Alvarez A (2013) Capabilities and limitations of dispersive liquid-liquid microextraction with solidification of floating organic drop for the extraction of organic pollutants from water samples. Anal Chim Acta 805:60–69
Bolzan CM, Caldas SS, Guimarães BS, Primel EG (2016) Dispersive liquid-liquid microextraction based on solidification of floating organic droplet for the determination of triazine and triazoles in mineral water samples. J Sep Sci 39:3410–3417
Pirsaheb M, Fattahi N, Shamsipur M, Khodadadi T (2013) Application of dispersive liquid-liquid microextraction based on solidification of floating organic drop for simultaneous determination of alachlor and atrazine in aqueous samples. J Sep Sci 36:684–689
Sanagi MM, Abbas HH, Ibrahim WAW, Aboul-Enien HY (2012) Dispersive liquid-liquid microextraction method based on solidification of floating organic droplet for the determination of triazine herbicides in water and sugarcane samples. Food Chem 133:557–562
Lima DL, Silva CP, Otero M, Esteves VI (2013) Low cost methodology for estrogens monitoring in water samples using dispersive liquid-liquid microextraction and HPLC with fluorescence detection. Talanta 115:980–985
Farajzadeh MA, Afshar Mogaddam MR, Aghdam SR, Nouri N, Bamorrowat M (2016) Application of elevated temperature-dispersive liquid-liquid microextraction for determination of organophosphorus pesticides residues in aqueous samples followed by gas chromatography-flame ionization detection. Food Chemi 212:198–204
Mirparizi E, Rajabi M, Bazregar M, Asghari A (2017) Centrifugeless dispersive liquid-liquid microextraction based on salting-out phenomenon as an efficient method for determination of phenolic compounds in environmental samples. Anal Bioanal Chem 409:3007–3016
Chen PS, Haung WY, Huang SD (2014) Analysis of triazine herbicides using an up-and- down-shaker-assisted dispersive liquid-liquid microextraction coupled with gas chromatography-mass spectrometry. J Chromatogr B 955–956:116–123
Lin ZB, Li JL, Zhang XY, Qiu MH, Huang ZB, Rao YL (2017) Ultrasound-assisted dispersive liquid-liquid microextraction for the determination of seven recreational drugs in human whole blood using gas chromatography-mass spectrometry. J Chromatogr B 1046:177–184
Ghazaghi M, Mousavi HZ, Shirkhanloo H, Rashidi A (2017) Stirring-controlled solidified floating solid-liquid drop microextraction as a new solid phase-enhanced liquid-phase microextraction method by exploiting magnetic carbon nanotube-nickel hybrid. Anal Chim Acta 951:78–88
Wang HZ, Hu L, Liu XY, Yin SJ, Lu RH, Zhang SB, Zhou WF, Gao HX (2017) Deep eutectic solvent-based ultrasound-assisted dispersive liquid-liquid microextraction coupled with high-performance liquid chromatography for the determination of ultraviolet filters in water samples. J Chromatogr A 1516:1–8
Xue JY, Li HC, Liu FM, Jiang WQ, Hou F (2016) Vortex-assisted matrix solid-liquid dispersive microextraction for the analysis of triazole fungicides in cotton seed and honeysuckle by gas chromatography. Food Chem 196:867–876
Qi XY (2010) Development of a matrix solid-phase dispersion-sonication extraction method for the determination of fungicides residues in ginseng extract. Food Chem 121:758–762
Quan C, Shang YG, Li SF, Tang SK, Huang T, Fang X (2010) Kinetic study of supercritical fluid extraction of organochlorine pesticides from ginseng by Simulink simulation. J Taiwan Inst Chem Eng 41:44–48
Huang XH, Zhao XH, Lu XT, Tian HP, Xu AJ, Liu Y, Jian Z (2014) Simultaneous determination of 50 residual pesticides in flos chrysanthemi using accelerated solvent extraction and gas chromatography. J Chromatogr B 967:1–7
Acknowledgements
This work was partly supported by the National Key Research and Development Program of China (2016YFD0200201), the National Natural Science Foundation of China (41807490), the Natural Science Research Project of High Education of Anhui (KJ2018A0128), and the University Youth Science Foundation of Anhui Agricultural University (2017zd04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Jiaying Xue declares that she has no conflict of interest. Author Dong Zhang declares that he has no conflict of interest. Author Xiangwei Wu declares that he has no conflict of interest. Author Dandan Pan declares that she has no conflict of interest. Author Rimao Hua declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xue, J., Zhang, D., Wu, X. et al. In-Tube Ultrasound Assisted Dispersive Solid–Liquid Microextraction Based on Self-Assembly and Solidification of an Alkanol-Based Floating Organic Droplet for Determination of Pyrethroid Insecticides in Chrysanthemum. Chromatographia 82, 695–704 (2019). https://doi.org/10.1007/s10337-018-3678-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3678-y