Abstract
A novel polyphenyl-grafted polysiloxane stationary phase named 3,4-bis(2-fluoro-5-(trifluoromethyl)phenyl)-2,5-diphenyl phenyl grafted polysiloxane stationary phase (FFMP) was synthesized through a Diels–Alder reaction with a high column efficiency (average number of plates: 3700 plates/m; achieved by naphthalene at 120 °C) and simultaneously coated on fused silica capillary tubes to prepare a gas chromatographic column with excellent performance. The column performance test results indicated that the FFMP columns could work properly up to 360 °C, as evidenced by the chromatogram of the polyethylene pyrolysis mixture. The thermogravimetric analysis curve showed that the decomposition temperature of the FFMP was up to 380 °C. The FFMP columns were also applied in the separation and analysis of multimixtures, such as Grob test mixtures, benzene mixtures and fatty acid esters, and as well as a medium polar stationary phase (according to the results of McReynolds constants, the sum of ∆I was 779.) The FFMF columns exhibited excellent separation selectivity for these substances because of the conjugated system formed by the polyphenyl side chain connected by single bonds. This conjugated system can promote the delocalization of π-electrons as well as enhance the forces of π–π interaction, and the dipole-induced dipole action between the FFMP stationary phase and the analytes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Capillary gas chromatography (CGC) has been widely applied in the fields of petrochemical engineering, environmental protection, and biological medicine because of its high sensitivity and quick analysis speed. At present, gas chromatography is a mature analytical technique, of which the application scope and depth are still being expanded [1]. In particular, the synthesis of new stationary phases with high resolution and thermal stability has broadened the application range of the stationary phases of CGC, such as metal–organic frameworks [2,3,4,5,6,7], carbon nanotubes [8,9,10], and ionic liquids [11, 12].
At present, thousands of gas chromatographic stationary phases have been synthesized, and a number of stationary phases have been gradually commercialized, because polysiloxane stationary phases are characterized by high thermal stability, good film formation, wide temperature range, low mass transfer resistance, and high column efficiency [13]. Many researchers have introduced different organic molecules into polysiloxane chains, which have been widely used for the selective separation of various mixtures, particularly polar mixtures [14]. The introduction of polarized phenyl groups with high resistance against oxidation into polysiloxane can enhance dipole-induced dipole action between stationary phases and polar substances, and improve the separation selectivity of stationary phases [15, 16]. Peaden et al. introduced different content of phenyl groups into methyl polysiloxane to obtain methylphenyl polysiloxane-fused silica capillary columns with high column effect and excellent thermal stability [17]. In the analysis of coal tar, the temperature of the stationary phase with 70% phenyl content could reach 400 °C; however, its minimum initial temperature was higher, thereby limiting its application. Lee et al. introduced biphenyl [13], naphthalene [13], phenoxy phenyl [13], alkyl benzene [18], and methoxy phenyl [19] into polysiloxane and then synthesized various polysiloxane stationary phases for gas chromatography. Although these stationary phases could separate aromatic substances, such as polycyclic aromatic hydrocarbons (PAHs), their temperatures were lower than 280 °C, even reaching below 200 °C [13]. Mayer et al. grafted n-octylmethyl, diphenyl, and polar trifluoro-propyl on the side chain of siloxanes to obtain a 50% octylmethyl—50% diphenyl polysiloxane stationary phase [20] and a tetramethyl-p-silphenylene-3,3,3-trifluoropropylmethyl dimethyl siloxane terpolymer stationary phase [21]. These stationary phases display excellent separation selectivity and thermal stability (360 °C) for separating mixtures of n-alkanes (C16–C36) and fatty acid esters (FAEs).
In view of the worsening environmental pollution in recent years, the study of environmental pollutants has become an even more important topic in various fields [22, 23]. In the field of CGC, studies mainly focused on the separation and analysis of environmental pollutants, such as PAHs and polychlorinated biphenyls. To improve the chromatographic performance of stationary phases, groups such as diphenyl phenyl [24], 3,4-bis(3,4,5-trifluorophenyl)-2,5-diphenyl phenyl [25], and 3,4-bis(trifluoromethyl phenyl)-2,5-diphenyl phenyl [26] were introduced into polysiloxanes to obtain a high thermal stability and excellent separation selectivity for aromatic substances. Therefore, the introduction of polyphenyl groups and polar groups could enhance not only the thermal stability of the stationary phase but also the forces between the stationary phases and the analytes, subsequently. Thus, through this analysis, the 3,4-bis(2-fluoro-5-(trifluoro-methyl phenyl)-2,5-diphenyl phenyl) grafted polysiloxane (FFMP) stationary phase was successfully synthesized and coated on fused silica capillaries to prepare capillary gas chromatographic columns. The polarizability for 3,4-bis(2-fluoro-5-trifluoromethyl phenyl)-2,5-diphenyl phenyl groups of the FFMP stationary phase was 50.13 Å3 (calculated according to Ref. [28]), which was considerably higher than that of benzene (10.40 Å3), biphenyl (20.05 Å3), and tetraphenyl phenyl (43.35 Å3). Therefore, a FFMP stationary phase containing a 3,4-bis(2-fluoro-5-trifluoromethyl phenyl)-2,5-diphenyl phenyl side chain with a high polarizability was expected to exhibit improved chromatographic selectivity. In addition, the chromatographic properties were evaluated in terms of column efficiency, polarity, and temperature resistance. The FFMP columns were used for the separation and analysis of PAHs, FAEs, and isomeric mixtures.
Experiment
Reagents and Apparatus
The reagents and chemicals used in this experiment, except for 2-fluoro-5-trifluorometh-ylbenzaldehyde. 2-fluoro-5-trifluoromethylbenzaldehyde, were of industrial grade and were purchased from Nanjing Chemical Reagent Co., Ltd. (Nanjing, China). Grob test mixture and polyethylene pyrolysis were prepared following the methods described in Refs. [29, 30], respectively. Fused silica capillary tubes (0.25 mm i.d.) for the preparation of capillary columns were prepared in our laboratory [30]. The commercial DB-17 column (30 m × 0.25 mm i.d.; film thickness: 0.5 µm) was acquired from Agilent Technologies (Palo Alto, USA).
The separation and analysis of various mixtures were performed using a capillary gas chromatograph (GC-2014, Shimadzu Corporation, Kyoto, Japan), and nitrogen (N2, 99.99% purity) was used as the carrier gas. To characterize the FFMP stationary phase, we used a Water 1515 GPC (Waters Corporation, Massachusetts, USA), a DPX 300 spectrometer (Bruker Analytische Messtechnik, Karlsruhe, Germany), a LCT-2 TGA system (Beijing Optical Instrument Factory, Beijing, China) and a MS50 mass spectrometer (British Karasto company, London, UK) to obtain the molecular weight of polymers, 1H nuclear magnetic resonance (NMR), thermogravimetric analysis (TGA) spectra and mass spectrometry, respectively.
Polymer Synthesis
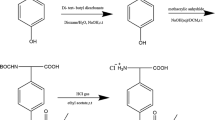
In this study, 1,2-bis(2-fluoro-5-(trifluoromethyl phenyl)-2-hydroxyethanone) (BFH) and 1,2-bis(2-fluoro-5-trifluoromethyl phenyl)ethane-1,2-dione (BFD) were synthesized following the method described in Ref. [31]. through the reaction principle of benzoin condensation. The FFMP stationary phase was synthesized through the Diels–Alder reaction principle. The synthesis route is shown in Fig. 1.
Synthesis of BFH
BFH was synthesized by condensing 2-fluoro-5-trifluoromethylbenzaldehyde (33.0 g, 172.0 mmol) with water (10.0 mL), thiamine (VB1, 4.0 g, 13.2 mmol), and 95% ethanol (30.0 mL) in an ice-water bath. Under stirring condition, sodium hydroxide ethanol solution (3.3 mol/L) was added dropwise to adjust the pH to 9.0–10.0. After 30 min, the reaction was conducted at 65 °C for 2 h. The reaction mixture was poured into ice-water for stratification. The lower oil phase was washed repeatedly with water until the supernatant was neutral to obtain an orange oily liquid, i.e., 25.0 g of 1,2-bis(2-fluoro-5-trifluoromethylphenyl)-2-hydroxyethanone in 75.8% yield.
Synthesis of BFD
BFD was synthesized by oxidizing BFH (20.0 g, 52.0 mmol) in the presence of copper sulfate (2 g, 12.6 mmol), ammonium nitrate (10.0 g, 60 mmol), water (4 mL), and acetic acid (50 mL). The reaction solution was heated to reflux for 2 h. After the reaction system was cooled, the reaction mixture was filtered, and the filter cake was washed thoroughly with water until the filtrate was neutral. The filter was subsequently recrystallized with ethanol to obtain 13.7 g of BFD as pale yellow crystals in 69.0% yield (m.p.: 82–84 °C).
Synthesis of 3,4-Bis(2-Fluoro-5-(Trifluoromethyl Phenyl)-2,5-Diphenylcyclopentadienone) (BFCD)
BFD (10.4 g, 30.0 mmol) was dissolved in 40.0 mL of ethanol with 1,3-diphenyl acetone (6.9 g, 33.0 mmol) under stirring condition, and the system was heated to reflux. Subsequently, a 4.0 mL solution of potassium hydroxide in ethanol (4.5 mol/L) was added dropwise to the mixture to obtain aubergine crystals, which were heated up to near boiling point for 1 h. After the reaction was completed, the mixture was cooled to room temperature. Approximately 13.2 g of aubergine crystals of BFCD was obtained by filtration and recrystallization with ethanol, with a yield of 78.9% (m.p.: 161–163 °C). 1H NMR (CDCl3, 300 MHz, δppm): 7.54–7.55 (d, 6H), 7.26–7.28 (m, 4H), and 7.03–7.07 (d, 4H); ESI–MS: m/z: 557.1123 [M + H]+, 574.1394 [M + Na]+, 579.0942 [M + NH4]+.
Synthesis of FFMP Stationary Phase
In this procedure, methylvinylpolysiloxane (vinyl chain content was 19.4%) was synthesized as previously described in Ref. [32]. The molecular weight was determined to be 65,600 by gel permeation chromatography (GPC). Exactly 4.3 g of methylvinylpolysiloxane (vinyl amount was 10.9 mmol) mixed with BFCD (6.7 g, 12.0 mmol) was dissolved in 50.0 mL of diphenyl ether, protected by nitrogen, and reacted at 220 °C for 48 h. The reaction solution turned from dark-brown to yellow-brown and formed a large number of bubbles. The reaction was terminated when the reaction mixture stopped bubbling, and diphenyl ether was removed through vacuum distillation to obtain a highly glutinous polymer. To purify, the crude polymer was dissolved in 20.0 mL of toluene, and the polymer was precipitated by adding 60 mL of methanol. This step was repeated for four times to remove low-molecular-weight polymers and unreacted BFCD. Subsequently, the solvent was removed through distillation under reduced pressure to produce 3.9 g of yellow gum with a yield of 50.2%. The molecular weight was determined to be 85,800 by GPC. The result of 1H NMR (CDCl3, 300 MHz, δppm) is shown in Fig. S1 (in supplemental): 7.28 (d, 2H), 7.18 (s, 1H), 7.02 (m, 4H), 7.00 (dd, 2H), 6.77 (d, 2H), 6.92 (m, 4H), and 6.69 (m, 2H). The peaks at 5.96 and 0.098 ppm corresponded to the chemical shifts of si-vinyl and Si-CH3 in the skeleton of polysiloxane.
Preparation of FFMP Columns
The fused silica capillary tubes (10 or 30 m in length were prepared in three replicates, i.d. 0.25 mm) were rinsed with 5.0 mL of dichloromethane and held at 250 °C for 2 h under nitrogen [33]. The columns were coated with 0.8% solution (v/v) of FFMP in dichloromethane. DCUP (the mass of DCUP accounted for 4% of the FFMP stationary phase) was added as a free radical initiator through the static method with 0.5 µm film thickness (df = dc/400, d is the capillary inner diameter (µm), and c is the volume percent concentration of FFMP stationary phase solution). After coating, the columns were maintained at 40 °C for 0.5 h, conditioned at a rate of 1 °C/min to 160 °C, maintained for 2 h to promote crosslinking, increased to 360 °C at the same rate, and then maintained for 12 h under nitrogen.
Results and Discussion
Chemical Composition of FFMP Polymer
The 1H NMR spectrum of the FFMP stationary phase in CDCl3 was recorded on a DPX 300 spectrometer. According to the 1H NMR spectrum data, the content of the 3,4-bis(2-fluoro-5-trifluoromethylphenyl)-2,5-diphenyl group in the FFMP was 16.2%, and the content of the residual vinyl group was 2.2% [30], which were obtained from the specific values of the peaks at 0.098 (attributed to Si-CH3), 5.85–6.12 (attributed to Si-CH = CH2), and 6.69–7.28 ppm (attributed to Ar-H).
Characteristics of the FFMP Column
The efficiency of the FFMP column was tested by naphthalene at a constant temperature of 120 °C. The result was expressed as the average number of plates, which was 3700 plates/m (3556–3827, k = 2.371 ± 0.10). The value of k slightly changed (k = 2.359 ± 0.10) after injection for 100 times, indicating that the film of the FFMP column was stable, and therefore, had potential to be suitable for long-term use. The coating efficiency of the FFMP column was 80.7% [25], implying that the FFMP stationary phase had an excellent film-forming capability and glorious cross-linking properties.
Thermal Stability of FFMP Stationary Phase
Under helium atmosphere, the temperature increased from 40 to 700 °C at 10 °C/min. The TGA curve of the FFMP is shown in Fig. S2 (in supplemental). The results indicated that the FFMP displayed a 1.7% weight loss when the temperature was increased to 360 °C and a 3.1% weight loss turned up at 380 °C, suggesting that the polyphenyl groups grafted on the polysiloxane chain could not only enhance the steric hindrance but also effectively reduce the macromolecular chain spiraling trend, thereby preventing the cyclization, rearrangement, and degradation of polysiloxanes under high temperatures [34].
Pyrolysis ethylene mixtures were separated by chromatographic columns prepared by the FFMP stationary phase, further demonstrating the thermal stability of the FFMP stationary phase for gas chromatographic analysis. As shown in Fig. S3 (in supplemental), the pyrolysis ethylene mixtures were separated on the FFMP column with symmetrical and sharp peaks, indicating that the FFMP column had a good separation performance for non-polar substances, such as aliphatic hydrocarbons. In addition, the baseline slightly drifted at 340 °C, and the column still exhibited a good separation performance when the temperature was increased to 360 °C. Thus, the FFMP column had an excellent thermal stability.
Separation Performance of FFMP Column
In this paper, the chromatographic performance of FFMP columns was characterized by the mixtures of Grob, aromatic hydrocarbons (MAHs), PAHs, aromatic isomers, FAEs, fatty alcohols and ethers, and the relevant chromatographic parameters were as follows: column length: 30 m, column diameter: 0.25 mm, film thickness: 0.5 µm, in addition, the chromatographic conditions for different mixtures are shown in Table 1.
Separation Performance for Grob Test Mixtures
The performance of the FFMP column was characterized by a Grob test mixture containing 12 components with different properties. As shown in Fig. 2, all chromatographic peaks were subjected to baseline separation because of the excellent coating performance of the FFMP stationary phase. All of their peaks were sharp and symmetrical, except for that of 2-ethylhexanoic acid (peak S), which showed a frontal peak because of overloading [30]. The octanol (ol) and 2,3-butanediol (D) peaks were significantly tailing (the tailing factor of octanol was 1.16), indicating the presence of weak reversible adsorption due to hydrogen bonding. In addition, the peak area ratio of 2,6-dimethylphenol (P) and 2,6-dimethylaniline (A) was 1.1:1. Moreover, the peak area of dicyclohexylamine (am) significantly reduced, indicating the presence of alkaline adsorption. Therefore, the FFMP column was useful for analyzing acidic substances.
McReynolds constants and Abraham system constants of FFMP column
In this paper, the polarity of the FFMP stationary phase was characterized by determining the McReynolds constants. As shown in Table 2, the five probe components were eluted in the order of Y′ (n-butanol) → X′ (benzene) →Z′ (2-amyl) → U′ (1-nitropropane) → S′ (pyridine). Compared with the OV-11 stationary phase (35% diphenyl-65% dimethylpolysiloxane) [35], the FFMP stationary phase had a slightly larger value of X′ possibly because of the conjugation system formed by the delocalized π-bond of multiple phenyl groups in the FFMP stationary phase, indicating that the FFMP stationary phase exerted a dipole-induced dipole effect, i.e., the dispersion force between the stationary phase and the solutes. The larger Z′ and S′ values indicated that the stationary phase had a strong interaction with solutes because the FFMP stationary phase was readily accessible to the protons of the analytes. Hydrogen bonds were generated between the stationary phase and the analytes, demonstrating that the FFMP stationary phase had a strong acting force and retention capacity for aromatic substances. In general, the total polarity (sum of ∆I) of the FFMP stationary phase was 779, which was comparable with the polarity of OV-11.
The Abraham system model is a reliable tool for characterizing diverse molecular interactions between probe compounds and stationary phases [34]. Table 2 lists the Abraham system constants of the FFMP, FPP (3,4-bis(4-fluorophenyl)-2,5-diphenyl phenyl grafted polysiloxanes, grafting amount was 11.9%.) [26], and DB-35 (35% diphenyl-65% dimethylpolysiloxane) [36] station phases under three temperatures (80, 100, and 120 °C). The solvation parameters e, s, a, b, and l represented the π–π and n–π electron interactions, dipole–dipole and induced dipole, alkaline hydrogen bonding, acidic hydrogen bonding, hole formation, and dispersion between the probe compounds and the stationary phase. As shown in Table 3, the dipole–dipole and dipole-induced dipole(s) were the primary forces between the FFMP stationary phases and the probe components, followed by dispersion (l) and alkaline hydrogen bonding (a)[37]. Compared with the FPP column, the FFMP column had obvious advantages in s, l, and a. These advantages may be due to the introduction of trifluoromethyl groups in polyphenyl. The selectivity of the FFMP stationary phase was enhanced. In addition, the solvation parameters of the FFMP column was close or even significantly better to those of DB-35 at the corresponding temperature (except for a), indicating that the polyphenyl groups played a major role in the forces of the stationary phase and the analytes.
Selectivity for Aromatic Substances
Selectivity for MAHs
Given that the polyphenyl side chains of the FFMP stationary phase could form a delocalized π-bond, the FFMP column was presumed to have a specific separation selectivity for aromatic substances. For the separation of MAH mixtures containing 19 types of monocyclic aromatic hydrocarbon substances, the results in Fig. 3a indicated that benzene substitutes, including trisubstituted benzene (1,2,4-trimethyl benzene, b.p. 168.0 °C), disubstituted benzene (1,3-dichlorobenzene, b.p. 172.0–173.0 °C), and aromatic isomers (o-nitrotoluene, b.p. 225.0 °C and p-nitrotoluene, b.p. 238.0 °C) achieved baseline separation within 40 min on FFMP column, and they possessed sharp and symmetrical peaks. This result indicates that a significant dipole-induced dipole and π–π force existed between the FFMP stationary phase and the analytes. While the separation of DB-17 (Fig. 3b) to MAHs showed that 3 pairs of MAHs did not realize baseline separation [o-nitroethylbenzene (peak 8, b.p. 174.0 °C) and n-butylbenzene (peak 9, b.p. 183.0 °C), 1,4-dibromobenzene (peak 11, b.p. 219.0 °C) and o-nitrotoluene (peak 12, b.p. 225.0 °C), p-nitrobromobenzene (peak 15, b.p. 256.0 °C) and 2,5-dichloronitrobenzene (peak 16, b.p. 267.0 °C)]. Therefore, the FFMP stationary phase was more suitable for the separation and analysis of MAHs.
Chromatograms of MAHs on FFMP column (a) and DB-17 column (b). Peaks: (1) methylbenzene; (2) chlorobenzene; (3) ethylbenzene; (4) m-dimethylbenzene; (5) p-chlorotoluene; (6) 1,2,4-trimethyl benzene; (7) 1,3-dichlorobenzene; (8) n-butylbenzene; (9) nitrobenzene; (10) 1,4-dibromobenzene; 11. o-nitrotoluene; 12. o-nitroethylbenzene; 13. p-nitrotoluene; 14. p-nitrochlorobenzene; 15. p-nitrobromobenzene; 16. 1,4-dichloro-2-nitrobenzene; 17. 3,5-dimethylnitrobenzene; 18. 2,4-dinitrotoluene; 19. 2,4-dinitrochlorobenzene
Selectivity for PAHs
PAHs are environmental pollutants that have raised considerable concern and have higher requirements for the gas chromatographic stationary phase because of their great difficulty in separation [38]. Figure 4a shows the separation of 22 types of PAHs on the FFMP column. Each component contained two–four benzene rings. All substances were baseline separation with perfect peak shapes. In particular, several substances with similar boiling and polarity, such as dibenzyl ether (peak 11, b.p. 298.0 °C) and fluorene (peak 12, b.p. 298.0 °C); phenanthrene (peak 15, b.p. 340.0 °C) and 4-nitrobiphenyl (peak 16, b.p. 340.0 °C). This result was due to the introduction of fluorine atoms and trifluoromethyl groups, which could increase the average molecular polarization of polyphenyl groups, thereby enhancing the conjugation, dispersion interactions, and dipole-induced dipole effects between the polar analytes and the FFMP stationary phase. But for DB-17 column (Fig. 4b), 2 pairs of PAHs [8-hydroxyquinoline (peak 8, b.p. 267.0 °C) and acenaphthylene (peak 9, b.p. 265.0 °C), phenanthrene (peak 15, b.p. 340.0 °C), and 4-nitro biphenyl (peak 16, b.p. 340.0 °C)] did not achieve baseline separation due to relatively close boiling point, and when the temperature rised to 320.0 °C, the baseline drifted obviously. Thus, the selectivity of the FFMP column to polar substances, such as PAHs, was ultimately improved.
Separation of PAHs on FFMP column (a) and DB-17 column (b). Peaks: (1) decahydronaphthalene; (2) tetralin; (3) naphthalene; (4) 2-methylnaphthalene; (5) 1-methylnaphthalene; (6) biphenyl; (7) diphenylmethane (8) 8-hydroxyquinoline; (9) acenaphthylene; (10) naphthyl ethyl ether; 11. dibenzyl ether; 12. fluorene; 13. 1,4-naphthoquinone; 14. α-nitronaphthalene; 15. phenanthrene; 16. 4-nitrobiphenyl; 17. benzil; 18. 9,10-phenanthrenequinone; 19. fluoranthene; 20. pyrene; 21. 1,2-benzanthracene; and 22. chrysene
Selectivity for Aromatic Isomers
Table 4 enumerated the separation factors (α) and capacity factors (k) of xylene, cresol, nitrotoluene, nitroaniline, nitrobenzaldehyde, and cyanobenzaldehyde aromatic isomers. As shown, six aromatic isomers were separated by the baseline. Among them, xylene isomers acted as weak polar aromatic isomers. Even though the boiling point of o-xylene (b.p. 139.1 °C) was close to that of p-xylene (b.p. 138.4 °C), it still exhibited a good separation effect on the FFMP column, indicating that the introduction of polar fluorine atoms and trifluoromethyl groups on the polyphenyl groups of the FFMP stationary phase could enhance the dipole-induced dipole interaction and dispersion forces between the stationary phase and the analytes. Consequently, the FFMP column became more sensitive to polar aromatic isomers or easily polarized aromatic isomers.
Selectivity for FAEs
In this section, 19 species of saturated fatty acid esters and partially unsaturated fatty acid esters were separated on the FFMP column. The results shown in Fig. 5a revealed that fatty acid esters were subjected to baseline separation and that the shapes of the peaks were satisfactory, sharp, and symmetrical. All saturated fatty acid esters were eluted based on the elevated boiling point, and unsaturated fatty acid esters (methyl methacrylate, peak 4, b.p. 100.0 °C) were usually eluted behind saturated fatty acid esters (butyl acetate, peak 3, b.p. 124.0 °C). The possible reason was the existence of forces between the C=C double bond in unsaturated fatty acid esters and the polyphenyl groups in the FFMP station phase, such as the dipole-induced dipole effect, which could improve the separation selectivity of the FFMP column. At the same time, we separated the same FAE mixtures on the DB-17 column, and the chromatogram is shown in Fig. 5b. Although most substances were separated by the baseline, peaks 14 (2-(2-ethoxyethoxy) ethylacetate, b.p. 218.0 °C) and 15 (ethyl cyanoacetate, b.p. 210.0 °C) appeared as co-elution peaks; and peaks 12 (ethyl valerate, b.p. 144.0 °C) and 13 (ethyl caproate, b.p. 145.0 °C) did not achieve baseline separation. Thus, the FFMP column was more suitable for the separation and analysis of FAEs.
Chromatograms of the separation of FAEs on FFMP column (a) and DB-17column (b). Peaks: (1) methyl acetate; (2) ethyl acetate; (3) butyl acetate; (4) methyl methacrylate; (5) isobutyl acetate; (6) methyl 3,3-dimethylacrylate; (7) ethyl butyrate; (8) butyl acetate; (9) amyl acetate; (10) isoamyl acetate; 11. allyl methacrylate; 12. ethyl valerate; 13. ethyl caproate; 14. 2-(2-ethoxyethoxy) ethylacetate; 15. ethyl cyanoacetate; 16. benzyl acetate 17. methyl decanoate; 18. methyl laurate; and 19. ethyl undecylenate
Selectivity for Fatty Alcohols
As polar compounds, fatty alcohols may inevitably exhibit peak tailing in the course of their separation. However, they can well reflect the separation ability of the station phase relative to polar materials [39]. The separation of fatty alcohols on the FFMP column is shown in Fig. 6a. All of the fatty alcohol substances underwent baseline separation, except for peaks 19 (1-heptanol, b.p. 176.5 °C), 20 (2-octanol, b.p. 178.0–179.0 °C), 34 (stearyl alcohol, b.p. 210.0 °C), and 35 (oleyl alcohol, b.p. 207.0 °C). The peaks were satisfactory with sharp and symmetrical shapes. These results indicated that the FFMP stationary phase and the hydroxyl groups of the fatty alcohols could form an appropriate hydrogen bond force to promote its separation selectivity for alcoholic substances. In addition, we separated the same fatty alcohol mixtures on the DB-17 column. The results in Fig. 6b illustrated that peaks 4 (t-butyl alcohol, b.p. 83.0 °C), 5 (1-propanol, b.p. 97.0 °C), 7 (allyl alcohol, b.p. 96.0–98.0 °C), 8 (n-butyl alcohol, b.p. 98.0 °C), 9 (2-methyl-2-butanol, b.p. 102.0 °C), 34, and 35 outflowed together. Peaks 15 (1-pentanol, b.p. 138.0 °C) and 14 (2-methyl-1-pentanol, b.p. 148.0 °C) did not achieve baseline separation, suggesting that its ability to separate aliphatic alcohols was considerably worse than that of the FFMP stationary phase. Therefore, the FFMP column has great potential application for the separation of aliphatic alcohols.
Chromatograms of the separation of fatty alcohols on FFMP column (a) and DB-17 column (b). Peaks: (1) methyl alcohol; (2) ethyl alcohol; (3) 2-propanol; (4) t-butyl alcohol; (5) 1-propanol; (6) trichloromethane; (7) allyl alcohol; (8) 2-butyl alcohol; (9) 2-methyl-2-butanol; (10) 2-methyl-1-propanol; 11. butyl alcohol; 12. 2-amyl alcohol; 13. 3-methyl-1-butanol; 14. 1-pentanol; 15. 2-methyl-1-pentanol; 16. 1-hexanol; 17. 4-hydroxy-4-methyl-2-penta–none; 18. 3-methyl-2-buten-1-ol; 19. 1-heptanol; 20. 2-octanol; 21. 2-ethylhexanol; 22. 1-octanol; 23. menthol; 24. 1-nonanol; 25. diethylene glycol monoethyl ether; 26. 1-decanol; 27. 1-undecanol; 28. 1-dodecanethiol; 29. lauryl alcohol; 30. myristyl alcohol; 31. 1-pentadecanol; 32. heptadecanol; 33. 1-hexadecanol; 34. stearyl alcohol; 35. oleyl alcohol; and 36. 1-icosanol
Selectivity for Ethers
Given that lone pair electrons were present in the oxygen atoms of ethers, a p–π conjugation with a large π-bond formed by polyphenylene occurred in the FFMP stationary phase, which enhanced the selectivity of the FFMP stationary phase to ether species. As shown in Fig. 7a, the FFMP column (Fig. 7a) had a good separation advantage for ether species. Anisole (Peak 15, 153.8 °C) had a longer retention time than bis(2-methoxyethyl) ether (peak 14, 162.0 °C), indicating that the FFMP stationary phase had a stronger π-π stacking effect with ethers containing benzene rings. However, for the DB-17 commodity column, the results in Fig. 7b displayed that multiple sets of similar boiling ether mixtures did not achieve baseline separation on the DB-17 commodity column. For instance, peaks 1 (diethyl ether, b.p. 34.6 °C), 2 (ethyl vinyl ether, b.p. 33.0 °C), 7 (ethyl propenyl ether, b.p. 67.0–76.0 °C), 8 (1,2-dimethoxyethane, b.p. 85.0 °C), 19 (ethyl propenyl ether, b.p. 187.8 °C), 20 (2-ethoxyethyl ether, b.p. 180.0–190.0 °C), 24 (4-methoxyphenol, b.p. 243.0 °C), and 25 (diphenyl ether, b.p. 259.0 °C) did not undergo baseline separation. Thus, the FFMP column was more suitable than the DB-17 commodity column for separating ethers.
Chromatogram of ethers on FFMP column (a) and DB-17 column (b). Peaks: (1) diethyl ether; (2) ethyl vinyl ether; (3) tetrahydrofuran; (4) isopropyl ether; (5) 2,3-dihydrofuran; (6) 2-methyl-tetrahydrofuran; (7) ethyl propenyl ether; (8) 1,2-dimethoxyethane; (9) allyl ether; (10) 1,4-dioxane; 11. ethylene glyc-ol diethyl ether; 12. ethylene glycol monomethyl ether; 13. dibutyl ether; 14. bis(2-methoxyethyl)ether; 15. anisole; 16. ethylene glycol monobutyl ether; 17. isopentyl ether; 18. dibutyl sulfide; 19. dichloroisopropyl ether; 20. 2-eth-oxyethyl ether; 21. 2,2′ -oxybis-ethanomonoethylether; 22. 2-(2-ethoxyethoxy)ethyl acetate; 23. di(ethyleneglycol)benzyl ether; 24. 4-methoxyphenol; 25. diphenyl ether; 26. 2-nitroanisole; 27.ethyl naphthyl ether; 28. benzyl ether
Conclusion
In this work, we synthesized the FFMP stationary phase by introducing fluorine atoms and trifluoromethyl groups in polyphenylene, which was grafted as a lateral chain onto polysiloxane, and coated on the surface of fused silica tubes to prepare gas chromatography columns with good film-forming property, high column efficiency, and excellent thermal stability. In addition, the Abraham system constants indicated that dipole-induced dipole, dispersion force, and hydrogen bonding were the major forces between the FFMP stationary phase and the analytes. As a medium polar stationary phase, the FFMP columns could be used for separating mixtures of MAHs, PAHs, aromatic isomers, FAEs, fatty alcohols, and ethers. Therefore, the development prospect of this type of stationary phase is relatively broad and applicable to the actual analysis of environmental pollutants, such as PAHs.
References
Ruo-Nong FU (2006) Evolution of gas chromatographic stationary phases. Chem Reag 28:11–15
Chen B, Liang C, Yang J, Contreras DS (2006) A microporous metal-organic framework for gas-chromatographic separation of alkanes. J Angew Chem Int Ed 118:1418–1421
Gu ZY, Yan XP (2010) Metal–organic framework MIL-101 for high resolution gas chromatographic separation of xylene isomers and ethylbenzene. J Angew Chem Int Ed 49:1477–1480
Chang N, Gu ZY, Yan XP (2010) Zeolitic imidazolate framework-8 nanocrystal coated capillary for molecular sieving of branched alkanes from linear alkanes along with high-resolution chromatographic separation of linear alkanes. J Am Chem Soc 132:13645–13647
Chang N, Yan XP (2012) Exploring reverse shape selectivity and molecular sieving effect of metal-organic framework UIO-66 coated capillary column for gas chromatographic separation. J Chromatogr A 1257:116–124
Xie SM, Zhang ZJ, Wang ZY, Yuan LM (2011) Chiral metal-organic frameworks for high resolution gas chromatographic separations. J Am Chem Soc 133:11892–11895
Gu ZY, Jiang JQ, Yan XP (2011) Fabrication of isoreticular metal–organic framework coated capillary columns for high-resolution gas chromatographic separation of persistent organic pollutants. J Anal Chem 83:5093–5100
Saridara C, Mitra S (2005) Chromatography on self-assembled carbon nanotubes. J Anal Chem 77:7094–7097
Karwa M, Mitra S (2006) Gas chromatography on self-assembled, single-walled carbon nanotubes. J Anal Chem 78:2064–2070
Gopalan AI, Lee KP, Ragupathy D (2009) Development of a stable cholesterol biosensor based on multi-walled carbon nanotubes-gold nanoparticles composite covered with a layer of chitosan-room-temperature ionic liquid network. J Biosens Bioelectron 24:2211–2217
Wasserscheid P, Bosmann A, Bolm C (2002) Synthesis and properties of ionic liquids derived from the chiral pool. J Chem Commun 25:200–201
Xiaoqi L, Yangliao Hu, Yaqing Fu (2012) Synthesis and characterization of Lu (111) ion imprinted polymer. J Inorg Organomet Polym 22:112–118
Kuei JC, Shelton JI, Castle LW (2015) Polarizable biphenyl polysiloxane stationary phase for capillary column gas chromatography. J Sep Sci 7:13–18
Haken JK (1977) Polysiloxane stationary phases in gas chromatography. J Chromatogr A 141:247–288
Ahnoff M, Johansson L (1984) Preparation and evaluation of capillary columns with phases with 5–33% phenyl substitution. J Chromatogr 19:151–154
Stark TJ, Larson PA, Dandeneau RD (1983) Selective phases for wall-coated open tubular columns. J Chromatogr A 279:31–40
Peaden PA, Wright BW, Lee ML (1982) The preparation of non-extractable methylphenylpolysiloxane stationary phases for capillary column gas chromatography. J Chromatogr 15:335–340
Bradshaw JS, Crowley SJ, Harper CW (2015) Autocrosslinkable methyl-2-phenylethylpoly-siloxane stationary phase for capillary column gas chromatography. J Sep Sci 7:89–92
Bradshaw JS, Adams NW, Johnson RS (2015) Preparation of polysiloxane stationary phases for capillary column chromatography: a new methoxyphenyl phase. J Sep Sci 8:678–683
Mayer BX, Kählig H, Rauter W (2003) A 50% n-octylmethyl, 50% diphenyl-polysiloxane as stationary phase with unique selectivity for gas chromatography. J Analyst 128:1238
Mayer BX, Rauter W, Kählig H (2015) A trifluoropropyl-containing silphenylene-siloxane terpolymer for high temperature gas chromatography. J Sep Sci 26:1436–1442
Santos FJ, Galceran MT (2002) The application of gas chromatography to environmental analysis. J Trends Anal Chem 21:672–685
Zuazagoitia D, Millán E, Garciaarrona R (2007) A screening method for polycyclic aromatic hydrocarbons determination in sediments by headspace SPME with GC-FID. J Chromatogr 66:773–777
Zhao P, Teng S, Yu M (2014) Synthesis and characterization of diphenyl-phenyl polysiloxane as a high-temperature gas chromatography stationary phase. J Anal Methods 7:1333–1338
Han X, He X, Wang H (2016) Fluoro-substituted tetraphenyl-phenyl grafted polysiloxanes as highly selective stationary phases for gas chromatography. J Chromatogr A 1449:118–128
Zhao PC, Liu L, Yu M, Niu N (2015) A 3,4-2 (trifluoromethyl phenyl)-2,5-diphenyl phenyl grafted polysiloxane as a stationary phase for gas chromatography. J Anal Methods 6278–6284
Miller KJ, Savchik J (1979) A new empirical method to calculate average molecular polarizabilities. J Am Chem Soc 101:7206–7213
Grob JK, Grob G, Grob K (1978) Comprehensive, standardized quality test for glass capillary columns. J Chromatogr A 156:1–20
Zhao PC, Niu YY, Yu M, Niu N, Wang H (2015) High temperature stationary phase of polysiloxane containing N, N′-bis (diphenylsilyl) tetramethylcyclodisilazane for gas chromatography. J RSC Adv 5:22399–22404
He X, Han X, Wang H (2016) Polysiloxanes-based stationary phases containing methoxy-substituted tetraphenyl–phenyl groups for gas chromotographic separations. J Rsc Adv 6:76514–76523
Friedman W, Gugig, Mehr L (1959) The synthesis of some alkoxy- and alkyl-substituted tetraphenylcyclopentadienones. J OrgChem 24:516–520
Feng SY, Zhang J, Li MJ, Zhu QZ (2004) Silicone polymers and their applications. Chemical Industry Press, Beijing, pp 79–80
Wang L, Wang X, Qi M et al (2014) Cucurbit [6]uril in combination with guanidinium ionic liquid as a new type of stationary phase for capillary gas chromatography. J Chromatogr A 1334:112–117
Thomas TH, Kendrick TC (2003) Thermal analysis of polysiloxanes. II. Thermal vacuum degradation of polysiloxanes with different substituents on silicon and in the main siloxane chain. J Polym Sci Part B Polym Phys 8:1823–1830
Grob K, Grob G (1981) Evaluation of capillary columns by separation number or plate number. J Chromatogr 207:291–297
Poole CF, Poole (2008) S K. Separation characteristics of wall-coated open-tubular columns for gas chromatography. J Chromatogr A 1184:254–280
Abraham MH, Poole CF, Poole SK (1999) Classification of stationary phases and other materials by gas chromatography. J Chromatogr 842:79–114
Zheng T, Luo Y, Cao X (2006) Advances in research of microbial degradation of high molecular weight polycyclic aromatic hydrocarbons-benzo(a)pyrene. J Appl Environ Biol 12:884–890
Wang XG, Qi ML, Fu RN (2014) Separation performance of cucurbit[7]uril in ionic liquid-based sol-gel coating as stationary phase for capillary gas chromatography. J Chromatogr A 1371:237–243
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 21275090).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, B., Liu, J., Li, X. et al. Synthesis and Applications of a Novel 3,4-Bis(2-Fluoro-5-Trifluoromethyl Phenyl)-2,5-Diphenyl Phenyl Grafted Polysiloxane Stationary Phase. Chromatographia 81, 1219–1229 (2018). https://doi.org/10.1007/s10337-018-3556-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3556-7