Abstract
A novel Lu(III) ion imprinted polymer (IIP) was synthesized by bulk polymerization using Lu(III) ion as a template, Lu(III)-4-vinylpyridine-acetylacetone complex as a functional monomer and ethylene glycol dimethacrylate as a crosslinker. The Lu(III) IIP was systematically characterized by adsorption test, UV–Vis spectrophotometry, FTIR spectroscopy, element analysis, X-ray diffraction, pore size analysis, SEM and TGA studies. The results showed that the maximum adsorption of Lu(III) IIP was 64.2 mg g−1 with the adsorption equilibrium time of 30 min and the optimum pH was 5.5. The synthesized Lu(III) IIP had a good selective recognition ability for Lu(III) ion compared with other ions and possessed a favorable heat stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Rare earth metals are of great importance with their widespread use in the manufacture industries such as metallurgy, machine, ceramics, catalysts, laser crystals, colour television tubes, capacitors and magnets. Therefore, the research for efficient separation methods for rare earth metals has attracted significant interest [1–5]. However, the effective separation of the different rare earth metals is still a challenge due to their close radius that lead to the similarities in physical and chemical properties. The liquid–liquid extraction method is reported to be an efficient method for the separating lanthanides. And solid phase extraction method has developed because of the lower consumption of reagents, higher enrichment factors and absence of emulsion. Among the absorbing materials of solid phase extraction, the imprinted materials synthesized by molecular imprinting technique are widely applied owing to their good selectivity and stability in different environments [6–12].
Molecular imprinting technique is a promising technique for the preparation of polymers which have specific recognition sites. Based on a synthetic organic polymer matrix, the imprints of the template molecule (i.e. metal ions, molecules) are created in the polymer. After the template is eluted from the rigid polymer network, recognition sites complementary to the template molecule in shape and size can be obtained. For metal ions, molecular imprinting can be also described more precisely as ion imprinted polymer (IIP). Some metal ion imprinted polymers have been used for metal ions adsorption, such as Cu(II) [13], Cd(II) [14, 15], Zn(II) [16] and rare earth ions imprinted polymers [17–24]. However, to the best of our knowledge, the research for Lu(III) IIP has not been reported.
Here we reported a novel method for the synthesis of Lu(III) IIP using Lu(III) ion as a template, Lu(III)–4-vinylpyridine –acetylacetone complex as a functional monomer and ethylene glycol dimethacrylate (EDMA) as a crosslinker. The characterization of Lu(III) IIP was systematically investigated by adsorption test, UV–Vis spectrophotometry, FTIR spectroscopy, element analysis, X-ray diffraction, pore size analysis, SEM and TGA studies. Moreover the selectivity of Lu(III) IIP was studied towards other ions.
2 Experimental
2.1 Instrument and Reagent
4-Vinylpyridine (VP), acetylacetone (Acac) and glycoldimethacrylate EDMA were obtained from Alfa (Tianjin, China). 2,2-azobisisobutyronitrile (AIBN) was purchased from Tianjin Chemical Reagent Company, China. All other chemicals were of analytical reagent grade.
The UV–Vis spectra were measured at room temperature with a Cintra10 UV–Vis spectrophotometer (GBC, Australia). The IR spectra were measured by a Avatar360 FTIR spectrophotometer (Nicolet, USA). Element analysis for the polymer was measured by Varioel element analysis instrument (Elementar, Germany). The X-ray diffraction patterns were obtained from Bruker D8 Advance (Bruker, Germany). The adsorption data were obtained from Asap 2020 M+C micro physical and chemical adsorption (Micromeritics, USA). Thermogravimetric analysis studies were carried out using a TGA-50H (Shimadzu, Japan). HITACHI S-4800 scanning electronic microscopy (Hitachi Co. Ltd., Tokyo, Japan) was used for morphological structure characterization of the polymers.
2.2 Preparation of Lu(III) Ion Imprinted Polymer (IIP)
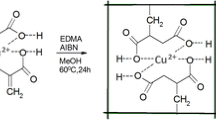
LuCl3·6H2O (1 mmol), acetylacetone (3 mmol), 4-vinylpyridine (4 mmol) and EDMA (16 mmol) and AIBN (0.24 mmol) were dissolved in 15 mL methanol. After fully dispersing and deoxygenating with sonication and nitrogen gas, the mixture was heated at 60 °C with water bath for 24 h. The resulting polymers were crushed, ground and sieved with a 0.2 mm sieve. Subsequently the synthesized polymers were treated with EDTA (0.1 mol L−1) for 2 h to remove the Lu(III) ion. The final Lu(III) IIP was dried in vacuum at 60 °C.
2.3 Preparation of Non-Lu(III) Ion Imprinted Polymer (N-Lu(III) IIP)
N-Lu(III) IIP was similarly prepared according to Lu(III) IIP in absence of Lu(III) ion.
2.4 Absorption Test
An amount of 50 mg of Lu(III) IIP was interacted with 10.0 mL different ion concentrations with various interaction times. Then the solution was centrifuged and filtrated to detect the ion concentrations left in solution with a UV–Vis spectrophotometer. The quantity of adsorption (Q) can be calculated based on the equation Q = (C 0 − C)V/w, in which Q is the quantity of adsorption (mg g−1), C 0 is the Lu(III) ion concentration of the solution before absorption (mg L−1), C is the Lu(III) ion concentration of the solution after absorption (mg L−1), w is the weight of the adsorbent (g), and V is the volume of the solution (L).
3 Results and Discussion
3.1 Absorption Test
3.1.1 Effect of Adsorption Time
Adsorption time of Lu(III) ion in Lu(III) IIP was investigated. Figure 1 shows the effect of time on the adsorption quantity of Lu(III) ion in the Lu(III) IIP. As can be seen from Fig. 1, Lu(III) ion adsorption quantity increases during 25 min, and then reaches an adsorption equilibrium at 30 min. This fast adsorption equilibrium is probably due to specific recognition sites for Lu(III) ion.
3.1.2 Effect of pH
The effect of pH on the a Lu(III) ion absorption quantity was investigated. Variation of pH from 3.0 to 8.0 was studied. As shown in Fig. 2, at pH 5.5, Lu(III) ion adsorption quantity reached its maximum from Fig. 2. The amino group of acetylacetone could be protonated at pH < 5, so Lu(III) ion adsorption quantity declined. However, at pH > 7, the Lu(III) ion could be hydrolyzed, and thus the Lu(III) ion adsorption quantity was decreased. Therefore, the optimum pH is 5.5.
3.1.3 Absorption Equilibrium and Adsorption Quantity
Equilibrium absorption isotherm is usually described by Langmuir model [25]:
In the Eq. 1, C eq is Lu(III) ion concentration at equilibrium, Q eq and Q max refer to metal absorption quantity at equilibrium and maximum metal absorption quantity (mg g−1) respectively, and K is the Langmuir isotherm constant.
Relation between C eq and Q eq is shown in Fig. 3. According to the experimental data, the curve of C eq /Q eq and C eq (Fig. 4) has a good linear relationship, demonstrating that the adsorption of Lu(III) IIPs for Lu(III) ion can be well fitted to the Langmuir model. The isotherm equation is as follows:
The adsorption quantity at equilibrium is 69.4 mg g−1 calculated by the Eq. 2, which is close to the maximum adsorption quantity measured by the experiment (64.2 mg g−1). On the contrary, the adsorption quantity of N-Lu(III) IIP for Lu(III) ion is 1.2 mg g−1, indicating the successful synthesis of the Lu(III) IIP.
3.2 Selectivity of the Imprinted Polymers
The selectivity of Lu(III) IIP for Lu(III) ion over four common metal ions and six rare earth ions were investigated. The selectivity coefficient (SLu(III)/M) is defined as [17]:
In the Eq. 3, D Lu and D M are the distribution ratios of the polymer with Lu(III) ion and the other metal ions respectively. These distribution ratios were calculated using the following formula:
In the Eq. 4, C i and C f are the concentrations of the metal ion (mg L−1) before and after the extraction respectively, v is the volume of the solution, and m is the mass of the polymer.
According to Eqs. 3 and 4, Table 1 shows that the Lu(III) ion absorption quantity of the Lu(III) IIP was higher than that of four common metal ions and six rare earth ions, which indicates that Lu(III) IIP synthesized has a good selectivity to the Lu(III) ion.
3.3 Characterization Studies
3.3.1 UV Spectra
The ternary complex of Lu(III) ion with acetylacetone and 4-vinylpyridine was synthesized according to the experimental section of 2.2. The binary complex of Lu(III) ion with Acac was prepared under the similar condition in the absence of 4-vinylpyridine.
The UV–Vis spectra of the ternary and binary complex, acetylacetone and 4-vinylpyridine were measured in methanol. As shown in Fig. 5, curve 1 is the absorption spectrum of acetylacetone with a peak at 269 nm using methanol as a reference. Curve 2 is the absorption spectrum of 4-vinylpyridine with a peak at 242 nm. The peak of binary complex of Lu(III) ion with Acac was red-shifted to 294 nm (see curve 3). The ternary complex of Lu(III) ion with acetylacetone and 4-vinylpyridine resulted in enhancement in the absorbance of the system, indicating the formation of ternary complex of Lu(III)–4-Vinylpyridine–acetylacetone and 4-Vinylpyridine acts as both the ligand for the ternary complex and functional monomer during the polymerization.
3.3.2 IR Spectra
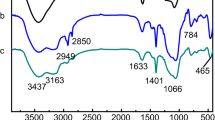
The IR spectra of N-Lu(III) IIP (curve 1), Lu(III) IIP (curve 2) and Lu(III) IIP without extraction (curve 3) are shown in Fig. 6.
The polymers have similar IR spectra because of the similar backbone. A weak bond near 1,600 cm−1 (νC=N) presented in Lu(III) IIP without extraction is shifted to a higher frequency of 1,636 cm−1 after extraction. The similar peak of 1,635 cm−1 is also can be seen in N-Lu(III) IIP. The shift towards lower frequency in Lu(III) IIP without extraction compared to Lu(III) IIP and N-Lu(III) IIP, and the decrease in strength of vibration in Lu(III) IIP without extraction compared to Lu(III) IIP could be attributed to the bonding between the Lu(III) ion and the nitrogen in the bond of C=N.
The bonds in the region of 3,700–3,500 cm−1 corresponds to the free –OH groups in acetylacetone, which appeared in N-Lu(III) IIP, Lu(III) IIP and Lu(III) IIP without extraction. The IR spectra of Lu(III) IIP without extraction and Lu(III) IIP have similar backbone, indicating that acetylacetone exists in the polymer after extraction.
3.3.3 Element Analysis
The CHN data of polymers were measured by a Varioel element analysis instrument. The results of Lu(III) IIP and Lu(III) IIP without extraction are as follows:
(i) Lu(III) IIP without extraction: C, 55.65%; H, 6.96%; N, 1.42%. (ii) Lu(III) IIP: C, 61.73%; H, 7.12%; N, 1.97%.
This element analysis is consistent with the IR spectra results, further confirming that acetylacetone is intact in Lu(III) IIP.
3.3.4 X-Ray Diffraction
Figure 7 shows the X-ray diffraction of the pure LuCl3 (curve 1) and Lu(III) IIP without extraction (curve 2) and Lu(III) IIP (curve 3) under the same condition. It is worth pointing out that the peaks of Lu(III) IIP compared to Lu(III) IIP without extraction have no peaks of pure LuCl3 which can be inferred that the method of extraction with EDTA (0.1 mol L−1) for 2 h could completely remove Lu(III) ion in the polymer.
3.4 Surface Area and Pore Size Analysis
Surface area and pore size of Lu(III) IIP were obtained from the instrument of Asap 2020 M+C micro physical and chemical adsorption. As shown in Table 2 and Fig. 8, Lu(III) IIP has a high pore specific surface area with the pore size of less than 80 Å.
3.5 Morphological Structure Characterization
The morphological structure characterization of the Lu(III) IIP and N-Lu(III) IIP were studied by SEM. As shown in Figs. 9 and 10, the morphological structure of N-Lu(III) IIP has about 1 μm particles with smooth surface, while the morphological structure of Lu(III) IIP has about 20 – 100 nm particles with small cavities, which can further explain that Lu(III) IIP has higher adsorption quantity than N-Lu(III) IIP.
3.6 Thermogravimetric Analysis (TGA)
The TGA curve of Lu(III) IIP in Fig. 11 showed that the polymer appeared to have a little weight loss between 24 and 300 °C due to the decomposition of acetylacetone. After 300 °C, Lu(III) IIP had a sharp weight loss because of the decomposition of the polymeric matrix. When the temperature was above 440 °C, the rate of weight loss of Lu-IIP reached 90%.
4 Conclusion
In this work, we have successfully synthesized Lu(III) IIP using Lu(III) ion as a template, Lu(III)–4-vinylpyridine–acetylacetone complex as a functional monomer and EDMA as a crosslinker. The characterization of the Lu(III) IIP was systematically studied by adsorption test, UV–Vis spectrophotometry, FTIR spectroscopy, element analysis, X-ray diffraction, pore size analysis, SEM and TGA. The results showed that the adsorption equilibrium time 30 min at the optimum pH 5.5, and the maximum adsorption of Lu(III) imprinted polymer was 64.2 mg g−1. The synthesized Lu(III) IIP has the properties of small cavity, large surface area, high adsorption capacity, fast adsorption rate, and favorable heat stability. The proposed method is simple and convenient, and has great potential application in the selective enrichment and separation of Lu(III) ion.
References
A. Ohashi, T. Hashimoto, H. Imura, K. Ohashi, Talanta 73, 893 (2007)
Z.F. Zhang, H.F. Li, F.Q. Guo, S.L. Meng, D.Q. Li, Sep. Purif. Technol. 63, 348 (2008)
Q. Jia, W.P. Liao, D.Q. Li, C.J. Niu, Anal. Chim. Acta 477, 251 (2003)
E.B. Marouna, H. Chebib, M.J. Leroy, A. Boos, G.J. Grandmont, Sep. Purif. Technol. 50, 220 (2006)
X.H. Luo, X.W. Huang, Z.W. Zhu, Z.Q. Long, Y. Liu, J. Rare Earth 27, 119 (2009)
X.D. Huang, H.F. Zou, X.M. Chen, Q.Z. Luo, L. Kong, J. Chromatogr. A 984, 273 (2003)
Q. Lei, X.W. He, W.Y. Li, Y.K. Zhang, J. Chromatogr. A 1187, 94 (2008)
M. Esteban, A. Fresenius, J. Anal. Chem. 370, 795 (2001)
F. Puoci, G. Cirillo, M. Curcio, F. Iemma, U.G. Spizzirri, N. Picci, Anal. Chim. Acta 593, 164 (2007)
D. Djozan, T. Baheri, J. Chromatogr. A 1166, 16 (2007)
J. Oxelbark, C.L. Quigley, S.A. Aureliano, J. Chromatogr. A 1160, 215 (2007)
F. Puoci, M. Curcio, G. Cirillo, F. Iemma, U.G. Spizzirri, N. Picci, Food Chem. 106, 836 (2008)
R. Say, E. Birlik, A. Ersoz, F. Yilmaz, T. Gedikbey, Anal. Chim. Acta 480, 251 (2003)
B. Gao, F. An, Y. Zhu, Polymer 48, 2288 (2007)
F. Li, H. Jiang, S. Zhang, Talanta 71, 1487 (2007)
K. Araki, T. Maruyama, N. Kamiya, M. Goto, J. Chromatogr. B 818, 141 (2005)
J.M. Lin, M. Yamada, Anal. Chem. 72, 148 (2000)
V.M. Biju, J.M. Gladis, T.P. Rao, Anal. Chim. Acta 478, 43 (2003)
N. Zhang, B. Hu, C.Z. Huang, Anal. Chim. Acta 597, 12 (2007)
R. Kala, V.M. Biju, T.P. Rao, Anal. Chim. Acta 549, 51 (2005)
P.G. Krishna, J.M. Gladis, T.P. Rao, G.R. Naidu, J. Mol. Recognit. 18, 109 (2005)
K. Araki, M. Yoshida, J. Chem. Eng. Jpn. 33, 665 (2000)
R. Kala, T.P. Rao, J. Sep. Sci. 29, 1281 (2006)
X.Q. Lai, Y.Q. Yang, J. Xue, Acta Chim. Sinica 67, 863 (2009)
T.Y. Guo, Y.Q. Xia, G.J. Hao, Biomaterials 25, 5905 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, X., Hu, Y., Fu, Y. et al. Synthesis and Characterization of Lu(III) Ion Imprinted Polymer. J Inorg Organomet Polym 22, 112–118 (2012). https://doi.org/10.1007/s10904-011-9570-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-011-9570-y