Abstract
In this paper, polythiophene/chitosan magnetic nanocomposite as a novel adsorbent is proposed for the preconcentration of triazines in aqueous samples prior to gas chromatography. The synthesized nanoparticles, magnetic chitosan and polythiophene–chitosan magnetic nanocomposite were characterized by scanning electron microscopy. The magnetic polythiophene–chitosan nanocomposite containing analytes could be removed from the sample solution by applying a permanent magnet. The major factors influencing the extraction efficiency including desorption conditions, nanocomposite components ratio, sorbent amount, extraction time, ionic strength and sample pH were optimized. The developed method proved to be rather convenient and offers sufficient sensitivity and good reproducibility. The limit of detection (S/N = 3) and limit of quantification (S/N = 10) of the method under optimized conditions were 10–30 and 100 ng L−1, respectively. Under the optimum conditions, good linearity was obtained within the range of 100–5000 ng L−1 for all triazines with correlation coefficients >0.9994. The relative standard deviation at a concentration level of 150 ng L−1 was 7–12 %. Furthermore, the method was successfully applied to the determination of triazines in real samples, where relative recovery percentages of 96–102 % were obtained. Compared with other methods, the current method is characterized by easy, fast separation and low detection limits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sample preparation is one of the vital steps in trace analysis methodologies. An improvement in sorbent-based extraction techniques has been performed during the last two decades, which has led to the development of some miniaturized extraction techniques. Synthesis of new sorbents is of great concern in the new decade and many groups around the world try to synthesize compounds with specific characteristics. Preparation of sorbents with special interactions or selectivity toward interested target analytes has also been taken into consideration in the field of analytical chemistry [1–3]. Common sorbent-based extraction methods for the extraction of triazine herbicides from water samples involve solid phase extraction (SPE) [4–6], solid phase microextraction (SPME) [7–9] and stir bar sorptive extraction (SBSE) [10, 11].
Although SPE is extensively applied, it is time consuming, tedious, and relatively expensive. In recent years, considerable efforts have been exerted to develop new techniques to overcome these drawbacks. Magnetic solid phase extraction (MSPE) is a new sample preparation method based on the use of magnetic adsorbents [12]. In MSPE technique, the adsorbent does not need to be packed into the SPE cartridge, therefore, the micro/nano-sorbents are capable of being exposed completely in the sample solution. Also, the magnetic sorbent could be subsequently removed from the sample solution by applying a permanent magnet. Since they can be easily recovered by a magnet, the particles are generally directly dispersed in sample solutions to achieve extraction. It not only enhances the extraction efficiency by increasing the contact between analytes and the sorbents, but also overcomes problems with conventional SPE, such as eliminating packing the columns and avoiding time-consuming process.
Also, magnetic nanoparticles can be collected and separated from liquid phase simply under a magnetic field, which avoids the tedious filtration or centrifugation procedure [13–15], and makes the particles easy to retrieve with low cost. These intriguing features of magnetic separation have led to its numerous applications in many research fields such as bio-separation [16–20]. It should be stressed that pure inorganic magnetic particles, such as Fe3O4, could be easily aggregated and are not suitable to be used for extracting organic compounds within the complex matrices. To overcome these problems, modification of the particles’ surface in different fashions is usually needed and has been proven to be quite efficient [21, 22]. Therefore, surface modification can not only improve their dispersibility but also provides an active surface to interact with certain molecules. To date, to impart surface reactivity of the magnetic nanoparticles, numerous types of natural or synthetic polymers, novel molecules, and inorganic materials have been coated on the surface of the magnetic nanoparticles. Among these adsorbents, alkyls [23–25], polymers [26–29], graphene [30–32], multiwalled carbon nanotubes [33], and surfactants [34–36] are commonly investigated. Accordingly, the modification of the magnetic material surfaces was employed to overcome these limitations. One group of the modifier compounds, which have been widely used, is conducting polymers (CPs) [37, 38]. Among the different CPs used as SPE sorbent, the great attentions have been focused on polythiophene (PTh), which is more stable than the other CPs in high temperature, air, moisture and various solvents [39–41]. Yamini and et al. [40, 41] used PTh-coated Fe3O4 as a MSPE sorbent. Fe3O4 nanoparticles have high density and low surface area that can affect their adsorptive properties [42]. To overcome these limitations and increase the prominent merits of the magnetic materials, in this work, chitosan can be used as a good support with the low aggregation properties during the growth of Fe3O4 nanoparticles. Therefore, chitosan can act not only as a “support” for the growth of Fe3O4 nanoparticles, but also as a “spacer” for the inhibition of nanoparticles aggregation.
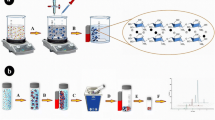
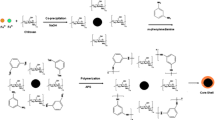
For the first time, we developed polythiophene/chitosan magnetic nanocomposite as the MSPE technique for the extraction and analysis of triazines in aqueous samples. First, magnetic nanoparticles and chitosan magnetic particles were synthesized through a facile co-precipitation, while several types of magnetic nanocomposites with different concentrations of thiophene (0.03–2 M) were coated on the surface of chitosan/Fe3O4 NPs using oxidized chemical polymerization. The capabilities of these sorbents for the extraction of triazines were examined. Extraction and desorption conditions were also optimized. To demonstrate the validation of the proposed method, the quantification limit, linearity and repeatability were investigated.
Experimental
Reagents
The selected triazines including of atrazine, ametryn and terbutryn were obtained from Fluka (Buchs, Switzerland). The stock standard solution of triazines (2000 mg L−1) was prepared with HPLC-grade methanol and stored and refrigerated at 4 °C. Then, the working standard solution was freshly prepared by diluting the mixed standard solution with distilled water to required concentrations. Other chemicals such as NaCl, FeCl3·6H2O, FeCl2·4H2O, HCl, NaOH and methanol, n-hexan, toluene, acetone, dichloromethane, acetonitrile and acetic acid were analytical grades and purchased from Merck (Darmstadt, Germany). The nitrogen gas with purity of 99.99 % was used for providing the inert atmosphere necessary for the synthesis of MNPs and their coating process. Also, ammonium peroxydisulfate (APS) was obtained from Merck (Darmstadt, Germany) and was used without further treatment while thiophene (Mississauga, Canada) was distilled under reduced pressure.
Preparation of Magnetic Fe3O4 Nanoparticles
Fe3O4 NPs were synthesized via alkaline precipitation of FeCl3 and FeCl2 [43]. Typically, in a closed stirred reactor with vigorous stirring at approximately 1000 rpm, 250 mL of a solution of 1.5 M sodium hydroxide in ultrapure water was purged with N2 gas for 30 min. After this period, a freshly prepared iron solution (5.2 g of FeCl3·6H2O and 2.0 g of FeCl2·4H2O and 0.85 mL of concentrated hydrochloric acid were dissolved in 25 mL of ultrapure water and purged by N2 to remove the oxygen) was added dropwise to the sodium hydroxide solution. The reaction proceeded during 60 min under a nitrogen atmosphere. After the reaction, the black product was separated from the reaction medium using a magnet and washed several times with ultrapure water and degassed with N2.
Preparation of Chitosan-Coated Magnetic Nanoparticles
The chitosan-magnetic nanoparticles (CS/MNPs) were prepared according to following process. First, FeCl3·6H2O (9.22 g) and FeCl2·4H2O (3.2 g) were dissolved in 100 mL deionized water under nitrogen gas with vigorous stirring at 45 °C. Then, 100 mL acetic acid aqueous solution (0.5 % v/v) containing 5 g L−1 CS was added to the solution under the nitrogen atmosphere. After being stirred for 30 min at 45 °C, then sodium hydroxide solution (1.5 M) was added drop by drop into the solution under vigorous stirring in 30 min under a nitrogen atmosphere. On the surface of Fe3O4 nanoparticles the mixed hemimicelle of CS formed and the color of solution changed from orange to brown immediately. After cooling to room temperature, the suspension was washed sequentially with deionized water (3 × 100 mL), methanol (3 × 100 mL), and deionized water (5 × 200 mL).
Preparation of Polythiophene/Chitosan Magnetic Nanocomposite
The preparation procedure of polythiophene/chitosan magnetic nanocomposite consisted of the following steps. First, chitosan/Fe3O4 sub microspheres with surface containing fictional reactive site (including −OH and −NH2 groups) were prepared according to the current method. The CS–MNPs have hydrophobic and hydrophilic moieties so they could facilitate the dissolution of thiophene monomers. Then, the polythiophene/chitosan magnetic nanocomposite was prepared by self-assembly polymerization method. Chitosan/magnetic nanoparticles (0.5 g) and different amount of thiophene monomers were successively added into a 250-mL three-necked round-bottom flask equipped with a condenser pipe and a stirring device. After deoxygenated by N2 for several minutes, the mixture was heated from room temperature to 60 °C, and then kept at this temperature for reaction time. Then suitable amount of APS, as initiator, was added to the solution and stirred for 1 h at this temperature and CS–PTh magnetic nanocomposites were obtained and the color of solution changed from brown to black immediately. After the sample was cooled to room temperature, the resulting polythiophene/chitosan magnetic nanocomposite were separated by means of external magnetic field and washed several times by redispersion in methanol and double distilled water. The washing procedure was continued until the filtrate become colorless.
Extraction Procedure
For the extraction purpose, 40 mg of polythiophene/chitosan magnetic nanocomposite were added into 50 mL double distillated water sample spiked with triazines to a final concentration of 1 mg L−1. Then the sample vial was sealed and the solution was stirred at the maximum stirring rate (1000 rpm) for 15 min. The supernatant was then removed and a magnet was paced on the outer wall of the vial to collect the polythiophene/chitosan magnetic nanocomposite. After performing the extraction, the polythiophene/chitosan magnetic nanocomposite was dried under nitrogen flow for about 30 s and the analytes were desorbed by 350 µL methanol at 5 min in ultrasonic bath. Then, the desorption solvent was evaporated under N2 flow until complete solvent drying. Finally, 10 µL methanol was added to the desorption vial and then 2 µL of desorbed solution was injected into the injection port of GC.
Instruments and Measurement
A gas chromatograph model Agilent 6820 with a split/splitless injection port and a flame ionization detection system, was used for optimization. The separation of analytes was carried by a column HP −5 MS (60 m, 0.25 mm i.d.) with 0.25 μm film thickness. The carrier gas was helium (99.99 %) at a flow rate of 1 mL min−1. The sample introduction was performed in the splitless mode and the split valve was kept closed for 3 min. The injector and detector temperatures were set at 260 and 290 °C, respectively. For real samples analysis and the quantitative survey, a Hewlett-Packard (HP, Palo Alto, CA, USA) HP 6890 series GC equipped with a split/splitless injector and a HP 5973 mass—selective detector system were used. The MS was operated in the EI mode (70 eV). Helium (99.999 %) was used as carrier gas and its flow-rate was adjusted to 1 mL min−1. The separation of the selected triazines was performed on a 60 m × 0. 25 mm HP −5 MS column with 0.25 μm film thickness. The GC–MS interface, ion source and quadrupole temperatures were set at 270, 230 and 150 °C, respectively. For quantitative analysis, the MS was set on the selected ion monitoring (SIM) mode and two characteristics ions for each compound were monitored (Table 1). The column temperature was programmed at 70 °C for 3 min, increased to 170 °C at a rate of 25 °C min−1 and kept at this temperature for 1 min and to 270 °C at 30 °C min−1 (hold for 1 min). The scanning electronic microscopy (SEM) images recorded using Cambridge Stereoscan 360 SEM Instrument (England) operating at 20 kV. The sample pH was measured by a Metrohm Herisan pH meter (Switzerland). A permanent magnet of NdFeB (80, 40 and 30 mm) model N48 with a magnetic field of 1.2 T was purchased from Ningbo Strong Magnet Material Co., Ltd. (Ningbo, China).
Results and Discussion
The objective of this study was to develop and characterize a magnetic solid phase extraction to perform preconcentration of triazines in a single step using new magnetic nanocomposite sorbent. Several parameters such as eluent type and volume, amount of sorbent, component ratio, adsorption and desorption time, sample pH and ionic strength were optimized to achieve the best extraction efficiency of analytes using polythiophene/chitosan magnetic sorbent. Each experiment was performed in triplicates. Double-distilled water spiked with 1 mg L−1 of triazines was used for optimization of the extraction parameters.
Characterization of Polythiophene/Chitosan Nanocomposite
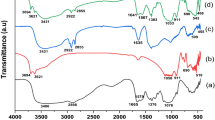
The morphology of magnetic nanoparticles, chitosan magnetic and polythiophene/chitosan magnetic nanocomposite were determined by SEM and shown in Fig. 1. The SEM image of the iron oxide NPs along with histogram plot show that the synthesized MNPs have rather high surface area and also the substructures with dimensions less than 50 nm could be observed (Fig. 1a, b). The synthesized magnetic nanoparticles, Fe3O4/chitosan and polythiophene/chitosan magnetic nanocomposite have large specific surface area and are more suitable for adsorption application (Fig. 1). Figure 1d shows that the final products exhibit slight aggregation as a result of surface modification by the attachment of PTh. This could be attributed to the fact that the reaction might occur on the particle surface and several PTh molecules were bound to chitosan on the magnetic particle.
Selection of Eluting Solvent
The eluting solvent is an important factor that affects the MSPE procedure. A suitable solvent can effectively elute the adsorbed analytes with the minimum volume and less interfering impurities co-eluted. In this experiment, six solvents including methanol, toluene, acetonitrile, n-hexane, dichloromethane and acetone were studied. The polar solvents such as ethanol, acetonitrile and methanol provided good results (Fig. 2a), however, there was higher intensity when using acetonitrile as eluting solvent. Therefore, acetonitrile was selected. The solvent volume and desorption time were investigated. The solvent volume increased from 50 to 500 µL (Fig. 2b), and the desorption time from 0.5 to 10 min under ultrasonic were studied. The result indicated that the ultrasonic time had clear effect on the eluting efficiency. The time of desorption should be as short as possible while carryover effects must be considered comprehensively. The result indicated that desorption could occur completely within 5 min and 350 µL of acetonitrile without carryover effect.
Sorbent Type
The structure and morphology of adsorbent are crucial parameters in the extraction approach. In this research, the extraction efficiency of Fe3O4 nanoparticles coated with CS and several types of PTh/CS in various components ratios along with undecorated magnetic nanoparticles were investigated by extracting selected triazines, model compounds, from aquatic media. According to Fig. 2c, the extraction capabilities of the CS–MNPs are also higher than the undecorated iron oxide nanoparticles. This result shows that CS is expected to play an important role in the extraction of triazines. Also, the effect of the thiophene concentration was considered in the range of 0.3–2 M, whereas the content of the CS–MNPs remained at a constant level of 0.5 g. The obtained results indicated that by increasing the thiophene concentration until 0.9 M, the peak intensity was enhanced. After this, it started to slowly decrease until 2 M of thiophene. Therefore, an amount of 0.9 M of thiophene was selected as the optimum value (Fig. 2d). Selecting this value caused high extraction efficiency. Also, this trend conforming that PTh has an important effect in the extraction process and this concentration was chosen as the optimum value. By increasing the thiophene content in reaction solution, the magnetic properties of CS–PTh magnetic nanocomposite was decreasing and can lead to the loss of sorbent during their collection by the external magnetic field.
Sample pH
Solution pH plays an important role for the adsorption of target compounds by affecting the existing form of analyte, and the charge species and density on the sorbents surface. The pKa values of selected triazines range from 1.6 to 4.1 [44]. The interested analytes, thus, remain neutral in double distilled water (pH = 6) and there is no need to perform any extra treatment before using this technique. Thus, different pH solutions of 1–11 were studied to acquire the optimized pH for maximum extraction. The experimental results (Fig. 3a) showed that the polythiophene/chitosan magnetic nanocomposite gave the best performance in neutral solution (pH = 7). The experimental results showed that the polythiophene/chitosan magnetic nanocomposite gave the best performance in neutral solution (pH = 7). This result can be easily described via the electrostatic forces between functional group of triazines (−NH) and functional groups in polythiophen-chitosan magnetic nanocomposite −NH2 and −OH at chitosan along with—S at thiophene) in the solutions having different pH values. Since, in strong acidic solutions, decrease in the extraction efficiency was probably due to the surface protonation of the adsorbent (sulfur, oxygen and nitrogen donor atoms) and triazines (nitrogen donor atom) by protons present in solution. At this region (pH <5) both of the analytes and sorbent have the same charge, an electrostatic repulsion force between them is expected to lower the extraction efficiency. As can be seen from the related figure, further increase of pH above 7 resulted in slight decrease of extraction, thus, most likely pH 7 was selected because provides the highest recoveries.
Ionic Strength
The salt addition effect has been commonly used in various extraction methodologies. Generally, addition of salt usually increases the ionic strength of the aqueous solution and would affect the solubility of organic solutes. This can be explained by the engagement of water molecules in the hydration spheres around the ionic salt. These hydration spheres reduce the concentration of water available to dissolve solute molecules. This should, then, drive additional solutes into extracting medium. Considering these facts, the influence of salt addition was studied in the range of 0–30 % (w/v) of NaCl. Generally, salt addition can decrease the solubility of analytes in the aqueous phase while enhancing their partitioning into the organic phase. However, in this work, salt addition had an adverse effect on the overall efficiency of the method and was, therefore, abandoned. There are three reasons for this phenomena including (1) the addition of NaCl suppressed the thickness of electrical adsorption layer at the sorbent solution interface, which led to the decrease of mixed hemimicelles formed on the sorbent surface [45]. (2) The presence of salt may increase the viscosity of solutions and reduce the adsorption ability of sorbent. At higher salt concentration the increase of viscosity affect the behavior of analytes and distorts the exponential manner. Diffusion coefficient depends on size and shape of molecule, interaction with solvent and viscosity of solvent. Relation between diffusion coefficient (D) and viscosity (η) can be expressed via the equation:
In which, k is Boltzmann constant, T is absolute temperature and r is radius of molecule. The amount of time required to attain equilibrium increased due to the rate of mass transfer of the analyte from the aqueous phase to the solid sorbent. Thus, the amount of time required to attain equilibrium decreased were performed without adding NaCl [46, 47]. (3) This may be due to the reduction of active site of sorbent [48]. In this work, due to adverse effect of ionic strength, a significant decrease in extraction efficiency of analytes was observed when the amount of salt exceeded. Consequently, salt addition had an adverse effect on the overall efficiency of the method and was, therefore, abandoned.
Effect of the Amount of Magnetic Nanocomposite
Fewer amounts of nano-adsorbents may be achieved more satisfactory results than micro-adsorbents because of their greater surface areas. To find the optimized amount of adsorbent for the extraction, the amounts of PTh/CS magnetic nanocomposite ranging from 2 to 100 mg were tested. The results show that only 40 mg of magnetic sorbent was needed to obtain maximum extraction of triazine under the same conditions. When the amount was over 40 mg, all the PTh/CS magnetic nanocomposite may not separate effectively in the same time, which leads to a decrease in recoveries. In addition, the small amount of magnetic PTh/CS magnetic nanocomposite justifies the minimal elution volumes for efficient release of the analyte from the sorbent. Therefore, 40 mg of new sorbent was used in the next experiments.
Effect of Extraction Time on the Adsorption Efficiency
The effect of extraction time on the adsorption of triazines in the range 3–35 min was studied (Fig. 3b). It could be seen that the adsorption equilibrium was achieved at about 15 min. The high surface area of MNPs along with uniform disperse of the sorbent throughout the sample could be the promising reasons for attaining fast extraction process. This is a superior benefit over the conventional SPE and other micro-extraction techniques, which generally need more than 30 min to reach the equilibrium. Consequently, an extraction time of 15 min was selected for the subsequent experiment.
Analytical Performance
The optimized magnetic solid phase extraction method based on the fabricated magnetic polythiophene–chitosan nanocomposite was evaluated by quantitative analysis of the spiked DDW samples. The linear dynamic range and other analytical parameters were determined based on method sensitivity. In this work, the two gas chromatography instruments with flame detector and mass spectrometer were used. A gas chromatography with flame ionization detection system was used for the optimization process. Since the sensitivity of flame detectors are much less compared to mass spectrometry. So we had a higher concentration (1000 ng mL−1) was used to optimize. However, triazines are recognized herbicides which have been broadly used in agriculture over the recent decades. Their high persistence and toxicity have required rigorous control of environmental contamination. Therefore, the presence of pesticides in surface waters is regulated by the European Directive 2008/105/EC that establishes a maximum permitted concentration of 2 μg L−1 for atrazine. Thus, sensitive methods for determining the low concentrations of triazine herbicides in environmental samples are required. In addition, the aim of this study was to develop a MSPE method based on PTh/chitosan magnetic nanocomposite for the determination of trace levels of triazines in water samples. Therefore, to improve quantification determination, a gas chromatograph equipped with a mass-selective detector system was used to evaluate the precision of the measurements, the limits of detection and the dynamic range of the method at range of tracer concentration. Some analytical features including linear range, correlation of determination, limit of detection, and repeatability were investigated. Analytical performance of the developed procedure is plotted in Table 2. Calibration curves were created by six different concentrations of the triazines in the range of 100–5000 ng L−1. The results indicate good linearity for all the analytes throughout the concentration range with determination coefficients (R 2 > 0.9994). Limit of detections (LODs) and limit of quantification (LOQ) based on S/N = 3 and 10, were 10–30 and 100 ng L−1, respectively. The relative standard deviation (RSD) was assessed in relations of repeatability (from five independent sample preparations, intra-day RSD) was 7–12 % at a concentration level of 150 ng L−1. To study the matrix effect, the developed method was applied to the tap water, Tehran rain water and Jajrood river samples. Acceptable relative recoveries in the range of 96–102 % for the selected analyte were achieved. The results in Table 2 confirm the validity of the proposed method. The chromatogram of analytes in real sample with spiking triazines at 450 ng L−1 is shown in Fig. 4. The reusability and stability of PTh/CS magnetic nanocomposite for the isolation of triazines was assessed by performing five consecutive separations–desorption cycles under the optimized conditions. There was no significant change in the performance of the sorbent during these cycles, indicating that the fabricated PTh–CS magnetic nanocomposite is a reusable and stable micro-solid phase sorbent for the extraction of triazines. The comparison of the current work with some other methods on the determination of triazines (Table 3) reveals that this method is either comparable or has rather pronounced advantages over them [30, 49–51]. Considering the results, the MSPE method along with applied new magnetic sorbent demonstrated to be a sensitive, efficient, reliable, and easy to use technique with good precision and dynamic linear range in extraction and preconcentration of the triazines from aqueous samples. Furthermore, simple, consuming small amounts of organic solvent and sorbent, high stability in different solution and fast fabrication of the polythiophene/chitosan magnetic nanocomposite is the key benefit of the proposed sorbent in comparison with other reported MNPs sorbents. Also, there is possibility of extraction of triazines from large volumes of sample in lower extraction times in comparison with conventional SPE sorbents.
Conclusions
A novel type of modified magnetic nanoparticles coated with polythiophene was synthesized and employed as magnetic solid phase extraction adsorbent for preconcentration of selected triazines in aqueous water. Polythiophene/CS magnetic nanocomposite could be easily produced in large quantity using the oxidized polymerization method. The separation of magnetic adsorbent containing triazines from the solution could be easily achieved by applying a permanent magnet. Moreover, the optimal method had attained acceptable analysis results of real sample with a little amount of adsorbents within a short period of time. Coating of MNPs with polythiophene not only can increase adsorption ability of the target analyte, but also improve stability of the NPs and their dispersibility in aqueous media. These advantages make the developed method a reliable and robust approach towards trace analysis of polar organic species in a variety of samples.
Change history
07 August 2018
The authors would like to call the reader’s attention to the fact that unfortunately the order of authors’ names was wrong in the original publication.
References
Basheer C, Alnedhary AA, Rao BSM, Valliyaveettil S, Lee HK (2006) Anal Chem 78:2853
Xu L, Lee HK (2008) J Chromatogr A 1192:203
Basheer C, Narasimhan K, Yin M, Zhao C, Choolani M, Lee HK (2008) J Chromatogr A 1186:358
Bagheri H, Saraji M, Barcelo D (2004) Chromatographia 59:283
Bagheri H, Mohammadi A (2003) J Chromatogr A 1015:23
Wang S, Zhao P, Min G, Fang G (2007) J Chromatogr A 1165:166
Hu X, Hu Y, Li G (2007) J Chromatogr A 11:1
Rocha C, Pappas EA, Huang C (2008) Environ Pollut 152:239
Bagheri H, Babanezhad E, Es-haghi A (2007) J Chromatogr A 1152:168
Sanchez-Ortega A, Unceta N, Gomez-Caballero A, Sampedro MC, Akesolo U, Goicolea MA, Barrio RJ (2009) Anal Chim Acta 641:110
Lokhnauth JK, Snow NH (2006) J Chromatogr A 1105:33
Yang L, Su P, Chen X, Zhang R, Yang Y (2015) Anal Methods 7:3246
Li QL, Lam MHW, Wu RSS, Jiang BW (2010) J Chromatogr A 1217:1219
Batlle X, Labarta A (2002) J Phys D Appl Phys 35:15
Zhang SX, Niu HY, Cai YQ, Shi YL (2010) Anal Chim Acta 665:167
Jeong U, Teng XW, Wang Y, Xia YN (2007) Adv Mater 19:33
Hsu CC, Whang CW (2009) J Chromatogr A 1216:8575
Gao Q, Luo D, Ding J, Feng YQ (2010) J Chromatogr A 1217:5602
Beveridge JS, Stephens JR, Latham AH, Williams ME (2009) Anal Chem 81:9618
Habibi MR, Ghasemi M (2011) J Magn Magn Mater 323:32
Girginova PI, Daniel-Da-Silva AL, Lopes CB, Figueira P, Otero M, Amaral VS, Pereira E, Trindade T (2010) J Colloid Interface Sci 345:234
Lu Y, Yin YD, Mayers BT, Xia YN (2002) Nano Lett 2:183
Li ZB, Huang DN, Fu CF, Wei BW, Yu WJ, Deng CH, Zhang XM (2011) J Chromatogr A 1218:6232
Zhang XL, Niu HY, Pan YY, Shi YL, Cai YQ (2010) Anal Chem 82:2363
Román I, Chisvert A, Canals A (2011) J Chromatogr A 1218:2467
Liu XY, Yin JJ, Zhu L, Zhao GH, Zhang HX (2011) Talanta 85:2451
Xu Z, Zhang J, Cong L, Song JM, Zhou J, Qiao XG (2011) J Sep Sci 34:46
Meng JR, Shi CY, Wei BW, Yu WJ, Deng CH, Zhang XM (2011) J Chromatogr A 1218:2841
Meng JR, Bu J, Deng CH, Zhang XM (2011) J Chromatogr A 1218:1585–1591
Zhao GY, Song SJ, Wang C, Wu QH, Wang Z (2011) Anal Chim Acta 708:155
Luo YB, Shi ZG, Gao Q, Feng YQ (2011) J Chromatogr A 1218:1353
Wu QH, Zhao GY, Feng C, Wang C, Wang Z (2011) J Chromatogr A 1218:7936
Pardasani D, Kanaujia PK, Purohit AK, Shrivastava AR, Dubey DK (2011) Talanta 86:248
Moliner-Martínez Y, Ribera A, Coronado E, Campíns-Falcó P (2011) J Chromatogr A 1218:2276
Bagheri H, Zandi O, Aghakhani A (2011) Anal Chim Acta 692:80
Eskandari H, Shariati MR (2011) Anal Chim Acta 704:146
Abolghasemi MM, Yousefi V (2014) J Sep Sci 37:120
Buszewski B, Olszowy P, Pikus S, Kozak M (2014) Monatshefte für Chemie Chem Mon 145:527
Li X, Li C, Chen J, Li C, Sun C (2008) J Chromatogr A 1198:7
Tahmasebi E, Yamini Y, Moradi M, Esrafili A (2013) Anal Chim Acta 770:68
Tahmasebi E, Yamini Y (2014) Microchim Acta 181:543
Hu C, Mou Z, Lu G, Chen N, Dong Z, Hu M, Qu L (2013) Phys Chem Chem Phys 15:13038
Xiaoli Z, Yali S, Yaqi C, Shifen M (2008) Environ Sci Technol 42:1201
Tomlin CDS (2000) The pesticide manual. British Crop Protection Council, Surrey
Huanga Y, Zhou Q, Xie G (2011) J Hazard Mater 193:82–89
Botero C, Kremer H, Froba AP, Leipertz A (2005) Diffus Fundam 2(67):1
Basheer C, Chong HG, Hii TM, Lee HK (2007) Anal Chem 79:6845
Ahmadi F, Asgharloo H, Sadeghi S (2009) J Chromatogr B 877:2945
Min G, Wang S, Zhu H, Fang G, Zhang Y (2008) Sci Total Environ 3906:79
Bagheri H, Es’haghi A, Es-haghi A, Mohammadkhani E (2013) Anal Chim Acta 792:59
Wang Y, Sun Y, Xu B, Li X, Jin R, Zhang H, Song D (2014) J Chromatogr A 1373:9
Acknowledgments
The Research Council and Graduates School of Islamic Azad University Central Tehran Branch are acknowledged for supporting the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animal performed by any of the authors.
Rights and permissions
About this article
Cite this article
Feizbakhsh, A., Ehteshami, S. Polythiophene–Chitosan Magnetic Nanocomposite as a Novel Sorbent for Disperse Magnetic Solid Phase Extraction of Triazine Herbicides in Aquatic Media. Chromatographia 79, 1177–1185 (2016). https://doi.org/10.1007/s10337-016-3134-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3134-9